Selective Serotonin Reuptake Inhibitors for the Treatment of Depression in Adults with Down Syndrome: A Preliminary Retrospective Chart Review Study
Abstract
:1. Background
2. Methods
2.1. Study Participants
2.2. Outcomes
2.3. Statistical Analysis
3. Results
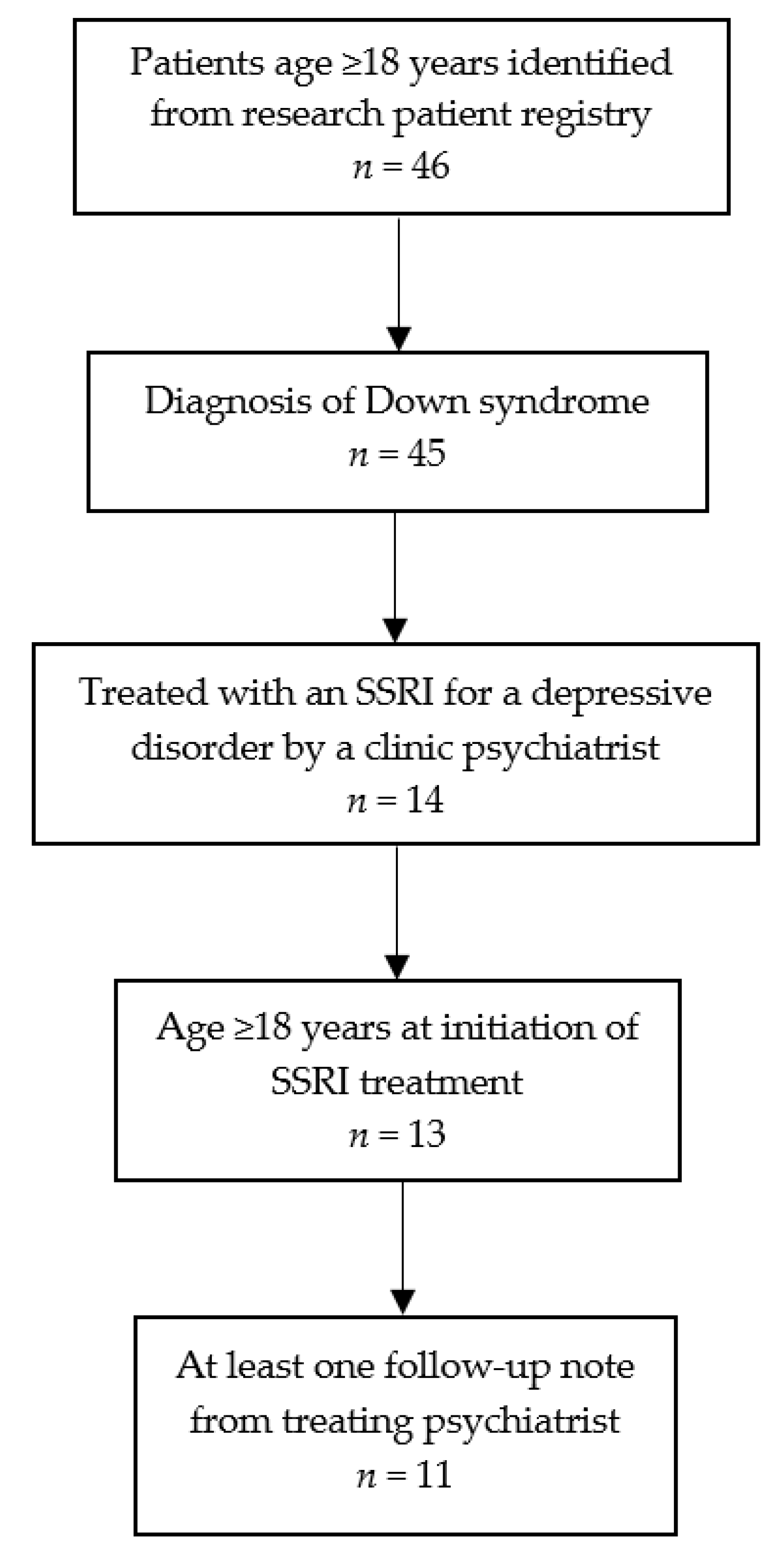
3.1. Study Flow
3.2. Demographic and Clinical Characteristics of the Sample
3.3. Characteristics of Depressive Episodes and SSRI Treatment
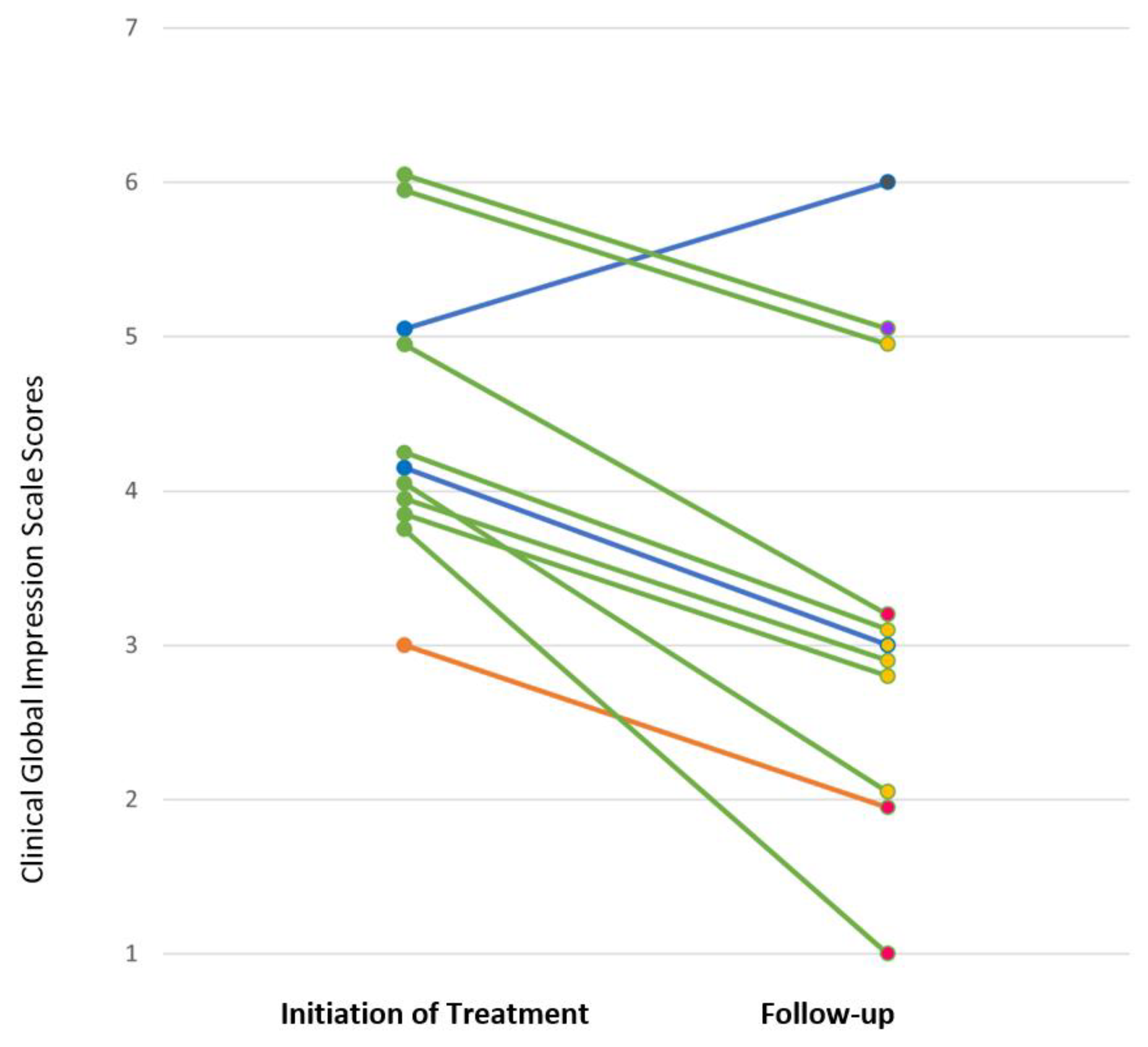
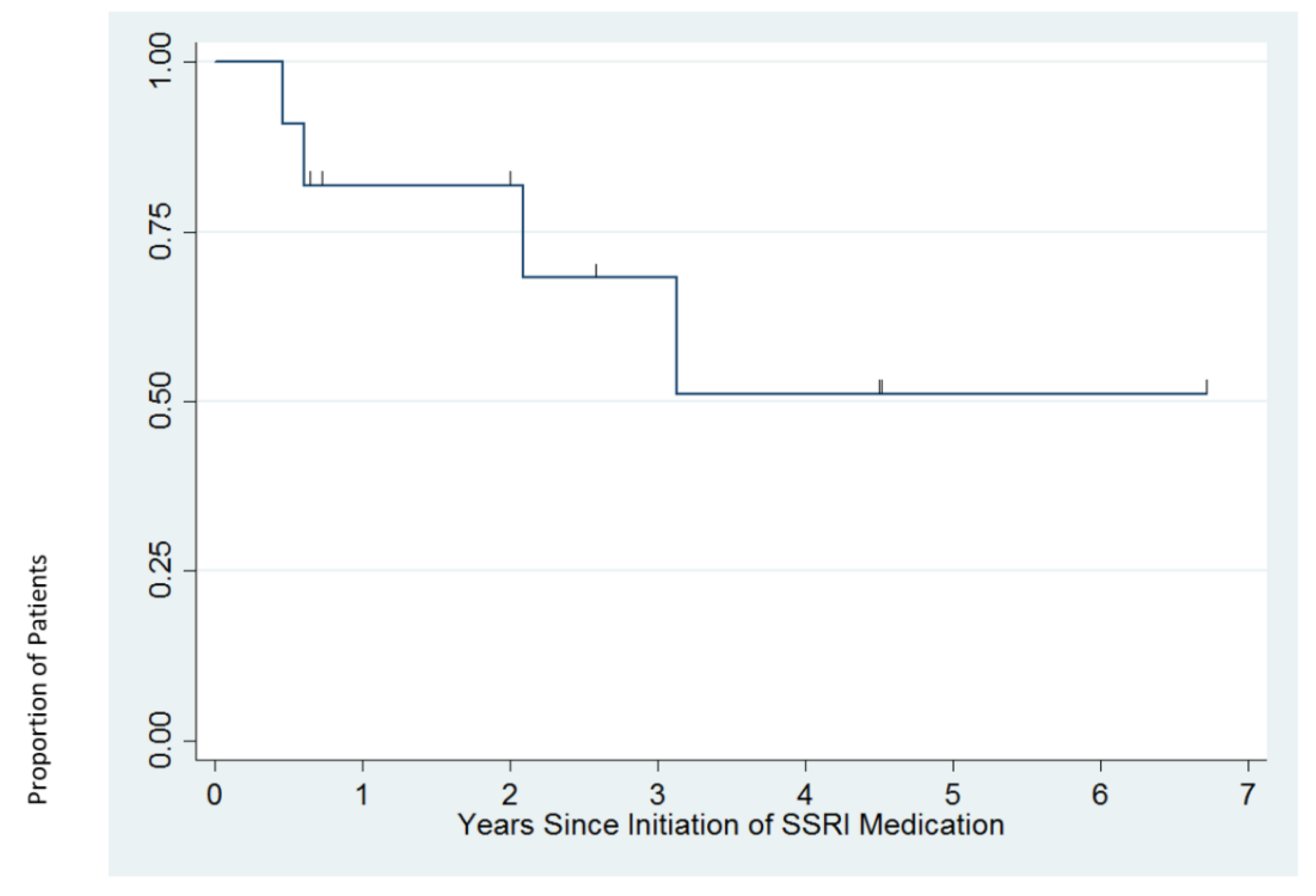
3.4. Response to SSRI Treatment
3.5. Adverse Effects
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosures
References
- CDC. Facts about Down Syndrome. Available online: https://www.cdc.gov/ncbddd/birthdefects/downsyndrome.html (accessed on 25 June 2020).
