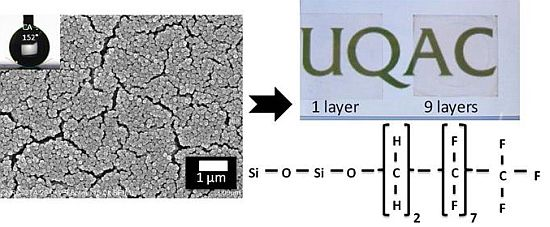
Fluorine Based Superhydrophobic Coatings
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
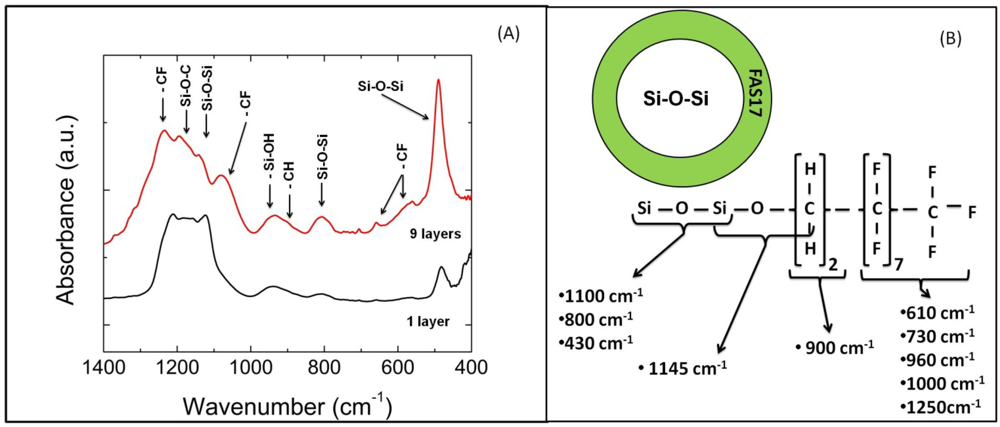
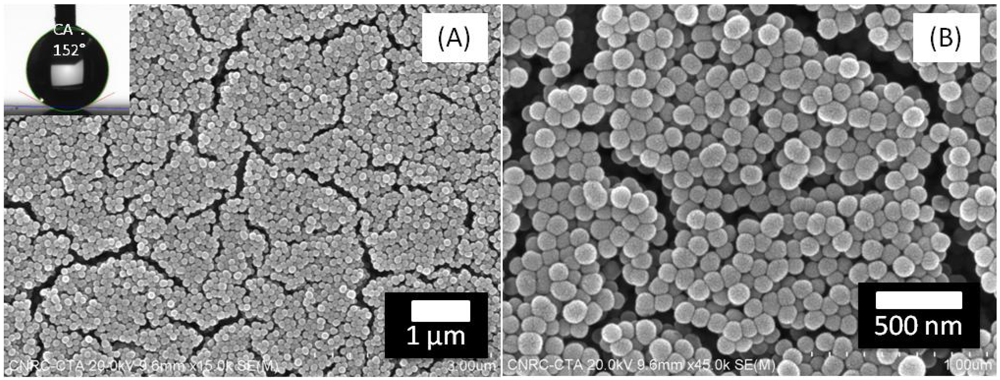
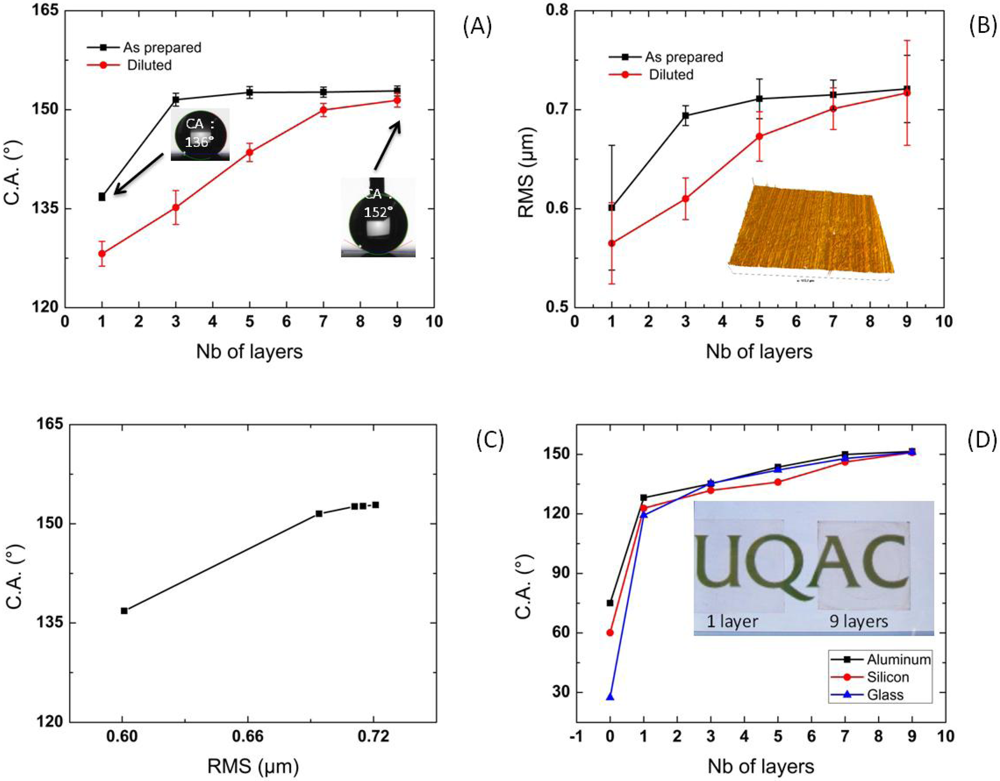
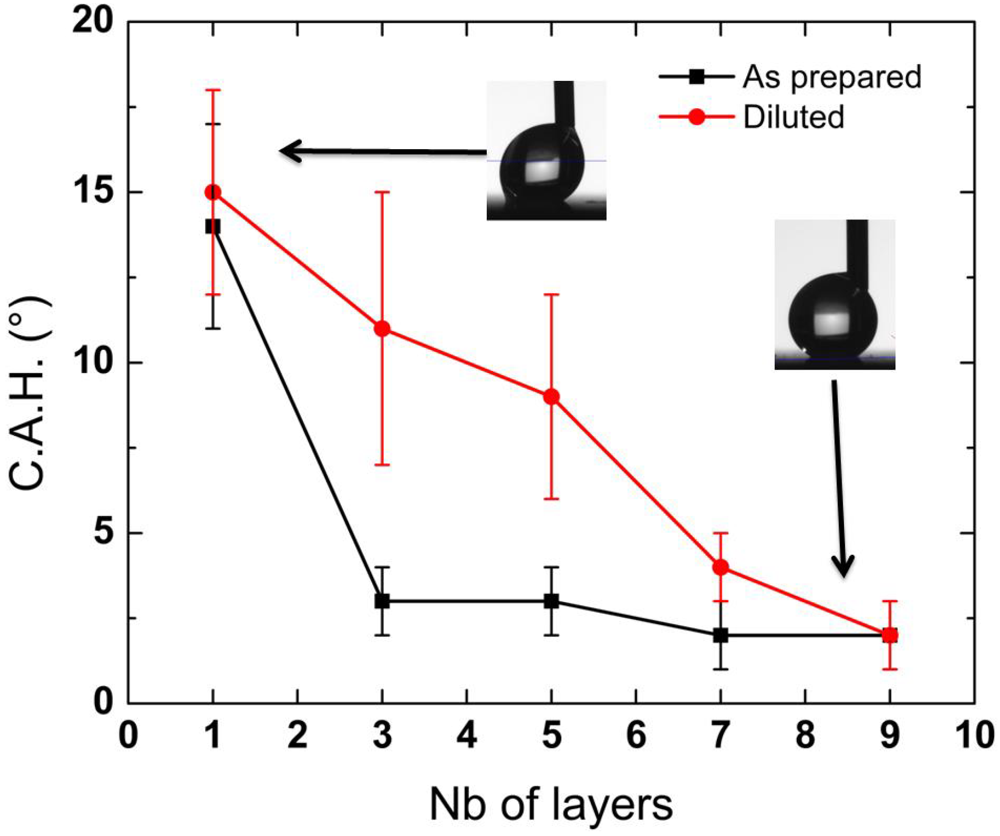
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Liu, X.; Liang, Y.; Zhou, F.; Liu, W. Extreme wettability and tunable adhesion: Biomimicking beyond nature? Soft Matter 2012, 8, 2070–2086. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Wang, Z.L. Controlled replication of butterfly wings for achieving tunable photonic properties. Nano Lett. 2006, 6, 2325–2331. [Google Scholar]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Botan. 1997, 79, 667–677. [Google Scholar]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar]
- Brassard, J.-D.; Sarkar, D.K.; Perron, J. Synthesis of monodisperse fluorinated silica nanoparticles and their superhydrophobic thin films. ACS Appl. Mater. Interfaces 2011, 3, 3583–3588. [Google Scholar]
- Huang, Y.; Sarkar, D.K.; Chen, X.G. A one-step process to engineer superhydrophobic copper surfaces. Mater. Lett. 2010, 64, 2722–2724. [Google Scholar]
- Safaee, A.; Sarkar, D.K.; Farzaneh, M. Superhydrophobic properties of silver-coated films on copper surface bye galvanic exchange reaction. Appl. Surf. Sci. 2008, 254, 2493–2498. [Google Scholar]
- Saleema, N.; Sarkar, D.K.; Gallant, D.; Paynter, R.W.; Chen, X.G. Chemical nature of superhydrophobic aluminum alloy surfaces produced via a one-step process using fluoroalkyl-silane in a base medium. ACS Appl. Mater. Interfaces 2011, 3, 4775–4781. [Google Scholar]
- Sarkar, D.K.; Farzaneh, M.; Paynter, R.W. Superhydrophobic properties of ultrathin rf-sputtered Teflon films coated etched aluminum surfaces. Mater. Lett. 2008, 62, 1226–1229. [Google Scholar]
- Sarkar, D.K.; Saleema, N. One-step fabrication process of superhydrophobic green coatings. Surf.Coat. Technol. 2010, 204, 2483–2486. [Google Scholar]
