Styrene-Based Copolymer for Polymer Membrane Modifications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
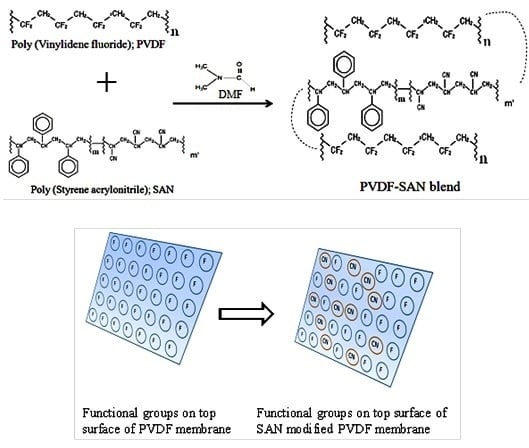
2.2. Preparation of PVDF/Styrene-Acrylonitrile Blend Membrane
2.3. Membrane Characterization
3. Results and Discussion
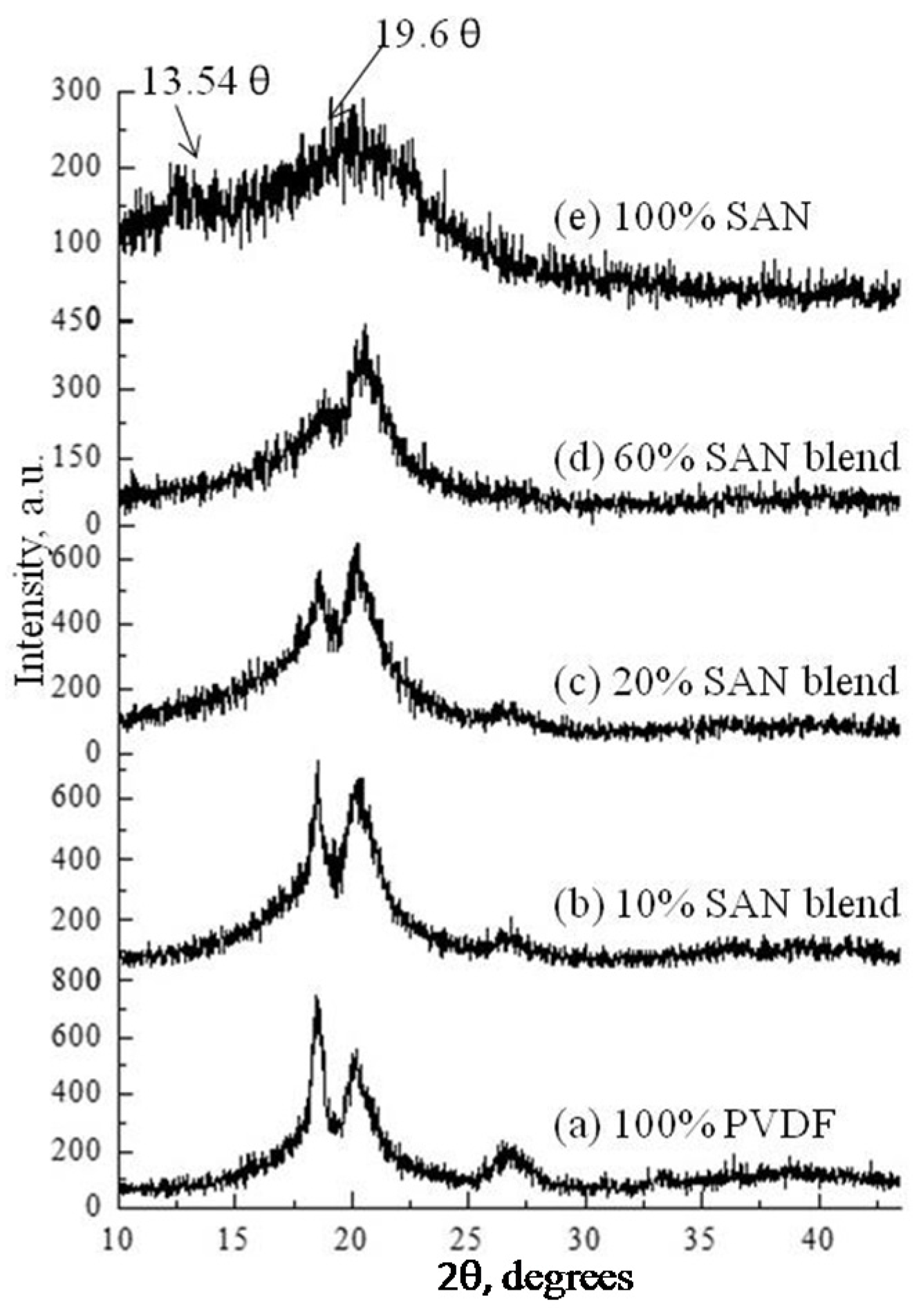
3.1. Study of Crystal Size and Crystallinity of Blend Membranes
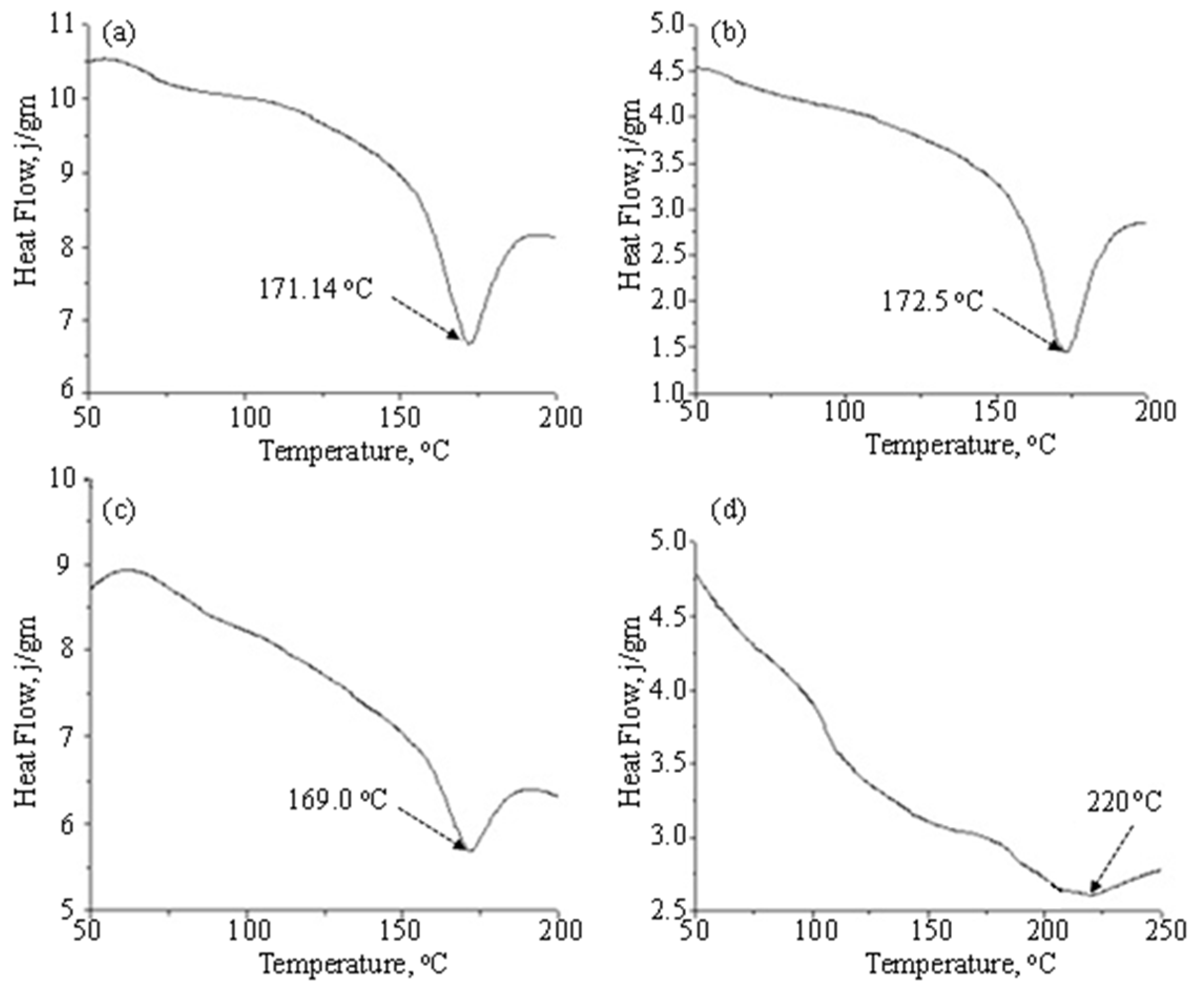
3.2. Study of Thermal Behavior of Blend Membranes
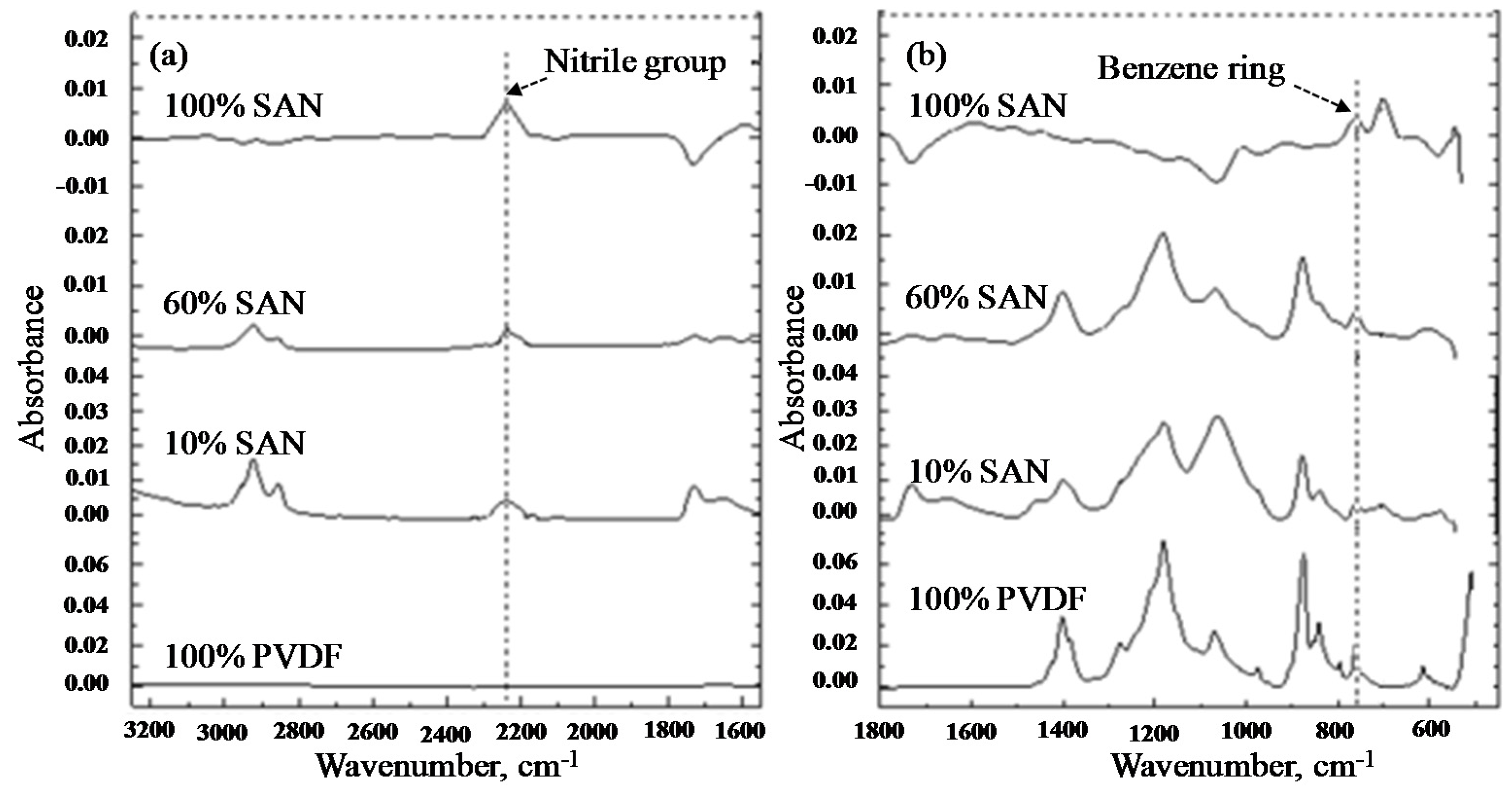
3.3. FTIR-ATR Analysis of Blend Membranes
3.4. Phase Analysis of Membranes
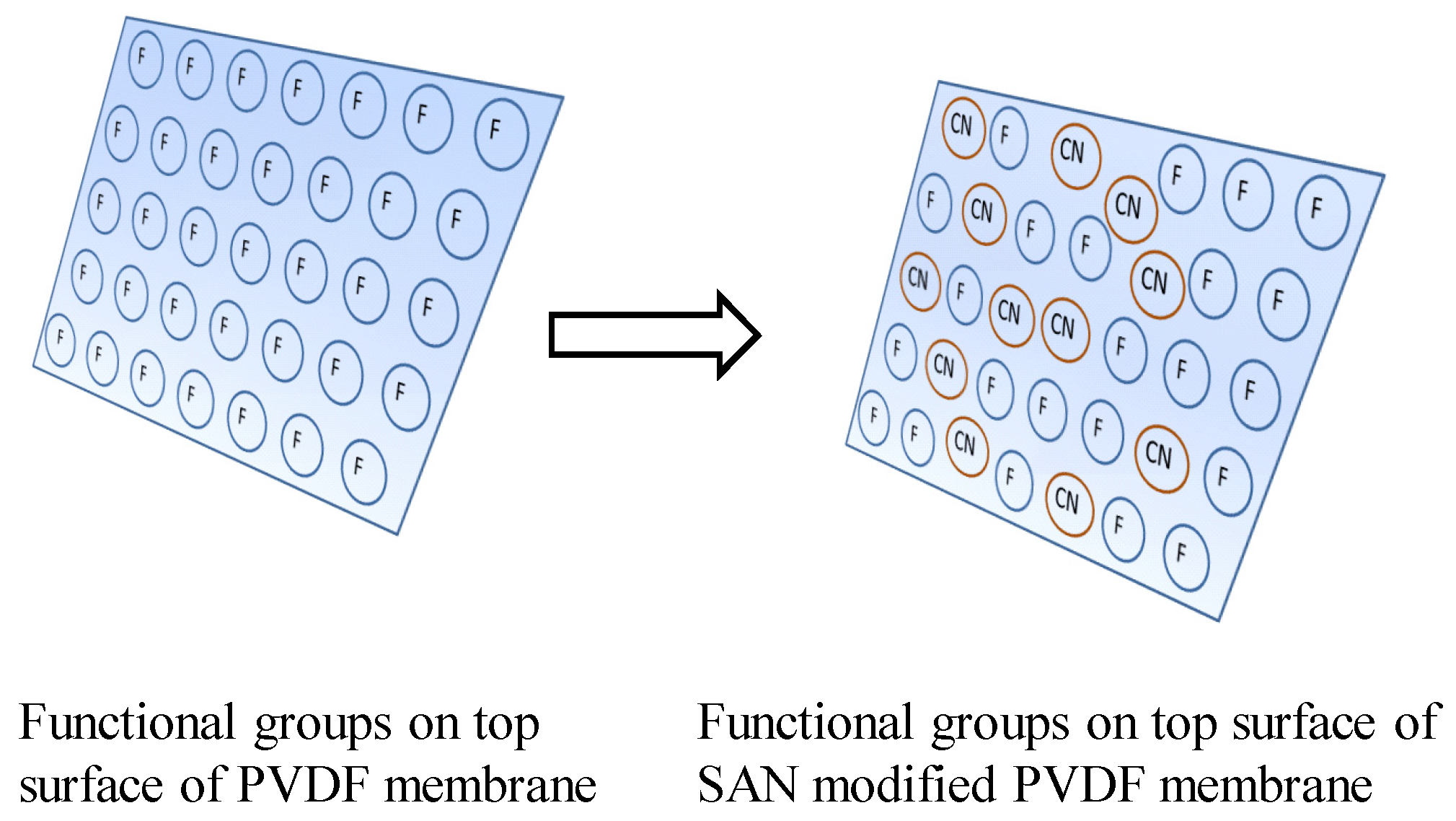
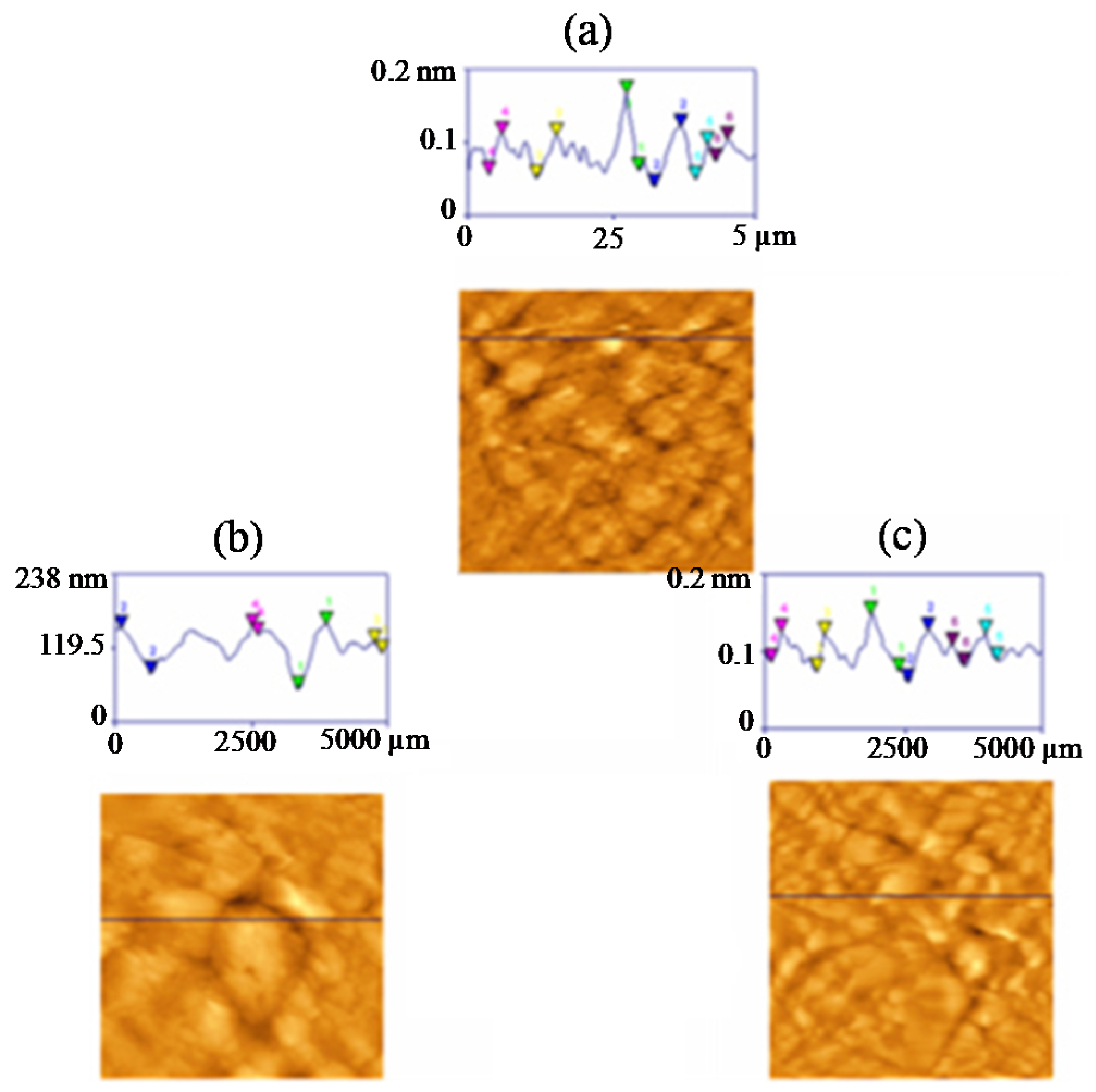
3.5. Surface Investigation of Styrene-Acrylonitrile-Modified PVDF Membranes
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Strathman, H.; Giono, L.; Drioli, E. Introduction to Membrane Science & Technology, 1st ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Horsfall, J.A.; Lovell, K.V. Synthesis and characterisation of sulfonic acid-containing ion exchange membranes based on hydrocarbon and fluorocarbon polymers. Eur. Polym. J. 2002, 38, 1671–1682. [Google Scholar] [CrossRef]