- Mai, C.T.; Isenburg, J.L.; Canfield, M.A.; Meyer, R.E.; Correa, A.; Alverson, C.J.; Lupo, P.J.; Riehle-Colarusso, T.; Cho, S.J.; Aggarwal, D.; et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019, 111, 1420–1435. [Google Scholar] [CrossRef]
- Ivan, D.L.; Cromwell, P. Clinical practice guidelines for management of children with Down syndrome: Part, I. J. Pediatric Health Care 2014, 28, 105–110. [Google Scholar] [CrossRef]
- Gath, A.; Gumley, D. Behavior problems in retarded children with special reference to Down’s syndrome. Br. J. Psychiatry 1986, 149, 156–161. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; Boyd, J. Psychopathology and young people with down’s syndrome: Childhood predictors and adult outcome of disorder. J. Intellect. Disabil. Res. 2001, 45, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.L.; McDougle, C.J. Pharmacotherapy of down syndrome. Expert Opin. Pharmacother. 2018, 19, 1875–1889. [Google Scholar] [CrossRef] [PubMed]
- Visootsak, J.; Sherman, S. Neuropsychiatric and behavioral aspects of trisomy 21. Curr. Psychiatry Rep. 2007, 9, 135–140. [Google Scholar] [CrossRef]
- Dykens, E.M. Psychiatric and behavioral disorders in persons with Down syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2007, 13, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.A.; Pueschel, S.M. Psychiatric disorders in persons with down syndrome. J. Nerv. Ment. Dis. 1991, 179, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A.; Collacott, R.A. Clinical features and diagnostic criteria of depression in Down’s syndrome. Br. J. Psychiatry 1994, 165, 399–403. [Google Scholar] [CrossRef]
- Dykens, E.M.; Shah, B.; Davis, B.; Baker, C.; Fife, T.; Fitzpatrick, J. Psychiatric disorders in adolescents and young adults with Down syndrome and other intellectual disabilities. J. Neurodev. Disord. 2015, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Mantry, D.; Cooper, S.A.; Smiley, E.; Morrison, J.; Allan, L.; Williamson, A.; Finlayson, J.; Jackson, A. The prevalence and incidence of mental ill-health in adults with Down syndrome. J. Intellect. Disabil. Res. 2008, 52, 141–155. [Google Scholar] [CrossRef]
- Startin, C.M.; D’Souza, H.; Ball, G.; Hamburg, S.; Hithersay, R.; Hughes, K.M.O.; Massand, E.; Karmiloff-Smith, A.; Thomas, M.S.C.; Strydom, A.; et al. Health comorbidities and cognitive abilities across the lifespan in down syndrome. J. Neurodev. Disord. 2020, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Zammit, S.; Allebeck, P.; David, A.S.; Dalman, C.; Hemmingsson, T.; Lundberg, I.; Lewis, G. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch. Gen. Psychiatry 2004, 61, 354–360. [Google Scholar] [CrossRef]
- Seidl, R.; Kaehler, S.T.; Prast, H.; Singewald, N.; Cairns, N.; Gratzer, M.; Lubec, G. Serotonin (5-HT) in brains of adult patients with Down syndrome. J. Neural Transm. Suppl. 1999, 57, 221–232. [Google Scholar] [CrossRef]
- Walker, J.C.; Dosen, A.; Buitelaar, J.K.; Janzing, J.G.E. Depression in Down syndrome: A review of the literature. Res. Dev. Disabil. 2011, 32, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Storm, W. Differential diagnosis and treatment of depressive features in Down’s syndrome: A case illustration. Res. Dev. Disabil. 1990, 11, 131–137. [Google Scholar] [CrossRef]
- Myers, B.A.; Pueschel, S.M. Major depression in a small group of adults with Down syndrome. Res. Dev. Disabil. 1995, 16, 285–299. [Google Scholar] [CrossRef]
- Szymanski, L.; Biederman, J. Depression and anorexia nervosa of persons with Down syndrome. Am. J. Ment. Defic. 1984, 89, 246–251. [Google Scholar]
- Schatzberg, A.; DeBattista, C. Manual of Clinical Psychopharmacology, 8th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2015. [Google Scholar]
- Kennedy, S.H.; Lam, R.W.; McIntyre, R.S.; Tourjman, S.V.; Bhat, V.; Blier, P.; Hasnain, M.; Jollant, F.; Levitt, A.J.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological treatments. Can. J. Psychiatry 2016, 61, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Denee, T.; Kerr, C.; Ming, T.; Wood, R.; Tritton, T.; Middleton-Dalby, C.; Massey, O.; Desai, M. Current treatments used in clinical practice for major depressive disorder and treatment resistant depression in England: A retrospective database study. J. Psychiatr. Res. 2021, 139, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Cascade, E.; Kalali, A.H.; Kennedy, S.H. Real-world data on SSRI antidepressant side effects. Psychiatry 2009, 6, 16–18. [Google Scholar]
- CGI—Clinical Global Impressions Scale. Available online: https://eprovide.mapi-trust.org/instruments/clinical-global-impressions-scale (accessed on 23 June 2020).