- Garcia, N.; Benito, E.; Tiemblo, P.; Hasan, M.M.B.; Synytska, A.; Stamm, M. Chemically guided topography in alkylsilane- and oligosiloxane-modified silica nanoparticle coatings: From very hydrophobic surfaces to “pearl” bouncing droplets. Soft Matter 2010, 6, 4768–4776. [Google Scholar]
- Taurino, R.; Fabbri, E.; Messori, M.; Pilati, F.; Pospiech, D.; Synytska, A. Facile preparation of superhydrophobic coatings by sol-gel processes. J. Colloid Interface Sci. 2008, 325, 149–156. [Google Scholar]
- Liu, H.; Szunerits, S.; Pisarek, M.; Xu, W.; Boukherroub, R. Preparation of superhydrophobic coatings on zinc, silicon, and steel by a solution-immersion technique. ACS Appl. Mater. Interfaces 2009, 1, 2086–2091. [Google Scholar] [CrossRef]
- Liu, H.; Szunerits, S.; Xu, W.; Boukherroub, R. Preparation of superhydrophobic coatings on zinc as effective corrosion barriers. ACS Appl. Mater. Interfaces 2009, 1, 1150–1153. [Google Scholar]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar]
- Bushnell, D.M.; Moore, K.J. Drag reduction in nature. Annu. Rev. Fluid Mech. 1991, 23, 65–79. [Google Scholar]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar]
- Carlborg, C.F.; van der Wijngaart, W. Sustained superhydrophobic friction reduction at high liquid pressures and large flows. Langmuir 2010, 27, 487–493. [Google Scholar]
- Carré, A.; Mittal, K.L. Superhydrophobic Surfaces; VSP: Leiden, The Netherlands, 2009. [Google Scholar]
- Karmouch, R.; Ross, G.G. Superhydrophobic wind turbine blade surfaces obtained by a simple deposition of silica nanoparticles embedded in epoxy. Appl. Surf. Sci. 2010, 257, 665–669. [Google Scholar]
- Verplanck, N.; Coffinier, Y.; Thomy, V.; Boukherroub, R. Wettability switching techniques on superhydrophobic surfaces. Nanoscale Res. Lett. 2007, 2, 577–596. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar]
- Chen, S.-L.; Dong, P.; Yang, G.-H.; Yang, J.-J. Kinetics of formation of monodisperse colloidal silica particles through the hydrolysis and condensation of tetraethylorthosilicate. Ind. Eng. Chem. Res. 1996, 35, 4487–4493. [Google Scholar]
