Barriers and Facilitators to the Implementation of Evidence-Based Lifestyle Management in Polycystic Ovary Syndrome: A Narrative Review
Abstract
:1. Introduction
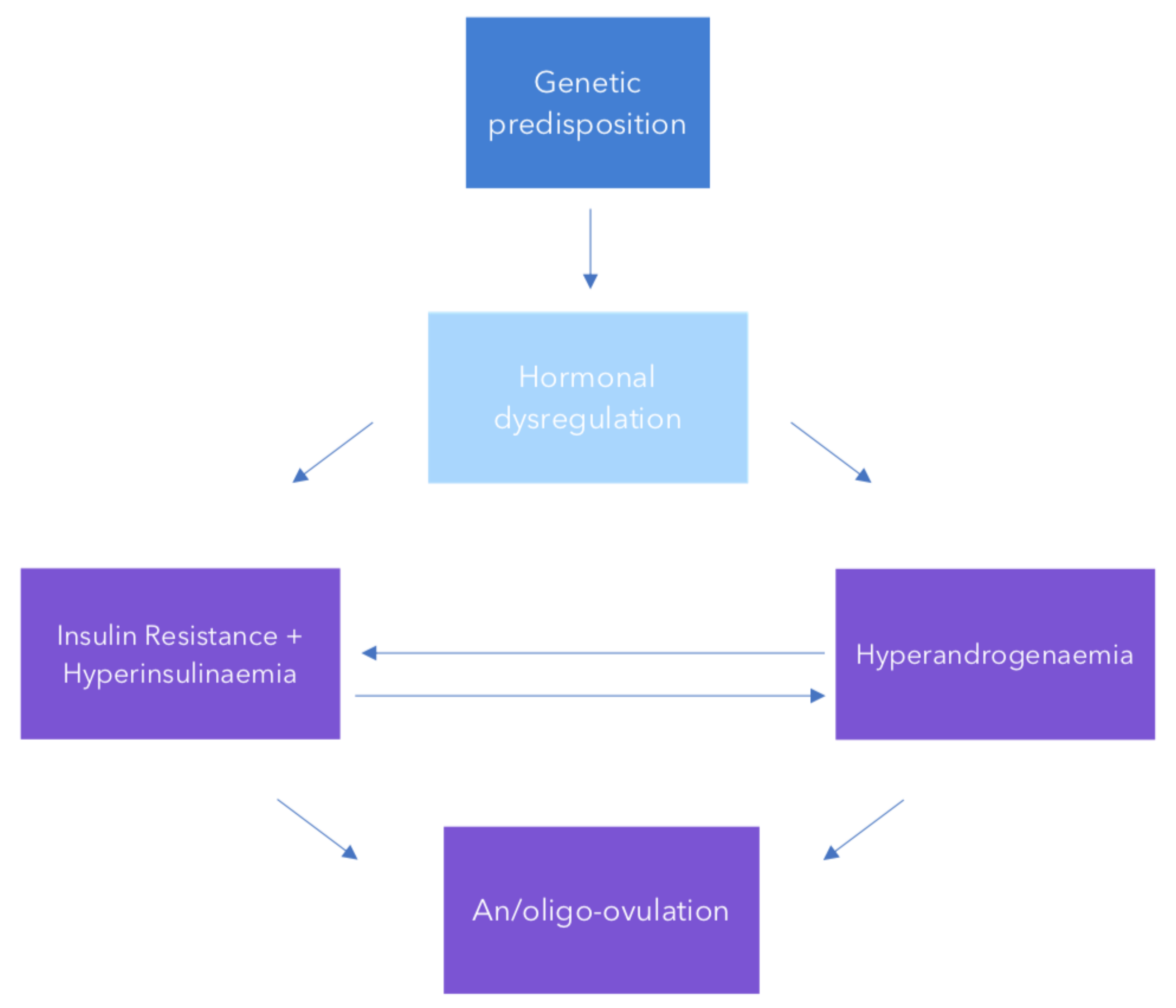
2. Pathogenesis and Aetiology
3. Obesity and Polycystic Ovary Syndrome
4. Management of Polycystic Ovary Syndrome
5. Lifestyle Management
6. Weight Management
7. Diet Intervention
8. Physical Activity
9. Behavioural Intervention
10. The Role of Healthcare Professionals in Lifestyle Management in Polycystic Ovary Syndrome
11. General Practitioners
12. Allied Health
13. Obstetricians-Gynaecologists
14. Endocrinologists
15. Barriers and Facilitators for Evidence Based Lifestyle Management
16. Knowledge and Practice Gaps
17. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Dunaif, A.; Segal, K.R.; Futterweit, W.; Dobrjansky, A. Profound Peripheral Insulin Resistance, Independent of Obesity, in Polycystic Ovary Syndrome. Diabetes 1989, 38, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Moreno-Asso, A.; McIlvenna, L.C.; Walters, K.A.; Rodgers, R.J. Molecular Mechanisms of Insulin Resistance in Polycystic Ovary Syndrome. Unraveling the Conundrum in Skeletal Muscle? J. Clin. Endocrinol. Metab. 2019, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Thornley, B.; Tomlinson, L.; Galletley, C.; Norman, R. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum. Reprod. 1998, 13, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber-Buchholz, M.-M.; Carey, D.G.P.; Norman, R.J. Restoration of Reproductive Potential by Lifestyle Modification in Obese Polycystic Ovary Syndrome: Role of Insulin Sensitivity and Luteinizing Hormone 1. J. Clin. Endocrinol. Metab. 1999, 84, 1470–1474. [Google Scholar] [CrossRef]
- Moran, L.J.; Noakes, M.; Clifton, P.M.; Tomlinson, L.; Norman, R. Dietary Composition in Restoring Reproductive and Metabolic Physiology in Overweight Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Moran, L.J.; Hutchison, S.K.; Norman, R.J.; Teede, H.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2011, 7, CD007506. [Google Scholar]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Avery, J.C.; Moore, V.M.; Davies, M.J.; Azziz, R.; Stener-Victorin, E.; Moran, L.J.; Robertson, S.A.; Stepto, N.K.; Norman, R.J.; et al. Complex diseases and co-morbidities: Polycystic ovary syndrome and type 2 diabetes mellitus. Endocr. Connect. 2019, 8, R71–R75. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Marin, C.; Hoq, L.; Badamgarav, E.; Song, P. Health Care-Related Economic Burden of the Polycystic Ovary Syndrome during the Reproductive Life Span. J. Clin. Endocrinol. Metab. 2005, 90, 4650–4658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domecq, J.P.; Prutsky, G.; Mullan, R.J.; Hazem, A.; Sundaresh, V.; Elamin, M.B.; Phung, O.J.; Wang, A.; Hoeger, K.; Pasquali, R.; et al. Lifestyle Modification Programs in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2013, 98, 4655–4663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haqq, L.; McFarlane, J.; Dieberg, G.; Smart, N. Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: A systematic review and meta-analysis. Endocr. Connect. 2014, 3, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Haqq, L.; McFarlane, J.; Dieberg, G.; Smart, N. The Effect of Lifestyle Intervention on Body Composition, Glycemic Control, and Cardiorespiratory Fitness in Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis. Int. J. Sport. Nutr. Exerc. Metab. 2015, 25, 533–540. [Google Scholar] [CrossRef]
- Naderpoor, N.; Shorakae, S.; De Courten, B.; Misso, M.L.; Moran, L.J.; Teede, H.J. Metformin and lifestyle modification in polycystic ovary syndrome: Systematic review and meta-analysis. Hum. Reprod. Updat. 2015, 21, 560–574. [Google Scholar] [CrossRef]