- Wang, I.F.; Ditter, J.F.; Zepf, R. Highly porous polyvinylidene difluoride membranes. US Patent 5834107A, 1998. [Google Scholar]
- Okuji, S.; Boldyryeva, H.; Takeda, Y.; Kishimoto, N. Characteristics of poly(vinylidene difluoride) modified by plasma-based ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Int. Mater. Atoms 2009, 267, 1557–1560. [Google Scholar] [CrossRef]
- Song, L.; Zhang, Z.; Song, S.; Gao, Z. Preparation and characterization of the modified Polyvinylidene Fluoride (PVDF) hollow fibre microfiltration membrane. J. Mater. Sci. Technol. 2007, 23, 55–60. [Google Scholar]
- Desai, N.V.; Rangarajan, R.; Rao, A.V.; Garg, D.K.; Ankleshwaria, B.V.; Mehta, M.H. Preliminary investigation of thin film composite reverse osmosis membranes developed from san as support membranes. J. Membr. Sci. 1992, 71, 201–210. [Google Scholar] [CrossRef]
- Radha, K.S.; Shobana, K.H.; Tarun, M.; Mohan, D. Studies on sulfonated styrene acrylonitrile and cellulose acetate blend ultrafiltration membranes. Desalin. Water Treat. 2014, 52, 459–469. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, C.K. Ultrafiltration membranes prepared from blends of polyethersulfone and poly(1-vinylpyrrolidone-co-styrene) copolymers. J. Membr. Sci. 2005, 262, 60–68. [Google Scholar] [CrossRef]