- Yang, H.; Pi, P.; Cai, Z.-Q.; Wen, X.; Wang, X.; Cheng, J.; Yang, Z.-R. Facile preparation of super-hydrophobic and super-oleophilic silica film on stainless steel mesh via sol-gel process. Appl. Surf. Sci. 2010, 256, 4095–4102. [Google Scholar]
- Bravo, J.; Zhai, L.; Wu, Z.; Cohen, R.E.; Rubner, M.F. Transparent superhydrophobic films based on silica nanoparticles. Langmuir 2007, 23, 7293–7298. [Google Scholar]
- Callies, M.; Chen, Y.; Marty, F.; Pépin, A.; Quéré, D. Microfabricated textured surfaces for super-hydrophobicity investigations. Microelectron. Eng. 2005, 78–79, 100–105. [Google Scholar] [CrossRef]
- Quéré, D. Non-sticking drops. Reports on Progress in Physics 2005, 68, 2495–2532. [Google Scholar]
- Sarkar, D.K.; Farzaneh, M.; Paynter, R.W. Superhydrophobic properties of ultrathin rf-sputtered Teflon films coated etched aluminum surfaces. Mater. Lett. 2008, 62, 1226–1229. [Google Scholar]
- Hozumi, A.; Takai, O. Preparation of ultra water-repellent films by microwave plasma-enhanced CVD. Thin Solid Films 1997, 303, 222–225. [Google Scholar]
- Latthe, S.S.; Imai, H.; Ganesan, V.; Rao, A.V. Superhydrophobic silica films by sol-gel co-precursor method. Appl. Surf. Sci. 2009, 256, 217–222. [Google Scholar]
- Teshima, K.; Sugimura, H.; Inoue, Y.; Takai, O. Gas barrier performance of surface-modified silica films with grafted organosilane molecules. Langmuir 2003, 19, 8331–8334. [Google Scholar]
- Zhao, Y.; Li, M.; Lu, Q.; Shi, Z. Superhydrophobic polyimide films with a hierarchical topography: Combined replica molding and layer-by-layer assembly. Langmuir 2008, 24, 12651–12657. [Google Scholar]
- Sarkar, D.K.; Brassard, D.; Khakani, M.A.E.; Ouellet, L. Dielectric properties of sol-gel derived high-k titanium silicate thin films. Thin Solid Films 2007, 515, 4788–4793. [Google Scholar]
- Hozumi, A.; Takai, O. Effect of hydrolysis groups in fluoro-alkyl silanes on water repellency of transparent two-layer hard-coatings. App. Surf. Sci. 1996, 103, 431–441. [Google Scholar]
- Latthe, S.S.; Imai, H.; Ganesan, V.; Rao, A.V. Superhydrophobic silica films by sol-gel co-precursor method. Appl. Surf. Sci. 2009, 256, 217–222. [Google Scholar]