- Busner, J.; Targum, S.D. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 2007, 4, 28. [Google Scholar] [PubMed]
- Rush, A.J.; Kraemer, H.C.; Sackeim, H.A.; Fava, M.; Trivedi, M.H.; Frank, E.; Ninan, P.T.; Thase, M.E.; Gelenberg, A.J.; Kupfer, D.J.; et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006, 31, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Capone, G.T.; Aidikoff, J.M.; Taylor, K.; Rykiel, N. Adolescents and young adults with down syndrome presenting to a medical clinic with depression: Co-morbid obstructive sleep apnea. Am. J. Med. Genet. Part A 2013, 161, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S. Essential Psychopharmacology: The Prescriber’s Guide; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
| Eligible for Inclusion n = 11 | |
|---|---|
| Sex, n (%) | |
| Female | 4 (36%) |
| Male | 7 (64%) |
| Age, mean (SD; range in years) | 27.2 (7.7; 18–46) |
| Race 1, n (%) | |
| White | 10 (91%) |
| Other | 1 (9%) |
| Intellectual Disability, n (%) | |
| Any intellectual disability | 11 (100%) |
| Mild | 2 (18%) |
| Moderate | 8 (73%) |
| Severe | 1 (9%) |
| Language Ability | |
| Full sentences | 7 (64%) |
| Phrase speech | 3 (27%) |
| Single words | 1 (9%) |
| Age at onset of first depressive episode, mean (SD; range in years) | 21.6 (10.1; 5–44) |
| Comorbid psychiatric diagnoses, n (%) | |
| Any diagnosis | 3 (27%) |
| Generalized anxiety disorder | 2 (18%) |
| Alzheimer’s disease | 1 (9%) |
| Comorbid medical diagnoses 2, n (%) | |
| Any diagnosis | 11 (100%) |
| Congenital heart disease | 7 (64%) |
| Obstructive sleep apnea | 4 (36%) |
| Hypothyroidism | 3 (27%) |
| Prior psychiatric medications, n (%) | |
| Any prior psychiatric medication | 5 (45%) |
| SSRI 3 | 3 (27%) |
| Second-generation antipsychotic | 2 (18%) |
| Buspirone | 2 (18%) |
| Donepezil | 1 (9%) |
| Eligible for Inclusion n = 11 | |
|---|---|
| Depressive Episode | |
| CGI-S score, n (%) | |
| 3: Mildly ill | 1 (9%) |
| 4: Moderately ill | 6 (55%) |
| 5: Markedly ill | 2 (18%) |
| 6: Severely ill | 2 (18%) |
| Suicidal ideation at baseline, n (%) | 1 (9%) |
| First depressive episode, n (%) | 7 (64%) |
| Psychotic features, n (%) | 2 (18%) |
| Treatment | |
| SSRI, n (%) | |
| Fluoxetine | 8 (73%) |
| Initial dose in mg, mean (SD; range) | 4.9 (0.4; 4.0–5.0) |
| Maximal dose in mg, mean (SD; range) | 25.5 (18.7; 14.0–70.0) |
| Sertraline 2 | 2 (18%) |
| Escitalopram 2 | 1 (9%) |
| Concomitant medication treatments 1, n (%) | |
| Any medication treatment | 5 (45%) |
| Second-generation antipsychotic | 3 (27%) |
| Buspirone | 2 (18%) |
| Concomitant non-medication treatments, n (%) | |
| Any non-medication treatment | 10 (91%) |
| Day program | 9 (82%) |
| Individual psychotherapy | 5 (45%) |
| Patient | SSRI | CGI-S (Baseline) | CGI-I (12 Weeks) | Follow-up Duration | Long-Term Treatment Course |
|---|---|---|---|---|---|
| 1 | sertraline | 5 | 5 | 37 weeks | Depression became more severe and psychotic features emerged over first 12 weeks of treatment. Ultimately did well on sertraline 62.5 mg per day and aripiprazole 6 mg per day. |
