Non-Immersive Virtual Reality to Improve Balance and Reduce Risk of Falls in People Diagnosed with Parkinson’s Disease: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Source Data and Search Strategy
2.3. Study Design
2.4. Study Screening: Inclusion and Exclusion Criteria
2.5. Data Extraction
2.6. Outcomes
2.7. Risk of Bias Assessment
3. Results
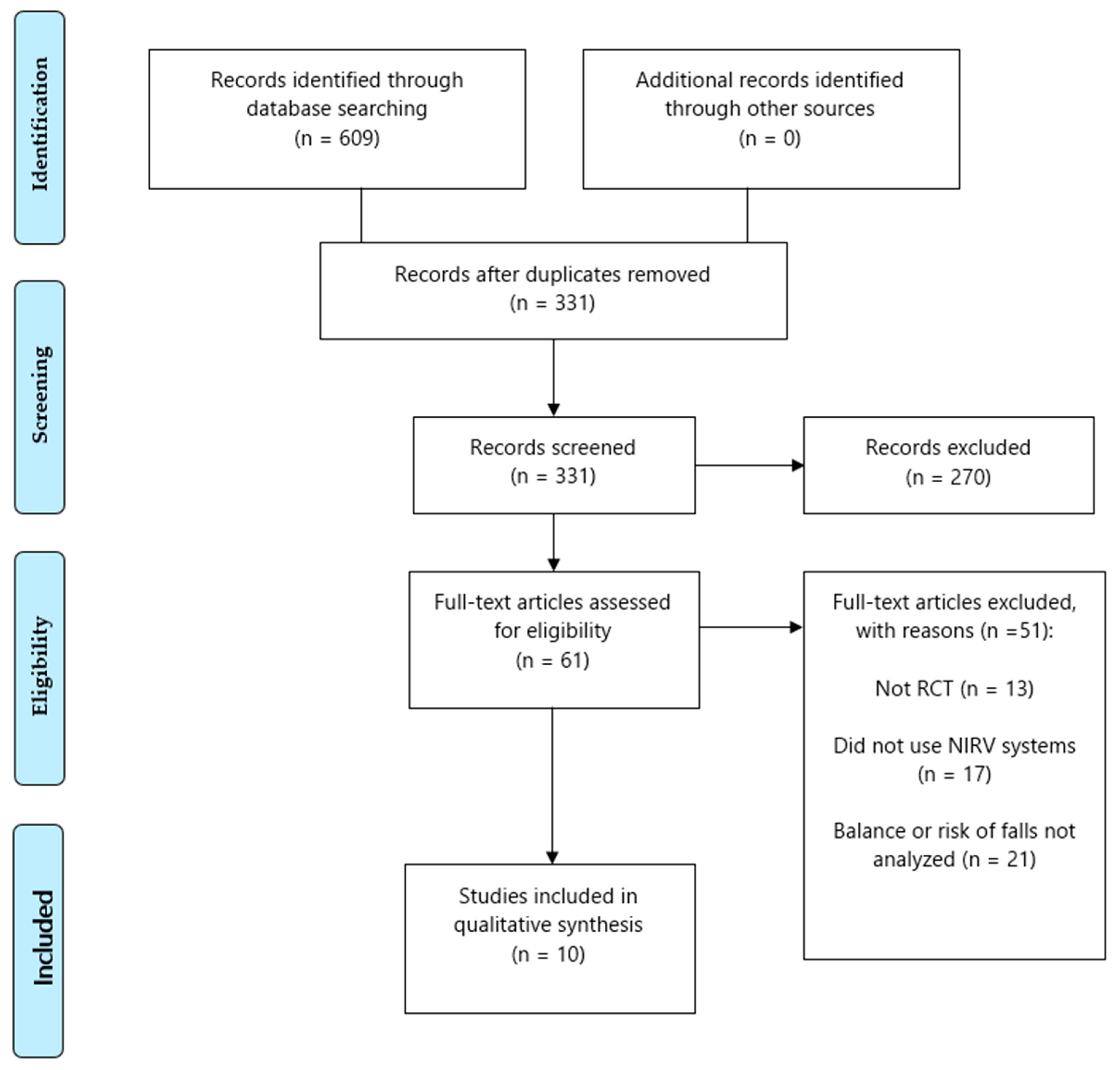
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Methodological Quality of Included Studies
3.4. Results of the Included Studies
3.4.1. Balance
3.4.2. Risk of Falls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Postuma, R.B.; Bloem, B.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Hardy, J.; Lang, A.; et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov. Disord. 2014, 29, 454–462. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Eeden, S.K.V.D.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. NPJ Park. Dis. 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Burillo, J.; Rivero-Celada, D.; Cabezón, A.S.-D.; Casado-Pellejero, J.; Alberdi-Viñas, J.; Alarcia-Alejos, R. Influencia de la estimulación cerebral profunda en la carga de cuidadores de pacientes con enfermedad de Parkinson. Neurología 2018, 33, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.C.; Nutt, J.G.; Holford, N.H.G. Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br. J. Clin. Pharmacol. 2012, 74, 267–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winser, S.J.; Kannan, P.; Bello, U.M.; Whitney, S.L. Measures of balance and falls risk prediction in people with Parkinson’s disease: A systematic review of psychometric properties. Clin. Rehabil. 2019, 33, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Grimbergen, Y.A.M.; Cramer, M.; Willemsen, M.; Zwinderman, A.H. Prospective assessment of falls in Parkinson’s disease. J. Neurol. 2001, 248, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, M.D.; Grimbergen, Y.A.; Slabbekoorn, M.; Bloem, B.R. Falling in Parkinson disease: More often due to postural instability than to environmental factors. Ned. Tijdschr. Geneeskd. 2000, 144, 2309–2314. [Google Scholar] [PubMed]
- Tomlinson, C.L.; Patel, S.; Meek, C.; Herd, C.; Clarke, C.; Stowe, R.; Shah, L.; Sackley, C.; Deane, K.; Wheatley, K.; et al. Physiotherapy intervention in Parkinson’s disease: Systematic review and meta-analysis. BMJ 2012, 345, e5004. [Google Scholar] [CrossRef] [Green Version]
- Obeso, A.J.; Oroz, M.C.R.; Goetz, C.G.; Marin, C.; Kordower, J.H.; Rodriguez, M.; Hirsch, E.; Farrer, M.; Schapira, A.; Halliday, G. Missing pieces in the Parkinson’s disease puzzle. Nat. Med. 2010, 16, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Manson, A.; Stirpe, P.; Schrag, A. Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J. Park. Dis. 2012, 2, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlskog, J.E.; Muenter, M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001, 16, 448–458. [Google Scholar] [CrossRef]