- Benham, J.L.; Yamamoto, J.M.; Friedenreich, C.M.; Rabi, D.M.; Sigal, R.J. Role of exercise training in polycystic ovary syndrome: A systematic review and meta-analysis. Clin. Obes. 2018, 8, 275–284. [Google Scholar] [CrossRef]
- Kite, C.; Lahart, I.M.; Afzal, I.; Broom, D.R.; Randeva, H.; Kyrou, I.; Brown, J.E. Exercise, or exercise and diet for the management of polycystic ovary syndrome: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 51. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, R.L.; Makris, A.; Randall, R.W.; Daniels, G.; Kistner, R.W.; Ryan, K.J. Insulin Stimulates Androgen Accumulation in Incubations of Ovarian Stroma Obtained from Women with Hyperandrogenism. J. Clin. Endocrinol. Metab. 1986, 62, 904–910. [Google Scholar] [CrossRef]
- Balen, A. The pathophysiology of polycystic ovary syndrome: Trying to understand PCOS and its endocrinology. Best Pr. Res. Clin. Obstet. Gynaecol. 2004, 18, 685–706. [Google Scholar] [CrossRef] [PubMed]
- Powers, L.P.; Nestler, J.E.; Matt, D.W.; Steingold, K.A.; Plymate, S.R.; Rittmaster, R.S.; Clore, J.N.; Blackard, W.G. A Direct Effect of Hyperinsulinemia on Serum Sex Hormone-Binding Globulin Levels in Obese Women with the Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 1991, 72, 83–89. [Google Scholar]
- Balen, A.H.; Conway, G.S.; Techatraisak, K.; Manning, P.J.; West, C.; Jacobs, H.S.; Kaltsas, G. Andrology: Polycystic ovary syndrome: The spectrum of the disorder in 1741 patients. Hum. Reprod. 1995, 10, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.; Mendoza, S.; Hamer, T.; Sosa, F.; Glueck, C. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metab. 1994, 43, 647–654. [Google Scholar] [CrossRef]
- Ezeh, U.; Yildiz, B.O.; Azziz, R. Referral Bias in Defining the Phenotype and Prevalence of Obesity in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E1088–E1096. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Kirubakaran, R.; Mykhalchenko, K.; Suturina, L.; Chernukha, G.; Diamond, M.P.; Azziz, R. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: Systematic review and meta-analysis. Fertil. Steril. 2016, 106, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, B.O.; Knochenhauer, E.S.; Azziz, R. Impact of Obesity on the Risk for Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Kiddy, D.S.; Sharp, P.S.; White, D.M.; Scanlon, M.F.; Mason, H.D.; Bray, C.S.; Polson, D.W.; Reed, M.J.; Franks, S. Differences in Clinical and Endocrine Features Between Obese and Non-Obese Subjects with Polycystic Ovary Syndrome: An Analysis of 263 Consecutive Cases. Clin. Endocrinol. 1990, 32, 213–220. [Google Scholar] [CrossRef]
- Teede, H.J.; Joham, A.E.; Paul, E.; Moran, L.J.; Loxton, D.; Jolley, D.; Lombard, C. Longitudinal weight gain in women identified with polycystic ovary syndrome: Results of an observational study in young women. Obes. 2013, 21, 1526–1532. [Google Scholar] [CrossRef]
- Lim, S.; Davies, M.; Norman, R.; Moran, L. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2012, 18, 618–637. [Google Scholar] [CrossRef]
- Deeks, A.A.; Gibson-Helm, M.E.; Teede, H.J. Anxiety and depression in polycystic ovary syndrome: A comprehensive investigation. Fertil. Steril. 2010, 93, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.; Pinkney, J.; Adams, L.; Stenhouse, E.; Bendall, A.; Corrigan, O.; Letherby, G. The diagnosis and lived experience of polycystic ovary syndrome: A qualitative study. J. Adv. Nurs. 2017, 73, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Ranasinha, S.; Zoungas, S.; Moran, L.; McNaughton, S.; Brown, W.; Teede, H. The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum. Reprod. 2013, 28, 2276–2283. [Google Scholar] [Green Version]