- Grassie, N.; Bain, D.R. Thermal degradation of copolymers of styrene and acrylonitrile. I. Preliminary investigation of changes in molecular weight and the formation of volatile products. J. Polym. Sci. Part A-1 Polym. Chem. 1970, 8, 2653–2664. [Google Scholar] [CrossRef]
- Radha, K.S.; Shobana, K.H.; Mohan, D.; Lakshmi Narayana, Y. Synthesis and characterization of styrene-acrylonitrile copolymer blend ultrafiltration membranes. Desalin. Water Treat. 2009, 12, 114–126. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Q.; Li, G.; Sun, Y.; Mao, Y. Phase behavior of blends of poly (vinyl methyl ether) with styrene-acrylonitrile in film. Mater. Lett. 2005, 59, 2680–2684. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Q.; Li, G.; Sun, Y.; Mao, Y. Phase behavior of near-critical PVME/SAN blend in film. Mater. Lett. 2006, 60, 589–593. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, X.J.; Chen, S.J.; Ma, W.Z.; Zhang, J.; Wang, S.L. Effect of solution-blended poly(styrene-co-acrylonitrile) copolymer on crystallization of poly(vinylidene fluoride). Exp. Polym. Lett. 2010, 4, 284–291. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Chen, S.; Wang, X. Crystallization behavior and hydrophilicity of poly (vinylidene fluoride) (PVDF)/poly (styrene-co-acrylonitrile) (SAN) blends. Coll. Polym. Sci. 2008, 286, 1193–1202. [Google Scholar] [CrossRef]
- Srivastava, H.P.; Arthanareeswaran, G.; Anantharaman, N.; Starov, V.M. Performance of modified poly(vinylidene fluoride) membrane for textile wastewater ultrafiltration. Desalination 2011, 282, 87–94. [Google Scholar] [CrossRef]
- Srivastava, H.P.; Arthanareeswaran, G.; Anantharaman, N.; Starov, V.M. Performance and properties of modified poly (vinylidene fluoride) membranes using general purpose polystyrene (GPPS) by dips method. Desalination 2011, 283, 169–177. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Park, Y.-S.; Yamazaki, Y. Low water/methanol permeable Nafion/CHP organic–inorganic composite membrane with high crystallinity. Eur. Polym. J. 2006, 42, 375–387. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, A.-Q.; Zhu, B.-K.; Du, C.-H.; Xu, Y.-Y. Polymorphism in porous poly(vinylidene fluoride) membranes formed via immersion precipitation process. J. Membr. Sci. 2008, 319, 169–175. [Google Scholar] [CrossRef]