- Heumann, R.; Moratalla, R.; Herrero, M.T.; Chakrabarty, K.; Drucker-Colín, R.; Garcia-Montes, J.R.; Simola, N.; Morelli, M. Dyskinesia in Parkinson’s disease: Mechanisms and current non-pharmacological interventions. J. Neurochem. 2014, 130, 472–489. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Fu, Z.; Le, W. Exercise and Parkinson’s disease. Int. Rev. Neurobiol. 2019, 147, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.C.; De Hemptinne, C.; Thompson, M.C.; Miocinovic, S.; Miller, A.M.; Gilron, R.; Ostrem, J.L.; Chizeck, H.J.; Starr, P.A. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J. Neural Eng. 2018, 15, 046006. [Google Scholar] [CrossRef] [PubMed]
- Elahi, B.; Chen, R.; Elahi, B. Effect of transcranial magnetic stimulation on Parkinson motor function-Systematic review of controlled clinical trials. Mov. Disord. 2008, 24, 357–363. [Google Scholar] [CrossRef]
- Lindvall, O. Treatment of Parkinson’s disease using cell transplantation. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuena, C.; Pedroli, E.; Trimarchi, P.D.; Gallucci, A.; Chiappini, M.; Goulene, K.; Gaggioli, A.; Riva, G.; Lattanzio, F.; Giunco, F.; et al. Usability issues of clinical and research applications of virtual reality in older people: A systematic review. Front. Hum. Neurosci. 2020, 14, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, B.; Requejo, P.; Flynn, S.; Rizzo, A.; Valero-Cuevas, F.; Baker, L.; Winstein, C. The potential of virtual reality and gaming to assist successful aging with disability. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Bohil, C.; Alicea, B.; Biocca, F.A. Virtual reality in neuroscience research and therapy. Nat. Rev. Neurosci. 2011, 12, 752–762. [Google Scholar] [CrossRef]
- Mujber, T.; Szecsi, T.; Hashmi, M. Virtual reality applications in manufacturing process simulation. J. Mater. Process. Technol. 2004, 156, 1834–1838. [Google Scholar] [CrossRef]
- Matijević, V.; Secić, A.; Mašić, V.; Sunić, M.; Kolak, Z.; Znika, M. Virtual reality in rehabilitation and therapy. Acta Clin. Croat. 2013, 52, 453–457. [Google Scholar] [PubMed]
- Keshner, E.A.; Weiss, P.T. Introduction to the special issue from the proceedings of the 2006 International Workshop on Virtual Reality in Rehabilitation. J. Neuroeng. Rehabil. 2007, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, S.B.; Bisconti, S.; Muthalib, M.; Spezialetti, M.; Cutini, S.; Ferrari, M.; Placidi, G.; Quaresima, V. A semi-immersive virtual reality incremental swing balance task activates prefrontal cortex: A functional near-infrared spectroscopy study. NeuroImage 2014, 85, 451–460. [Google Scholar] [CrossRef] [PubMed]
- De Miguel-Rubio, A.; Rubio, M.D.; Alba-Rueda, A.; Salazar, A.; Moral-Munoz, A.J.; Lucena-Anton, D. Virtual reality systems for upper limb motor function recovery in patients with spinal cord injury: Systematic review and meta-analysis. JMIR Mhealth Uhealth 2020, 8, e22537. [Google Scholar] [CrossRef] [PubMed]