- Shorakae, S.; Jona, E.; De Courten, B.; Lambert, G.W.; Lambert, E.A.; Phillips, S.E.; Clarke, I.J.; Teede, H.J.; Henry, B.A. Brown adipose tissue thermogenesis in polycystic ovary syndrome. Clin. Endocrinol. 2018, 90, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, A.L.; Naessén, S.; Stridsberg, M.; Byström, B.; Holte, J. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2004, 19, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Noakes, M.; Clifton, P.M.; Wittert, G.A.; Tomlinson, L.; Galletly, C.; Luscombe, N.D.; Norman, R. Ghrelin and Measures of Satiety Are Altered in Polycystic Ovary Syndrome but Not Differentially Affected by Diet Composition. J. Clin. Endocrinol. Metab. 2004, 89, 3337–3344. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Noakes, M.; Clifton, P.M.; Wittert, G.A.; Le Roux, C.W.A.; Ghatei, M.; Bloom, S.R.; Norman, R.J. Postprandial ghrelin, cholecystokinin, peptide YY, and appetite before and after weight loss in overweight women with and without polycystic ovary syndrome. Am. J. Clin. Nutr. 2007, 86, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wu, L.; Chang, F.; Cao, G. Low circulating ghrelin levels in women with polycystic ovary syndrome: A systematic review and meta-analysis. Endocr. J. 2016, 63, 93–100. [Google Scholar] [CrossRef]
- Hutchison, S.K.; Teede, H.J.; Rachoń, D.; Harrison, C.L.; Strauss, B.J.; Stepto, N.K. Effect of exercise training on insulin sensitivity, mitochondria and computed tomography muscle attenuation in overweight women with and without polycystic ovary syndrome. Diabetol. 2012, 55, 1424–1434. [Google Scholar] [CrossRef]
- Hansen, S.L.; Svendsen, P.F.; Jeppesen, J.F.; Hoeg, L.D.; Andersen, N.R.; Kristensen, J.M.; Nilas, L.; Lundsgaard, A.M.; Wojtaszewski, J.F.P.; Madsbad, S.; et al. Molecular mechanisms in skeletal muscle underlying insulin resistance women who are lean with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 1841–1854. [Google Scholar] [CrossRef]
- Auble, B.; Elder, D.; Gross, A.; Hillman, J.B. Differences in the Management of Adolescents with Polycystic Ovary Syndrome across Pediatric Specialties. J. Pediatr. Adolesc. Gynecol. 2013, 26, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Helm, M.; Dokras, A.; Karro, H.; Piltonen, T.; Teede, H.J. Knowledge and Practices Regarding Polycystic Ovary Syndrome among Physicians in Europe, North America, and Internationally: An Online Questionnaire-Based Study. Semin. Reprod. Med. 2018, 36, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cussons, A.J.; Stuckey, B.G.A.; Walsh, J.P.; Burke, V.; Norman, R.J. Polycystic ovarian syndrome: Marked differences between endocrinologists and gynaecologists in diagnosis and management. Clin. Endocrinol. 2005, 62, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Webb, V.L.; Moran, C.H.; Bailer, B.A. Lifestyle modification for obesity: New developments in diet, physical activity, and behavior therapy. Circulation 2012, 125, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Love, J.G.; McKenzie, J.S.; Nikokavoura, E.A.; Broom, J.; Rolland, C.; Johnston, K.L. The experiences of women with polycystic ovary syndrome on a very low-calorie diet. Int. J. Women’s Heal. 2016, 8, 299–310. [Google Scholar] [CrossRef]
- Kiddy, D.S.; Seppälä, M.; Koistinen, R.; James, V.H.T.; Reed, M.J.; Franks, S.; Hamilton-Fairley, D.; Hamilton-Fairley, D. Diet-Induced Changes in Sex Hormone Binding Globulin and Free Testosterone in Women with Normal or Polycystic Ovaries: Correlation with Serum Insulin and Insulin-Like Growth Factor-I. Clin. Endocrinol. 1989, 31, 757–764. [Google Scholar] [CrossRef]