- Mark, J.E. Polymer Data Handbook, 2nd ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- ICDD data base entry No. 53-1895. Available online: http://www.icdd.com/translation/pdf2.htm (accessed on 19 May 2016).
- Rajabzadeh, S.; Maruyama, T.; Ohmukai, Y.; Sotani, T.; Matsuyama, H. Preparation of PVDF/PMMA blend hollow fiber membrane via thermally induced phase separation (TIPS) method. Sep. Purif. Technol. 2009, 66, 76–83. [Google Scholar] [CrossRef]
- Lin, D.J.; Chang, C.L.; Lee, C.K.; Cheng, L.P. Preparation and characterization of microporous PVDF/PMMA composite membranes by phase inversion in water/DMSO solutions. Eur. Polym. J. 2006, 42, 2407–2418. [Google Scholar] [CrossRef]
- Gregorio, R., Jr.; Borges, D.S. Effect of crystallization rate on the formation of the polymorphs of solution cast poly(vinylidene fluoride). Polymer 2008, 49, 4009–4016. [Google Scholar] [CrossRef]
- Gregorio, J.R.; Nociti, N.C.P.D.S. Effect of PMMA addition on the solution crystallization of the alpha and beta phases of poly(vinylidene fluoride) (PVDF). J. Phys. D Appl. Phys. 1995, 28. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; D’Asti, V.; Piaggio, P. Preparation and properties of novel organic–inorganic porous membranes. Sep. Purif. Technol. 2001, 22–23, 269–275. [Google Scholar] [CrossRef]