- Cameirão, M.S.; i Badia, S.B.; Oller, E.D.; Verschure, P.F. Neurorehabilitation using the virtual reality based Rehabilitation Gaming System: Methodology, design, psychometrics, usability and validation. J. Neuroeng. Rehabil. 2010, 7, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paszkiel, S. Control based on brain-bomputer interface technology for video-gaming with virtual reality techniques. J. Autom. Mob. Robot. Intell. Syst. 2016, 10, 3–7. [Google Scholar] [CrossRef]
- Téllez, P.D.; Moral-Munoz, J.A.; Fernández, E.C.; Couso, A.S.; Lucena-Anton, D. Efectos de la realidad virtual sobre el equilibrio y la marcha en el ictus: Revisión sistemática y metaanálisis. Rev. Neurol. 2019, 69, 223–234. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2019. [Google Scholar]
- Santos, C.M.D.C.; Pimenta, C.A.D.M.; Nobre, M.R.C. The PICO strategy for the research question construction and evidence search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ba, L.J.V.; Perera, S.; Studenski, S.A. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J. Am. Geriatr. Soc. 2011, 59, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta- analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Sherringtonab, C.; Herbert, R.; Maher, C.; Mmoseleyad, A. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man. Ther. 2000, 5, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Macedo, L.G.; Elkins, M.; Maher, C.; Moseley, A.M.; Herbert, R.; Sherrington, C. There was evidence of convergent and construct validity of Physiotherapy Evidence Database quality scale for physiotherapy trials. J. Clin. Epidemiol. 2010, 63, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Del Din, S.; Galna, B.; Lord, S.; Nieuwboer, A.; Bekkers, E.M.J.; Pelosin, E.; Avanzino, L.; Bloem, B.R.; Rikkert, M.G.M.O.; Nieuwhof, F.; et al. Falls risk in relation to activity exposure in high-risk older adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2020, 75, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Pelosin, E.; Cerulli, C.; Ogliastro, C.; LaGravinese, G.; Mori, L.; Bonassi, G.; Mirelman, A.; Hausdorff, J.M.; Abbruzzese, G.; Marchese, R.; et al. A multimodal training modulates short-afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson’s disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 75, 722–728. [Google Scholar] [CrossRef]
- Santos, P.; Machado, T.; Santos, L.; Ribeiro, N.; Melo, A. Efficacy of the Nintendo Wii combination with Conventional Exercises in the rehabilitation of individuals with Parkinson’s disease: A randomized clinical trial. Neurorehabilitation 2019, 45, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, C.; Liu, J.; Wang, L.; Ma, J.; Li, G.; Gan, L.; Shang, X.; Wu, Z. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s Disease patients: A randomized controlled trial. Med. Sci. Monit. 2019, 25, 4186–4192. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.; Geroin, C.; Dimitrova, E.; Boldrini, P.; Waldner, A.; Bonadiman, S.; Picelli, A.; Regazzo, S.; Stirbu, E.; Primon, D.; et al. Virtual reality telerehabilitation for postural instability in Parkinson’s Disease: A multicenter, single-blind, randomized, controlled trial. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet 2016, 388, 1170–1182. [Google Scholar] [CrossRef] [Green Version]