- Crosignani, P.G.; Colombo, M.; Vegetti, W.; Somigliana, E.; Gessati, A.; Ragni, G. Overweight and obese anovulatory patients with polycystic ovaries: Parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum. Reprod. 2003, 18, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Kiddy, D.S.; Bush, A.; Short, F.; Anyaoku, V.; Reed, M.J.; Franks, S.; Hamilton-Fairley, D.; Hamilton-Fairley, D. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin. Endocrinol. 1992, 36, 105–111. [Google Scholar] [CrossRef]
- Thomson, R.L.; Buckley, J.D.; Lim, S.S.; Noakes, M.; Clifton, P.M.; Norman, R.J.; Brinkworth, G.D. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil. Steril. 2010, 94, 1812–1816. [Google Scholar] [CrossRef]
- Herman, W.H. The cost-effectiveness of diabetes prevention: Results from the Diabetes Prevention Program and the Diabetes Prevention Program Outcomes Study. Clin. Diabetes Endocrinol. 2015, 1, 9. [Google Scholar] [CrossRef]
- Kramer, F.M.; Jeffery, R.W.; Forster, J.L.; Snell, M.K. Long-term follow-up of behavioral treatment for obesity: Patterns of weight regain among men and women. Int. J. Obes. 1989, 13, 123–136. [Google Scholar]
- Kraschnewski, J.L.; Boan, J.; Esposito, J.; Sherwood, N.E.; Lehman, E.B.; Kephart, D.K.; Sciamanna, C.N. Long-term weight loss maintenance in the United States. Int. J. Obes. 2010, 34, 1644–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greaves, C.; Poltawski, L.; Garside, R.; Briscoe, S. Understanding the challenge of weight loss maintenance: A systematic review and synthesis of qualitative research on weight loss maintenance. Health Psychol. Rev. 2017, 11, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.A.; Steinbeck, K.S.; Atkinson, F.S.; Petocz, P.; Brand-Miller, J.C. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am. J. Clin. Nutr. 2010, 92, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorensen, L.B.; Soe, M.; Halkier, K.H.; Stigsby, B.; Astrup, A. Effects of increased dietary protein-to-carbohydrate ratios in women with polycystic ovary syndrome. Am. J. Clin. Nutr. 2012, 95, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Honas, J.J.; Early, J.L.; Frederickson, D.D.; O’Brien, M.S.; O’Brien, M.S. Predictors of Attrition in a Large Clinic-Based Weight-Loss Program. Obes. Res. 2003, 11, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Teede, H.; Skouteris, H.; Linardon, J.; Hill, B.; Moran, L. Lifestyle and Behavioral Management of Polycystic Ovary Syndrome. J. Women’s Heal. 2017, 26, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Banting, L.K.; Gibson-Helm, M.; Polman, R.; Teede, H.J.; Stepto, N.K. Physical activity and mental health in women with Polycystic Ovary Syndrome. BMC Women’s Heal. 2014, 14, 51. [Google Scholar] [CrossRef]
- Alvarado, M.; Murphy, M.M.; Guell, C. Barriers and facilitators to physical activity amongst overweight and obese women in an Afro-Caribbean population: A qualitative study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 387. [Google Scholar] [CrossRef]
- Lim, S.S.; Norman, R.J.; Clifton, P.M.; Noakes, M. Hyperandrogenemia, psychological distress, and food cravings in young women. Physiol. Behav. 2009, 98, 276–280. [Google Scholar] [CrossRef]
- Geier, L.; Bekx, M.; Connor, E.L. Factors Contributing to Initial Weight Loss Among Adolescents with Polycystic Ovary Syndrome. J. Pediatr. Adolesc. Gynecol. 2012, 25, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Bruner, B.; Chad, K.; Chizen, D. Effects of exercise and nutritional counseling in women with polycystic ovary syndrome. Appl. Physiol. Nutr. Metab. 2006, 31, 384–391. [Google Scholar] [CrossRef] [Green Version]
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013. [Google Scholar]