- Nozawa, K.; Gailhanou, H.; Raison, L.; Panizza, P.; Ushiki, H.; Sellier, E.; Delville, J.P.; Delville, M.H. Smart control of monodisperse stöber silica particles: Effect of reactant addition rate on growth process. Langmuir 2004, 21, 1516–1523. [Google Scholar]
- Qu, A.L.; Wen, X.F.; Pi, P.H.; Cheng, J.; Yang, Z.R. Study on superhydrophobicity of composite silica film surface. Wuji Cailiao Xuebao/J. Inorg. Mater. 2008, 23, 373–378. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Chen, X.G. Fabrication of superhydrophobic surfaces on aluminum alloy via electrodeposition of copper followed by electrochemical modification. Nano Micro Lett. 2011, 3, 160–165. [Google Scholar]
- Dufour, R.; Harnois, M.; Coffinier, Y.; Thomy, V.; Boukherroub, R.; Senez, V. Engineering sticky superomniphobic surfaces on transparent and flexible PDMS substrate. Langmuir 2010, 26, 17242–17247. [Google Scholar]
- Ke, Q.; Fu, W.; Jin, H.; Zhang, L.; Tang, T.; Zhang, J. Fabrication of mechanically robust superhydrophobic surfaces based on silica micro-nanoparticles and polydimethylsiloxane. Surf. Coat. Technol. 2011, 205, 4910–4914. [Google Scholar]
- Lakshmi, R.V.; Bharathidasan, T.; Basu, B.J. Superhydrophobic sol–gel nanocomposite coatings with enhanced hardness. Appl. Surf. Sci. 2011, 257, 10421–10426. [Google Scholar]
- Ou, J.; Liu, M.; Li, W.; Wang, F.; Xue, M.; Li, C. Corrosion behavior of superhydrophobic surfaces of Ti alloys in NaCl solutions. Appl. Surf. Sci. 2012, 258, 4724–4728. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brassard, J.-D.; Sarkar, D.K.; Perron, J. Fluorine Based Superhydrophobic Coatings. Appl. Sci. 2012, 2, 453-464. https://doi.org/10.3390/app2020453
Brassard J-D, Sarkar DK, Perron J. Fluorine Based Superhydrophobic Coatings. Applied Sciences. 2012; 2(2):453-464. https://doi.org/10.3390/app2020453
Chicago/Turabian StyleBrassard, Jean-Denis, D.K. Sarkar, and Jean Perron. 2012. "Fluorine Based Superhydrophobic Coatings" Applied Sciences 2, no. 2: 453-464. https://doi.org/10.3390/app2020453