- Negrini, S.; Bissolotti, L.; Ferraris, A.; Noro, F.; Bishop, M.D.; Villafañe, J.H. Nintendo Wii Fit for balance rehabilitation in patients with Parkinson’s disease: A comparative study. J. Bodyw. Mov. Ther. 2017, 21, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-C.; Wang, H.-K.; Wu, R.-M.; Lo, C.-S.; Lin, K.-H. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: A randomized controlled trial. J. Formos. Med. Assoc. 2016, 115, 734–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.-Y.; Lee, D.-K.; Song, H.-S. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J. Phys. Ther. Sci. 2015, 27, 145–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.-Y.; Yang, Y.-R.; Cheng, S.-J.; Wu, Y.-R.; Fuh, J.-L.; Wang, R.-Y. Virtual reality–based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s Disease. Neurorehabil. Neural Repair 2014, 29, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Godfrey, A.; Galna, B.; Mhiripiri, D.; Burn, D.; Rochester, L. Ambulatory activity in incident Parkinson’s: More than meets the eye? J. Neurol. 2013, 260, 2964–2972. [Google Scholar] [CrossRef]
- Crosbie, J.H.; Lennon, S.; Basford, J.R.; McDonough, P.S.M. Virtual reality in stroke rehabilitation: Still more virtual than real. Disabil. Rehabil. 2007, 29, 1139–1146. [Google Scholar] [CrossRef]
- Oh, Y.-B.; Kim, G.-W.; Han, K.-S.; Won, Y.H.; Park, S.-H.; Seo, J.-H.; Ko, M.-H. Efficacy of virtual reality combined with real instrument training for patients with stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2019, 100, 1400–1408. [Google Scholar] [CrossRef] [Green Version]
- Ögün, M.N.; Kurul, R.; Yaşar, M.F.; Turkoglu, S.A.; Avci, Ş.; Yildiz, N. Effect of Leap Motion-based 3D Immersive Virtual Reality Usage on Upper Extremity Function in Ischemic Stroke Patients. Arq. Neuro Psiquiatr. 2019, 77, 681–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeşilyaprak, S.S.; Yıldırım, M.S.; Tomruk, M.; Ertekin, Ö.; Algun, Z.C. Comparison of the effects of virtual reality-based balance exercises and conventional exercises on balance and fall risk in older adults living in nursing homes in Turkey. Physiother. Theory Pr. 2016, 32, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, J.; Paidi, G.; Alshowaikh, K.; Jayarathna, A.I.; Salibindla, D.; Karpinska-Leydier, K.; Ergin, H.E. Virtual reality intervention to help improve motor function in patients undergoing rehabilitation for Cerebral Palsy, Parkinson’s Disease, or Stroke: A systematic review of randomized controlled trials. Cureus 2021, 13, 16763. [Google Scholar] [CrossRef]
| Participants | Interventions | Comparisons | Outcomes | Study Design |
|---|---|---|---|---|