- Jeanes, Y.M.; Barr, S.; Smith, K.; Hart, K.H. Jeanes, Y. Dietary management of women with polycystic ovary syndrome in the United Kingdom: The role of dietitians. J. Hum. Nutr. Diet. 2009, 22, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. The International PCOS Network Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin. Endocrinol. 2018, 89, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; Elkind-Hirsch, K.; Ravussin, E. Aerobic Exercise in Women with Polycystic Ovary Syndrome Improves Ovarian Morphology Independent of Changes in Body Composition. Fertil. Steril. 2011, 95, 2696–2699. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, S.K.; Stepto, N.K.; Harrison, C.L.; Moran, L.J.; Strauss, B.J.; Teede, H.J. Effects of Exercise on Insulin Resistance and Body Composition in Overweight and Obese Women with and without Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E48–E56. [Google Scholar] [CrossRef] [Green Version]
- Palomba, S.; Giallauria, F.; Falbo, A.; Russo, T.; Oppedisano, R.; Tolino, A.; Colao, A.; Vigorito, C.; Zullo, F.; Orio, F. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: A 24-week pilot study. Hum. Reprod. 2008, 23, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Patten, R.K.; Tassone, E.C.; Misso, M.L.; Brennan, L.; Boyle, J.; Boyle, R.A.; Harrison, C.L.; Hirschberg, A.L.; Marsh, K.; et al. Exercise Recommendations for Women with Polycystic Ovary Syndrome: Is the Evidence Enough? Sports Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Gennat, H.; O’Rourke, P.; Del Mar, C. Exercise for overweight or obesity. Cochrane Database Syst. Rev. 2006, 4, Cd003817. [Google Scholar] [CrossRef] [PubMed]
- Butryn, M.L.; Webb, V.; Wadden, T.A. Behavioral treatment of obesity. Psychiatr. Clin. North Am. 2011, 34, 841–859. [Google Scholar] [CrossRef]
- Shaw, K.; O’Rourke, P.; Del Mar, C.; Kenardy, J. Psychological interventions for overweight or obesity. Cochrane Database Syst. Rev. 2005, 18, Cd003818. [Google Scholar] [CrossRef]
- Greaves, C.J.; Sheppard, K.E.; Abraham, C.; Hardeman, W.; Roden, M.; Evans, P.H.; Schwarz, P.; IMAGE Study Group. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Heal. 2011, 11, 119. [Google Scholar]
- Tay, C.T.; Moran, L.J.; Wijeyaratne, C.N.; Redman, L.M.; Norman, R.J.; Teede, H.J.; Joham, A.E. Integrated Model of Care for Polycystic Ovary Syndrome. Semin. Reprod. Med. 2018, 36, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.W.; Bergomi, E.J.; Dollahite, J.S.; Sobal, J.; Hoeger, K.M.; Lujan, M.E. Trust in Physicians and Medical Experience Beliefs Differ Between Women with and without Polycystic Ovary Syndrome. J. Endocr. Soc. 2018, 2, 1001–1009. [Google Scholar] [CrossRef]
- Katon, W.; Von Korff, M.; Lin, E.; Simon, G. Rethinking practitioner roles in chronic illness: The specialist, primary care physician, and the practice nurse. Gen. Hosp. Psychiatry 2001, 23, 138–144. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Boyle, J.A.; Garad, R.M.; McAllister, V.; Downes, L.; Gibson-Helm, M.; Hart, R.J.; Rombauts, L.; Moran, L.; et al. Translation and implementation of the Australian-led PCOS guideline: Clinical summary and translation resources from the International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Med. J. Australia 2018, 209, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.O.; Nowson, C.A. Patient recall of receiving lifestyle advice for overweight and hypertension from their General Practitioner. BMC Fam. Pract. 2010, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Tham, M.; Young, D. The role of the General Practitioner in weight management in primary care—A cross sectional study in General Practice. BMC Fam. Pract. 2008, 9, 66. [Google Scholar] [CrossRef]
- Deloitte Access Economic. Value of Accredited Exercise Physiologists in Australia. Deloitte Access Economics: Sydney, Australia; 2015. Available online: https://www.essa.org.au/wp-content/uploads/2015/10/Deloitte-Report-2015_Value-of-AEPs-in-Australia_Summary (accessed on 27 January 2019).