- Gregorio, R. Determination of the α, β, and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Macchi, P.; Davoli, M.; Drioli, E. Poly(vinylidene fluoride) membranes by phase inversion: The role the casting and coagulation conditions play in their morphology, crystalline structure and properties. Eur. Polym. J. 2007, 43, 1557–1572. [Google Scholar] [CrossRef]
- Richard Bowen, W.; Hilal, N.; Lovitt, R.W.; Williams, P.M. Atomic force microscope studies of membranes: Surface pore structures of cyclopore and anopore membranes. J. Membr. Sci. 1996, 110, 233–238. [Google Scholar] [CrossRef]



| Membrane | Polymer Composition (wt. %) | Solvent (wt. %) | Crystallite Size (t) (A°) | Residual Strain (ε) | |
|---|---|---|---|---|---|
| PVDF | Styrene-Acrylonitrile | ||||
| 100% PVDF | 17.5 | - | 82.5 | 174.40 | 0.08 |
| 10% styrene-acrylonitrile | 15.75 | 1.75 | 82.5 | 41.5 | 0.031 |
| 20% styrene-acrylonitrile | 14.0 | 3.5 | 82.5 | 31.1 | 0.053 |
| 60% styrene-acrylonitrile | 7 | 10.5 | 82.5 | 15.6 | 1.07 |
| 100% styrene-acrylonitrile | - | 17.5 | 82.5 | 9.6 | 1.24 |
| Membranes | Lorentz Function | Gauss Function | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crystalline Area | Amorphous Area | Crystalline Area | Amorphous Area | |||||||