| Patients with Parkinson’s | NIVR | Fall prevention education, treadmill, conventional exercise, and sensory integration balance training | Index of falls, balance, functional mobility and motor status | Randomized clinical trials |
| Database | Search strategy |
|---|---|
| PubMed Medline | (parkinson disease[mh] OR parkinson disease[tiab] OR parkinson’s disease[tiab] OR “parkinson”[tiab]) AND (virtual reality[mh] OR virtual reality[tiab] OR virtual reality exposure therapy[mh] OR “non-immersive virtual reality”[tiab] OR “Nintendo”[tiab] OR “Xbox” [tiab] OR videogam *[tiab] OR exergame *[tiab]) AND (postural balance[mh] OR postural balance[tiab] OR “balance”[tiab] OR postural control[tiab] OR accidental falls[mh] OR accidental falls[tiab] OR fall *[tiab] OR risk of fall *[tiab]) |
| PEDro | Parkinson * virtual reality |
| Web of Science | TS = (Parkinson * AND (videogame * OR exergame * OR virtual reality) AND (balance or fall *)) |
| SCOPUS | (TITLE-ABS-KEY (parkinson OR “Parkinson’s disease”)) AND (TITLE-ABS-KEY (“virtual reality” OR “exergames”)) AND (TITLE-ABS-KEY (“balance” OR “fall”)) |
| CINAHL | (MH “Parkinson Disease”) AND ((MM “Virtual Reality Exposure Therapy”) OR (MM “Virtual Reality”) OR (MM “Exergames”)) AND ((MM “Balance, Postural”) OR (MM “Balance Training, Physical”) OR (MM “Accidental Falls”)) |
| DIALNET | Parkinson * AND (“virtual reality” OR exergame *) AND (balance OR fall *) |
| SciELO | Parkinson * AND (“virtual reality” OR exergame *) AND (balance OR fall *) |
| Study | Participants (N) | Age (years) | Design | Evaluation | Outcomes | Measuring Instrument | Results |
|---|---|---|---|---|---|---|---|
| Del Din et al. (2020) [37] | 128 | 71.68 ± 6.4 | CG = 62 EG = 66 | T0 = Baseline T1 = 6 wk | Number of falls | FRA | The FRA index decreased significantly in the CG and EG (p ≤ 0.035). |
| Pelosin et al. (2020) [38] | 24 | 71.9 ± 4.1 | CG = 14 EG = 10 | T0 = Baseline T1 = 6 w kT2 = 12 wk | Number of falls | Schedule | The EG and CG showed a significant time training interaction (F 1.33 = 7.39, p = 0.012). EG = TM + VR reduced the number of falls (p < 0.001) with respect to CG = TM. |
| Santos et al. (2019) [39] | 45 | 64.3 ± 8.5 | CG = 15 EG1 = 15 EG2 = 15 | T0 = Baseline T1 = 8 wk | Balance Risk of falls | BBS TUG | No statistically significant differences between GG, EG1 and EG2 with respect to BBS (p = 0.968) and TUG (p = 0.824). |
| Significant differences found in pre and post intervention analyses of all outcomes. | |||||||
| The effect size was larger for EG2 = NW + CE in all functional tests. | |||||||
| Feng et al. (2019) [40] | 28 | 66.93 ± 4.64 67.47 ± 4.79 | CG = 14 EG = 14 | T0 = Baseline T1 = 12 wk | Balance Risk of falls | BBS TUG | After Tx, BBS and TUG scores improved significantly in both groups (p < 0.005). The EG = VR showed improved performance compared to the CG = CP on BBS, TUG and Unified Parkinson’s Disease Rating Scale (p < 0.005). |