- Dokras, A.; Saini, S.; Gibson-Helm, M.; Schulkin, J.; Cooney, L.; Teede, H. Gaps in knowledge among physicians regarding diagnostic criteria and management of polycystic ovary syndrome. Fertil. Steril. 2017, 107, 1380–1386. [Google Scholar] [CrossRef]
- Humphreys, L.; Costarelli, V. Implementation of dietary and general lifestyle advice among women with polycystic ovarian syndrome. J. R. Soc. Promot. Health 2008, 128, 190–195. [Google Scholar] [CrossRef]
- Gibson-Helm, M.; Teede, H.; Dunaif, A.; Dokras, A. Delayed Diagnosis and a Lack of Information Associated with Dissatisfaction in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2016, 102, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Adamson, L.; Brown, W.J.; Byles, J.; Chojenta, C.; Dobson, A.; Fitzgerald, D.; Hockey, R.; Loxton, D.; Powers, J.R.; Spallek, M.; et al. Women’s Weight: Findings from the Australian Longitudinal Study on Women’s Health; Australian Government Department of Health and Ageing: Sydney, Australia, 2007. [Google Scholar]
- Gibson-Helm, M.E.; Lucas, I.M.; Boyle, J.A.; Teede, H.J. Women’s experiences of polycystic ovary syndrome diagnosis. Fam. Pract. 2014, 31, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Mann, L. From “silos” to seamless healthcare: Bringing hospitals and GPs back together again. Med. J. Aust. 2005; 182, 34–37. [Google Scholar]
- Nicholas, L.G.; Pond, C.D.; Roberts, D.C. Dietitian–General practitioner interface: A pilot study on what influences the provision of effective nutrition management. Am. J. Clin. Nutr. 2003, 77, 1039S–1042S. [Google Scholar] [CrossRef] [PubMed]
- Kushner, R. Barriers to Providing Nutrition Counseling by Physicians: A Survey of Primary Care Practitioners. Prev. Med. 1995, 24, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Engel, H.; Timperio, A.; Cooper, C.; Crawford, D. Obesity Management: Australian General Practitioners’ Attitudes and Practices. Obes. Res. 2000, 8, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Epling, J.W.; Morley, C.P.; Ploutz-Snyder, R. Family physician attitudes in managing obesity: A cross-sectional survey study. BMC Res. Notes 2011, 4, 473. [Google Scholar] [CrossRef] [PubMed]
- Salinas, G.D.; Glauser, T.A.; Williamson, J.C.; Rao, G.; Abdolrasulnia, M. Primary Care Physician Attitudes and Practice Patterns in the Management of Obese Adults: Results from a National Survey. Postgrad. Med. 2011, 123, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Lambe, B.; Collins, C. A qualitative study of lifestyle counselling in general practice in Ireland. Fam. Pract. 2010, 27, 219–223. [Google Scholar] [CrossRef]
- Jansink, R.; Braspenning, J.; Van Der Weijden, T.; Elwyn, G.; Grol, R. Primary care nurses struggle with lifestyle counseling in diabetes care: A qualitative analysis. BMC Fam. Pract. 2010, 11, 41. [Google Scholar] [CrossRef]
- Passey, M.; Fanaian, M.; Lyle, D.; Harris, M.F. Assessment and management of lifestyle risk factors in rural and urban general practices in Australia. Aust. J. Prim. Health 2010, 16, 81–86. [Google Scholar] [CrossRef]
- Kim, K.K.; Yeong, L.-L.; Caterson, I.D.; Harris, M.F. Analysis of factors influencing general practitioners’ decision to refer obese patients in Australia: A qualitative study. BMC Fam. Pract. 2015, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Ampt, A.J.; Amoroso, C.; Harris, M.F.; McKenzie, S.H.; Rose, V.K.; Taggart, J.R. Attitudes, norms and controls influencing lifestyle risk factor management in general practice. BMC Fam. Pract. 2009, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Teede, H.; Moran, L. Analysis of the barriers and enablers to implementing lifestyle management practices for women with PCOS in Singapore. BMC Res. Notes 2016, 9, 1526. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.; Pais-Ribeiro, J.; Maia, A.; Teixeira, F. A qualitative study of GPs’ views towards obesity: Are they fighting or giving up? Public Health 2015, 129, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.A.; Roepke, N.; Stevenin, B.; Dubois, A.M.; Ahn, S.M. Physician knowledge about and perceptions of obesity management. Obes. Res. Clin. Pract. 2015, 9, 573–583. [Google Scholar] [CrossRef] [PubMed]