| 2 | sertraline | 4 | 2 | 6.7 years | Depression continued to improve after 12 weeks with gradual upward dose titration. Doing well on sertraline 125 mg per day at last follow-up. |
| 3 | escitalopram | 3 | 1 | 2.1 years | Depression responded well to escitalopram 12.5 mg per day for ~2 years, but anxiety persisted. Higher doses of escitalopram were associated with fatigue, tearfulness, and irritability. Escitalopram was switched to another SSRI, which also caused tearfulness. The patient was doing well without medications for several months at last follow-up. |
| 4 | fluoxetine | 5 | 1 | 2.0 years | Continued to do well on fluoxetine 14 mg per day at last follow up. |
| 5 | fluoxetine | 4 | 2 | 4.5 years | Continued to do well on fluoxetine 15 mg per day and aripiprazole 2 mg per day at last follow-up. Did not tolerate aripiprazole taper due to irritability. |
| 6 | fluoxetine | 6 | 3 | 2.6 years | Experienced 25–30% improvement on fluoxetine 55 mg per day. Adjunctive buspirone 7.5 mg BID resulted in remission after six months of treatment. |
| 7 | fluoxetine | 4 | 2 | 4.5 years | After six months of fluoxetine 20 mg per day, experienced mania, and fluoxetine was discontinued. Ultimately did well on carbamazepine 300 mg BID and guanfacine 0.5 mg BID. |
| 8 | fluoxetine | 4 | 2 | 24 weeks | Experienced 50% improvement on fluoxetine 20 mg per day. When fluoxetine was increased to 25 mg, experienced agitation, irritability, and anger. Follow-up data after fluoxetine was tapered is not available. |
| 9 | fluoxetine | 6 | 2 | 33 weeks | Experienced moderate improvement on fluoxetine 15 mg per day. |
| 10 | fluoxetine | 4 | 2 | 4.5 years | Depression responded to fluoxetine 20 mg per day. After two years of stability, fluoxetine was tapered and depression recurred. Symptoms resolved when fluoxetine was increased back to 20 mg per day. |
| 11 | fluoxetine | 4 | 1 | 3.1 years | After three years of stability on fluoxetine 30 mg, fluoxetine was tapered and discontinued without return of depression. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thom, R.P.; Palumbo, M.L.; Thompson, C.; McDougle, C.J.; Ravichandran, C.T. Selective Serotonin Reuptake Inhibitors for the Treatment of Depression in Adults with Down Syndrome: A Preliminary Retrospective Chart Review Study. Brain Sci. 2021, 11, 1216. https://doi.org/10.3390/brainsci11091216
Thom RP, Palumbo ML, Thompson C, McDougle CJ, Ravichandran CT. Selective Serotonin Reuptake Inhibitors for the Treatment of Depression in Adults with Down Syndrome: A Preliminary Retrospective Chart Review Study. Brain Sciences. 2021; 11(9):1216. https://doi.org/10.3390/brainsci11091216
Chicago/Turabian StyleThom, Robyn P., Michelle L. Palumbo, Claire Thompson, Christopher J. McDougle, and Caitlin T. Ravichandran. 2021. "Selective Serotonin Reuptake Inhibitors for the Treatment of Depression in Adults with Down Syndrome: A Preliminary Retrospective Chart Review Study" Brain Sciences 11, no. 9: 1216. https://doi.org/10.3390/brainsci11091216