| Gandolfi et al. (2017) [41] | 76 | 69.84 ± 9.41 67.45 ± 7.18 | CG = 38 EG = 38 | T0 = Baseline T1 = 7 wk T2 = 11 wk | Balance Balance confidence activities Number of falls | BBS ABC Self-reported | There were significant differences between the groups, with the EG = home VR showing improvement in the BBS (p = 0.04). |
| No significant differences between the groups for ABC and number of falls. Significant pre/post-test differences in EG = home VR with respect to the number of falls (p = 0.034). | |||||||
| Mirelman et al. (2016) [42] | 130 | 73 ± 5 74 ± 5 | CG = 64 EG = 66 | T0 = Baseline T1 = 6 wk T2 = 30 wk | Number of falls | Incidence | The number of falls was lower in the EG = TM + VR than in the CG = TM in patients diagnosed with Parkinson’s (p = 0.001). |
| Negrini et al. (2016) [43] | 27 | 67 ± 9 66 ± 8 | CG = 11 EG = 16 | T0 = Baseline T1 = 5 wk T2 = 9 wk | Balance Risk of falls | BBS TT FRA | The post hoc analysis showed significant differences between groups in the pre-test, post-test and follow-up (p < 0.02) on BBS and FRA, but no significant difference between the pre-test and follow-up in the Tinetti test (p = 0.2) in the EG. |
| No significant differences between the intervention groups (p> 0.005). | |||||||
| The effect size was large in BBS (d = 0.9); moderate in TT (d = 0.4) and small in FRA (d < 0.2) after the intervention. | |||||||
| Yang et al. (2016) [44] | 23 | 72.5 ± 8.4 75.4 ± 6.3 | CG = 12 EG = 11 | T0 = Baseline T1 = 6 wk T2 = 8 wk | Balance Risk of falls | BBS TUG | Both groups obtained better results in relation to BBS and TUG after the intervention and at 8 weeks of follow-up (p < 0.001). |
| No significant differences between the groups after the test and at 8 weeks of follow-up. | |||||||
| Lee et al. (2015) [45] | 20 | 70.1 ± 3.3 68.4 ± 2.9 | CG = 10 EG = 10 | T0 = Baseline T1 = 6 wk | Balance | BBS | After 6 wk of Tx, BBS improved significantly in the EG (46.0 ± 1.3 to 48.1 ± 3.0; p < 0.05), but showed no significant improvement in the CG (45.0 ± 1.3 to 45.4 ± 1.5; p > 0.05). |
| Liao et al. (2015) [46] | 36 | 64.6 ± 8.6 65.1 ± 6.7 67.3 ± 7.1 | CG = 12 EG1 = 12 EG2 = 12 | T0 = Baseline T1 = 6 wk T2 = 10 wk | Dynamic balance Sensory organization Risk of fallsNumber of falls | MV/SOT TUG FES-I | EG1 and EG2 showed significant improvements in MV/SOT compared to the CG after treatment and at 1 month of follow-up (p < 0.001). |
| EG1 and EG2 showed significant improvements compared to the CG relative to follow-up (p < 0.001). | |||||||
| No significant differences between EG1 and EG2 relative to FES-I. | |||||||
| EG2 showed significant improvements in SOT, TUG, FES-I with respect to CG. |
| Study | Intervention | Type of NIVR | Time per Session | Frequency | Duration of Treatment |
|---|---|---|---|---|---|
| Del Din et al. (2020) [37] | CG = TM EG = TM + NIVR | Large screen Modified Microsoft Kinect | 40 min | 3/wk | 6 wk |
| Pelosin et al. (2020) [38] | CG = TM EG = TM + NIVR | Large screen Modified Microsoft Kinect | 45 min | 3/wk | 6 wk |
| Santos et al. (2019) [39] | CG = CE EG1 = NIVR EG2 = NIVR + CE | Nintendo Wii Fit | 50 min | 2/wk | 8 wk |