| Author | Intervention Type | Control | Number of Included Papers | Total Number of Participants (n) | Population and PCOS Diagnostic Criteria | Key Findings |
|---|---|---|---|---|---|---|
| Moran et al. [9] | Structured dietary, exercise or behavioural intervention or any combination | Minimal intervention | 6 | n = 164 | PCOS (Rotterdam or NIH) | Anthropometric: ↓ weight, waist, WHR; NC BMI |
| Cardiovascular-Respiratory: NA | ||||||
| Metabolic: ↓OGTT insulin, FI; NC glucose, cholesterol | ||||||
| Reproductive: ↓ TT, hirsutism; NC FAI, SHBG | ||||||
| Psychological: NA | ||||||
| Domecq et al. [13] | Diet, physical exercise or combination diet and physical exercise | Metformin or minimal intervention | 10 | n = 610 | PCOS (criteria unspecified) | Lifestyle vs. Minimal intervention |
| Anthropometric: NA | ||||||
| Cardiovascular-Respiratory: NA | ||||||
| Metabolic: ↓ FBG, FBI, direct correlation between BMI and FBG, no significant trend between BMI and FBG | ||||||
| Reproductive: ↓ FGS | ||||||
| Psychological: NA | ||||||
| Lifestyle vs. Metformin | ||||||
| Anthropometric: NA | ||||||
| Cardiovascular-Respiratory: NA | ||||||
| Metabolic: NSD FBG, FBI | ||||||
| Reproductive: NSD hirsutism score pregnancy rate | ||||||
| Psychological: NA | ||||||
| Haqq et al. [14] | Physical exercise alone or combination diet and physical exercise | Usual care i.e., No active intervention, metformin, untreated controls, placebo, healthy diet only | 7 | n = 206 | PCOS (criteria unspecified) | Lifestyle Intervention (Combinatorial) |
| Anthropometric: NA | ||||||
| Cardiovascular-Respiratory: NA | ||||||
| Metabolic: NA | ||||||
| Reproductive:↑ FSH, SHBG; ↓ TT, androstenedione, FAI, FGS; NSD LH | ||||||
| Psychological: NA | ||||||
| Exercise Alone | ||||||
| Anthropometric: NA | ||||||
| Cardiovascular-Respiratory: NA | ||||||
| Metabolic: NA | ||||||
| Reproductive:↑ FSH, SHBG, free testosterone; ↓ TT, androstenedione, FGS; NSD LH, FAI, E2, LH:FSH | ||||||
| Psychological: NA | ||||||
| Haqq et al. [15] | Physical exercise alone or combination diet and physical exercise | Usual care Sedentary control, placebo, diet only, or metformin | 12 | n = 668 | PCOS (criteria unspecified) | Lifestyle intervention (Combinatorial) |
| Anthropometric: ↓ BMI, BM, WC, WHR, BF% | ||||||
| Cardiovascular-Respiratory: ↑ VO2 max | ||||||
| Metabolic: ↓ CRP; NSD insulin, glucose, IR, TG, TC, LDL, HDL | ||||||
| Reproductive: NA | ||||||
| Psychological: NA | ||||||
| Exercise alone | ||||||
| Anthropometric: ↓ BMI, WC | ||||||
| Cardiovascular-Respiratory: ↓ RHR; ↑ VO2 max | ||||||
| Metabolic: NA | ||||||
| Reproductive: NA | ||||||
| Psychological: NA | ||||||
| Naderpoor et al. [16] | Lifestyle and metformin or metformin alone | Lifestyle or lifestyle and placebo | 12 | n = 608 | PCOS (Rotterdam) | Lifestyle + Metformin vs. Lifestyle ± Placebo 6/12 |
| Anthropometric: ↓ BMI, SAT; NSD WC, WHR, VAT | ||||||
| Cardiovascular-Respiratory: NSD SBP, DBP | ||||||
| Metabolic: NSD TC, HDL, LDL, TG, markers of IR, FBG | ||||||
| Reproductive: ↑ menstrual frequency over 6/12 and 12/12; NC acne, FG score, FAI, SHBG, DHEAS, LH | ||||||
| Psychological: NSD QOL | ||||||
| Lifestyle±Placebo vs. Metformin | ||||||
| Anthropometric: NSD BMI; ↓WC | ||||||
| Cardiovascular-Respiratory: NA | ||||||
| Metabolic: NSD FBG, insulin AUC, glucose AUC | ||||||
| Reproductive: ↑ SHBG, TT; NSD FAI, menstrual cycles 6 to 12/12 | ||||||
| Psychological: NA | ||||||