| Area | FWHM | Area | FWHM | Crystallinity (%) | Area | FWHM | Area | FWHM | Crystallinity (%) | |
| 100% PVDF | 273.2 | 0.37 | 814.6 | 2.60 | 25.11 | 225.5 | 0.47 | 706.4 | 2.54 | 24.20 |
| 10% styrene-acrylonitrile | 137.5 | 0.25 | 616.9 | 1.83 | 18.25 | 203.7 | 0.54 | 965.6 | 4.01 | 17.42 |
| 20% styrene-acrylonitrile | 224.9 | 0.62 | 1130.6 | 3.87 | 16.59 | 176.7 | 0.73 | 1070.6 | 4.71 | 14.17 |
| 60% styrene-acrylonitrile | 102.7 | 0.42 | 657.1 | 3.75 | 13.51 | 69.6 | 0.54 | 653.9 | 4.23 | 9.62 |
| 100% styrene-acrylonitrile | 64.6 | 0.99 | 1305.2 | 7.73 | 4.72 | 44.3 | 0.8 | 819.9 | 7.31 | 5.13 |
| Membrane | Melting Pt. (Tm), °C | , J/g | PVDF Content | , PVDF, J/g |
|---|---|---|---|---|
| 100% PVDF | 171.40 | 60.77 | 1.00 | 60.77 |
| 10% styrene-acrylonitrile | 172.5 | 37.60 | 0.90 | 41.77 |
| 60% styrene-acrylonitrile | 169 | 10.93 | 0.40 | 27.31 |
| Membrane | Td (Start) °C | Res. wt. % (Start) | Td (Peak) °C | Td (Final) °C | Res. wt. % (Finish) | ΔTd | Δ Res wt. % |
|---|---|---|---|---|---|---|---|
| 100% PVDF | 417.5 | 97.5 | 477.5 | 495 | 30.4 | 77.5 | 67.1 |
| 10% styrene-acrylonitrile | 368 | 97.2 | 467 | 493 | 36.4 | 125 | 60.8 |