| Feng et al. (2019) [40] | CG = CP EG = NIVR | Nintendo Wii Fit | 45 min | 5/wk | 12 wk |
| Gandolfi et al. (2017) [41] | CG = clinical SIBT EG = home NIVR | Nintendo Wii Fit | 50 min | 3/wk | 7 wk |
| Mirelman et al. (2016) [42] | CG = TM EG = TM + NIVR | Large screen Modified Microsoft Kinect | 45 min | 3/wk | 6 wk |
| Negrini et al. (2016) [43] | CG = NIVR 10 ss EG = NIVR 15 ss | Nintendo Wii Fit | 30 min | CG = 2/wk EG = 3/wk | 5 wk |
| Yang et al. (2016) [44] | CG = CE | Touch screen | 50 min | 2/wk | 6 wk |
| EG = home NIVR | Virtual balance training system | ||||
| Lee et al. (2015) [45] | CG = NDT + FES EG = NDT + FES + NIVR | Nintendo Wii Fit | 45 min 75 min | 5/wk | 6 wk |
| Liao et al. (2015) [46] | CG = FPE | Nintendo Wii Fit | 60 min | 2/wk | 6 wk |
| EG1 = CE + TM | |||||
| EG2 = NIVR + TM |
| Study | Criterion | Total Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Del Din et al. (2020) [37] | NO | YES | NO | YES | NO | NO | NO | NO | YES | YES | YES | 5 |
| Pelosin et al. (2020) [38] | YES | YES | YES | NO | NO | NO | YES | NO | NO | YES | NO | 4 |
| Santos et al. (2019) [39] | YES | YES | NO | YES | NO | NO | YES | YES | YES | YES | YES | 7 |
| Feng et al. (2019) [40] | YES | YES | NO | YES | NO | NO | YES | YES | YES | YES | YES | 7 |
| Gandolfi et al. (2017) [41] | YES | YES | NO | YES | NO | NO | YES | YES | NO | YES | YES | 6 |
| Mirelman et al. (2016) [42] | YES | YES | YES | YES | NO | NO | YES | YES | YES | YES | YES | 8 |
| Negrini et al. (2016) [43] | YES | NO | NO | NO | NO | YES | YES | YES | YES | YES | YES | 6 |
| Yang et al. (2016) [44] | YES | YES | NO | YES | NO | NO | YES | YES | YES | YES | YES | 7 |
| Lee et al. (2015) [45] | NO | YES | NO | YES | NO | NO | NO | NO | NO | YES | YES | 4 |
| Liao et al. (2015) [46] | YES | YES | YES | YES | NO | NO | YES | YES | NO | YES | YES | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-López, H.; Obrero-Gaitán, E.; Castro-Sánchez, A.M.; Lara-Palomo, I.C.; Nieto-Escamez, F.A.; Cortés-Pérez, I. Non-Immersive Virtual Reality to Improve Balance and Reduce Risk of Falls in People Diagnosed with Parkinson’s Disease: A Systematic Review. Brain Sci. 2021, 11, 1435. https://doi.org/10.3390/brainsci11111435
García-López H, Obrero-Gaitán E, Castro-Sánchez AM, Lara-Palomo IC, Nieto-Escamez FA, Cortés-Pérez I. Non-Immersive Virtual Reality to Improve Balance and Reduce Risk of Falls in People Diagnosed with Parkinson’s Disease: A Systematic Review. Brain Sciences. 2021; 11(11):1435. https://doi.org/10.3390/brainsci11111435
Chicago/Turabian StyleGarcía-López, Héctor, Esteban Obrero-Gaitán, Adelaida María Castro-Sánchez, Inmaculada Carmen Lara-Palomo, Francisco Antonio Nieto-Escamez, and Irene Cortés-Pérez. 2021. "Non-Immersive Virtual Reality to Improve Balance and Reduce Risk of Falls in People Diagnosed with Parkinson’s Disease: A Systematic Review" Brain Sciences 11, no. 11: 1435. https://doi.org/10.3390/brainsci11111435