| Benham et al. [17] | Aerobic exercise, resistance training, combination of aerobic and resistance training both with and without behavioural and diet interventions | Diet intervention alone or standard care | 14 | n = 617 | PCOS (Rotterdam NIH, NIH Phenotypes, physician diagnosed and unreported) | Meta-analysis |
| Anthropometric: ↓WC; NC BMI, BF% | ||||||
| Cardiovascular-Respiratory: ↓ SBP; NC VO2 max, DBP | ||||||
| Metabolic: ↓ FI, TC, LDL, TGs; ↑ HDL; NC FBG | ||||||
| Semi-quantitative Analysis | ||||||
| Reproductive: NC/↑ pregnancy ↑ovulation rate/cycles, menstrual frequency/regularity menstrual cycle length | ||||||
| Psychological: NA | ||||||
| Kite et al. [18] | Exercise or | Control or diet alone | 18 | n = 758 | PCOS (Rotterdam NIH, physician diagnosed) | Exercise vs. Control—Change from Baseline |
| Anthropometric: NSD BMI, WHR, ↓WC, FM, FFM, BF% | ||||||
| Cardiovascular-Respiratory: NSD SBP, DBP, VO2 max, RHR | ||||||
| Exercise and diet | Control or diet alone | Metabolic: NSD FBG; ↓ FI, HOMA-IR, TC, LDL, TG; NC HDL | ||||
| Reproductive: NSD TT, SHBG, FT, FAI, FGS, E2, DHEA-S, LH, FSH, LH:FSH ratio, PG, Prolactin, AMH, Adiponectin. | ||||||
| Psychological: NA |
| Barriers to Lifestyle and Obesity Interventions | Studies with Identified Barrier(s) | Potential Facilitators and Solutions |
|---|---|---|
| Health System Level | ||
| Lack of time | Kushner et al. [88] Campbell et al. [89] Nicholas et al. [87] Jansink et al. [93] Lambe et al. [92] Passey et al. [94] Salinas et al. [91] Epling et al. [90] | |
| Lack of reimbursement | Kushner et al. [88] Passey et al. [94] Lambe et al. [92] Salinas et al. [91] Epling et al. [90] Ko et al. [97] |
|
| Limited access to and availability of allied health providers and other members of the multidisciplinary care team | Nicholas et al. [87] Passey et al. [94] Epling et al. [90] Teixeira et al. [98] Glauser et al. [99] Kim et al. [95] |
|
| Provider location | Kushner et al. [88] Kim et al. [95] |
|
| Lack of coordination between healthcare providers | Jansink et al. [93] Kim et al. [95] |
|
| Lack of high quality and affordable material for patient education | Jansink et al. [93] Kushner et al. [88] |
|
| Health Professional Level | ||
| Self-perceived lack of expertise and training | Kushner et al. [88] Nicholas et al. [87] Ampt et al. [96] Jansink et al. [93] Lambe et al. [92] Salinas et al. [91] Glauser et al. [99] |
|
| Perception of low patient motivation, responsibility, and/or compliance | Kushner et al. [88] Campbell et al. [90] Nicholas et al. [87] Ampt et al. [96] Lambe et al. [92] Jansink et al. [93] Salinas et al. [91] Epling et al. [90] Glauser et al. [99] Kim et al. [95] Teixeira et al. [98] Ko et al. [97] |
|
| Poor patient-provider partnership | Jansink et al. [93] |
|
| Reluctance to or fear of offending patient | Jansink et al. [93] Lambe et al. [92] Glauser et al. [99] |
|
| Motivation to implement based on provider’s own interests, expectations and experiences | Jansink et al. [93] Kim et al. [95] Teixeira et al. [98] |
|
| Limited patient time and priority to regularly attend consultations | Teixeira et al. [98] Ko et al. [97] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blackshaw, L.C.D.; Chhour, I.; Stepto, N.K.; Lim, S.S. Barriers and Facilitators to the Implementation of Evidence-Based Lifestyle Management in Polycystic Ovary Syndrome: A Narrative Review. Med. Sci. 2019, 7, 76. https://doi.org/10.3390/medsci7070076
Blackshaw LCD, Chhour I, Stepto NK, Lim SS. Barriers and Facilitators to the Implementation of Evidence-Based Lifestyle Management in Polycystic Ovary Syndrome: A Narrative Review. Medical Sciences. 2019; 7(7):76. https://doi.org/10.3390/medsci7070076
Chicago/Turabian StyleBlackshaw, Lucinda C. D., Irene Chhour, Nigel K. Stepto, and Siew S. Lim. 2019. "Barriers and Facilitators to the Implementation of Evidence-Based Lifestyle Management in Polycystic Ovary Syndrome: A Narrative Review" Medical Sciences 7, no. 7: 76. https://doi.org/10.3390/medsci7070076