| 20% styrene-acrylonitrile | 355 | 97 | 460 | 491.5 | 38 | 136.5 | 59.0 |
| 60% styrene-acrylonitrile | 350 | 96.1 | 469 | 480 | 18.5 | 130 | 77.6 |
| 100% styrene-acrylonitrile | 340 | 95.5 | 405 | 430 | 3.5 | 90 | 92.0 |
| Membrane | Average Roughness (nm) | Roughness RMS (nm) | Mean Height (nm) | Peak-Valley Difference (nm) |
|---|---|---|---|---|
| 100% PVDF | 17.5 | 59.1 | 64.4 | 143 |
| 60% styrene-acrylonitrile | 11.5 | 34.5 | 51.42 | 218 |
| 100% styrene-acrylonitrile | 7.8 | 30.05 | 29.67 | 251 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, H.; Lade, H.; Paul, D.; Arthanareeswaran, G.; Kweon, J.H. Styrene-Based Copolymer for Polymer Membrane Modifications. Appl. Sci. 2016, 6, 159. https://doi.org/10.3390/app6060159
Srivastava H, Lade H, Paul D, Arthanareeswaran G, Kweon JH. Styrene-Based Copolymer for Polymer Membrane Modifications. Applied Sciences. 2016; 6(6):159. https://doi.org/10.3390/app6060159
Chicago/Turabian StyleSrivastava, Harsha, Harshad Lade, Diby Paul, G. Arthanareeswaran, and Ji Hyang Kweon. 2016. "Styrene-Based Copolymer for Polymer Membrane Modifications" Applied Sciences 6, no. 6: 159. https://doi.org/10.3390/app6060159