Characterization of Newly Discovered Phosphorite Deposits in Al-Tafeh, Jordan
Abstract
1. Introduction
2. Methodology
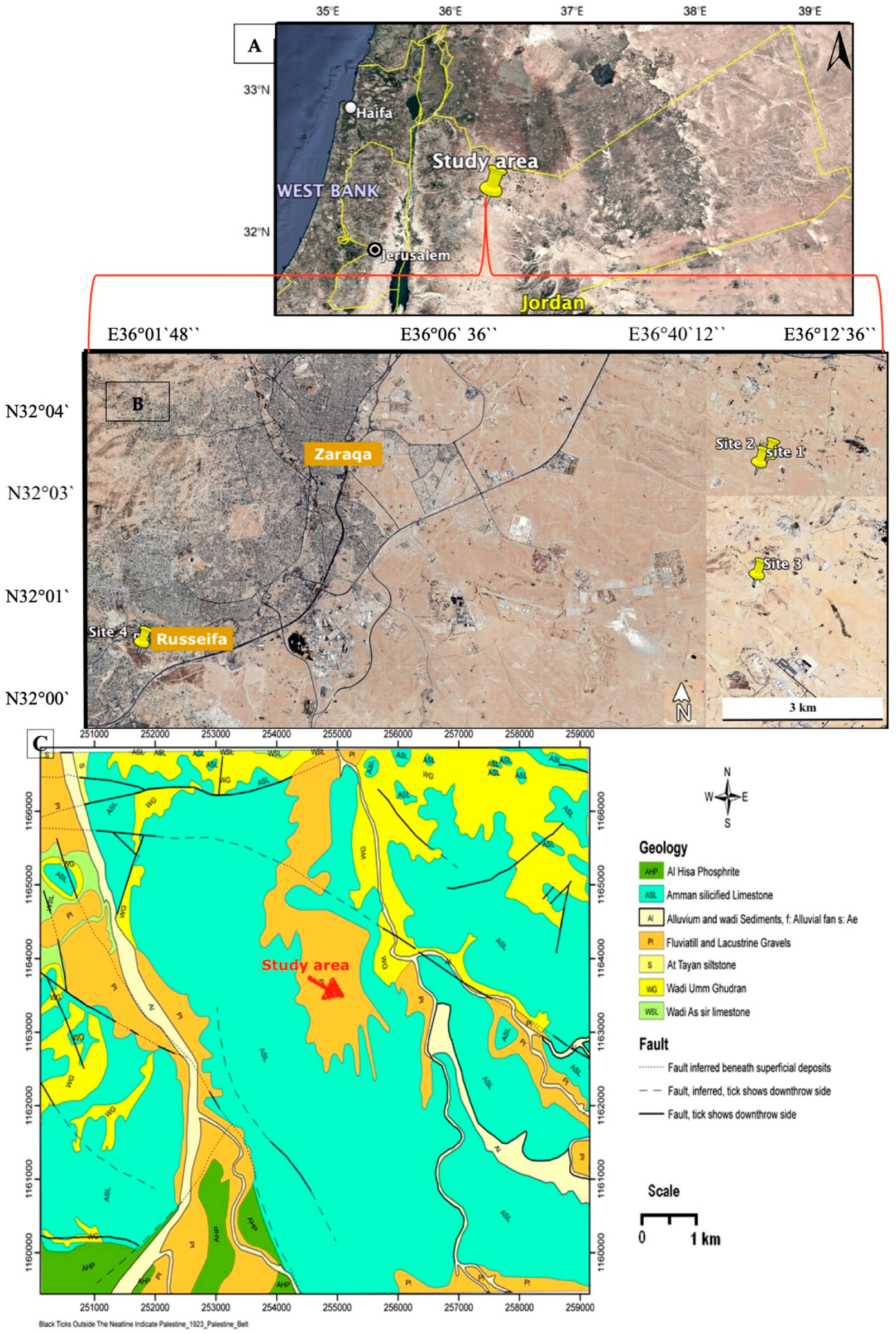
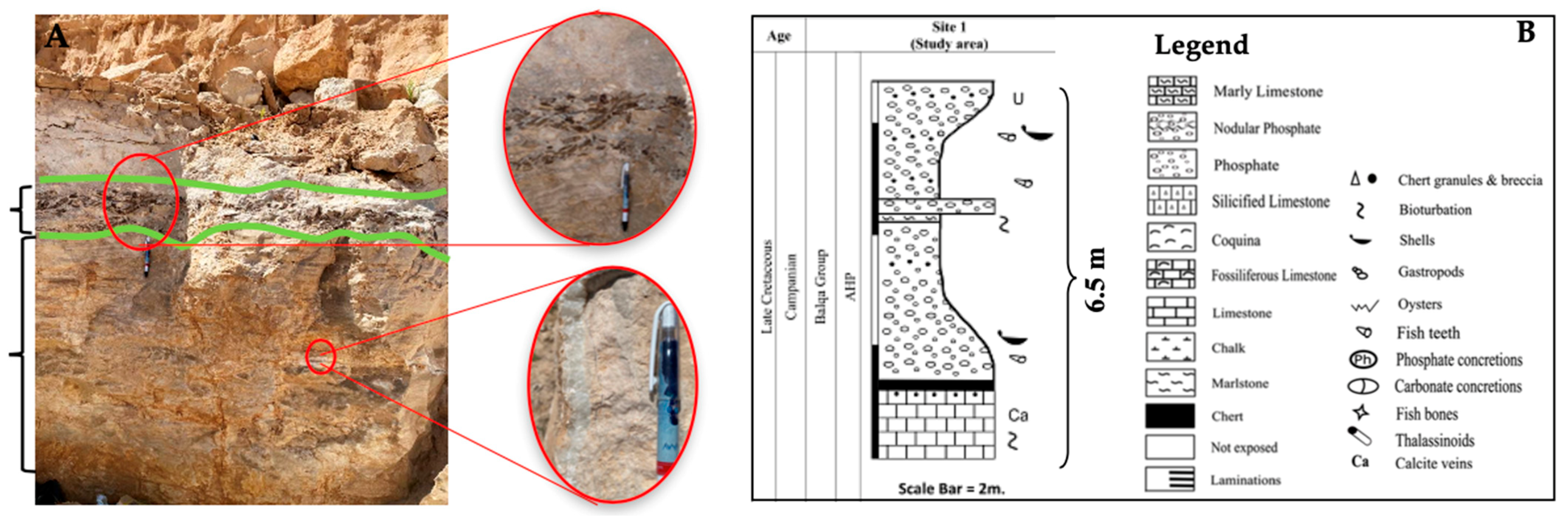
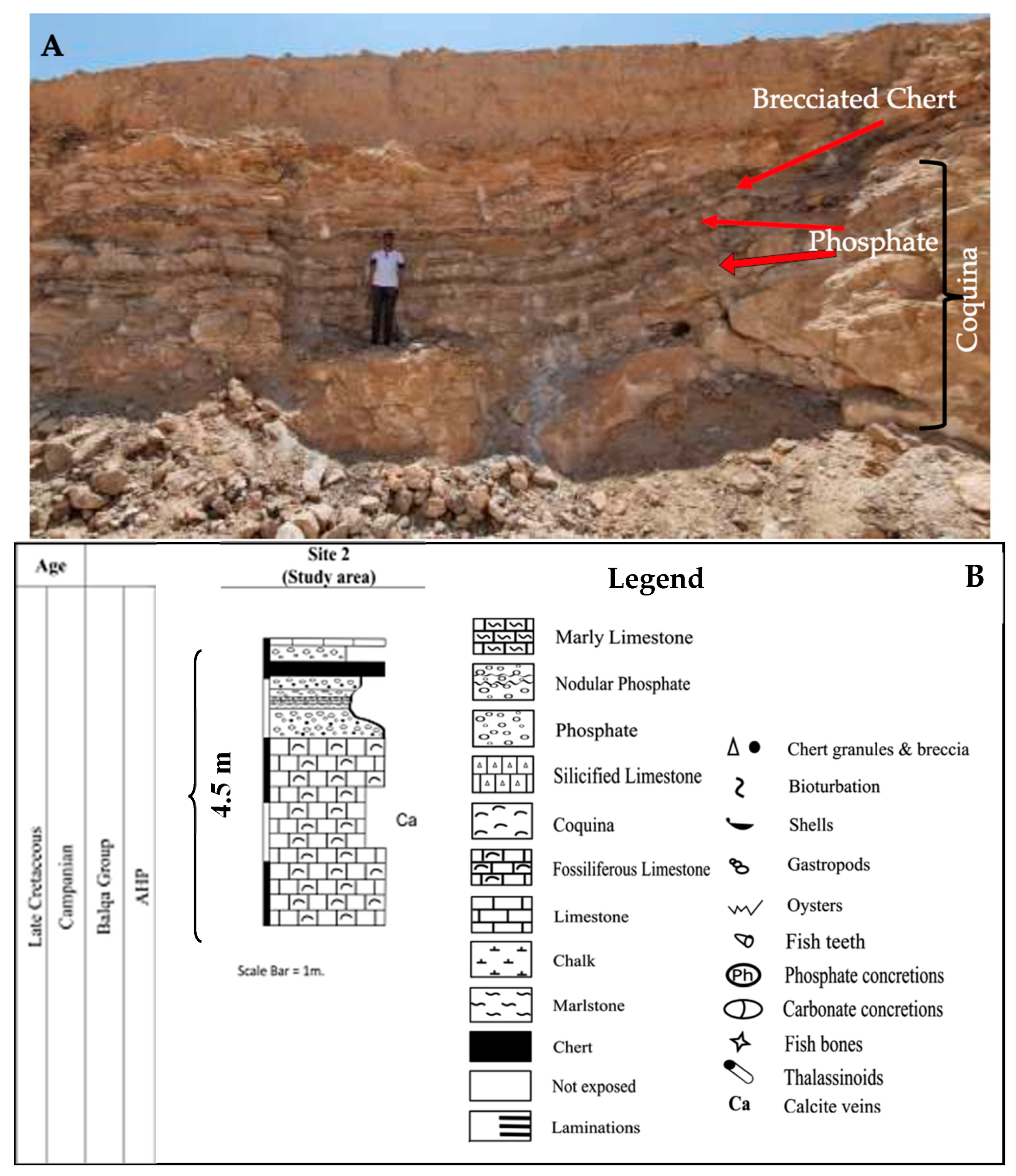
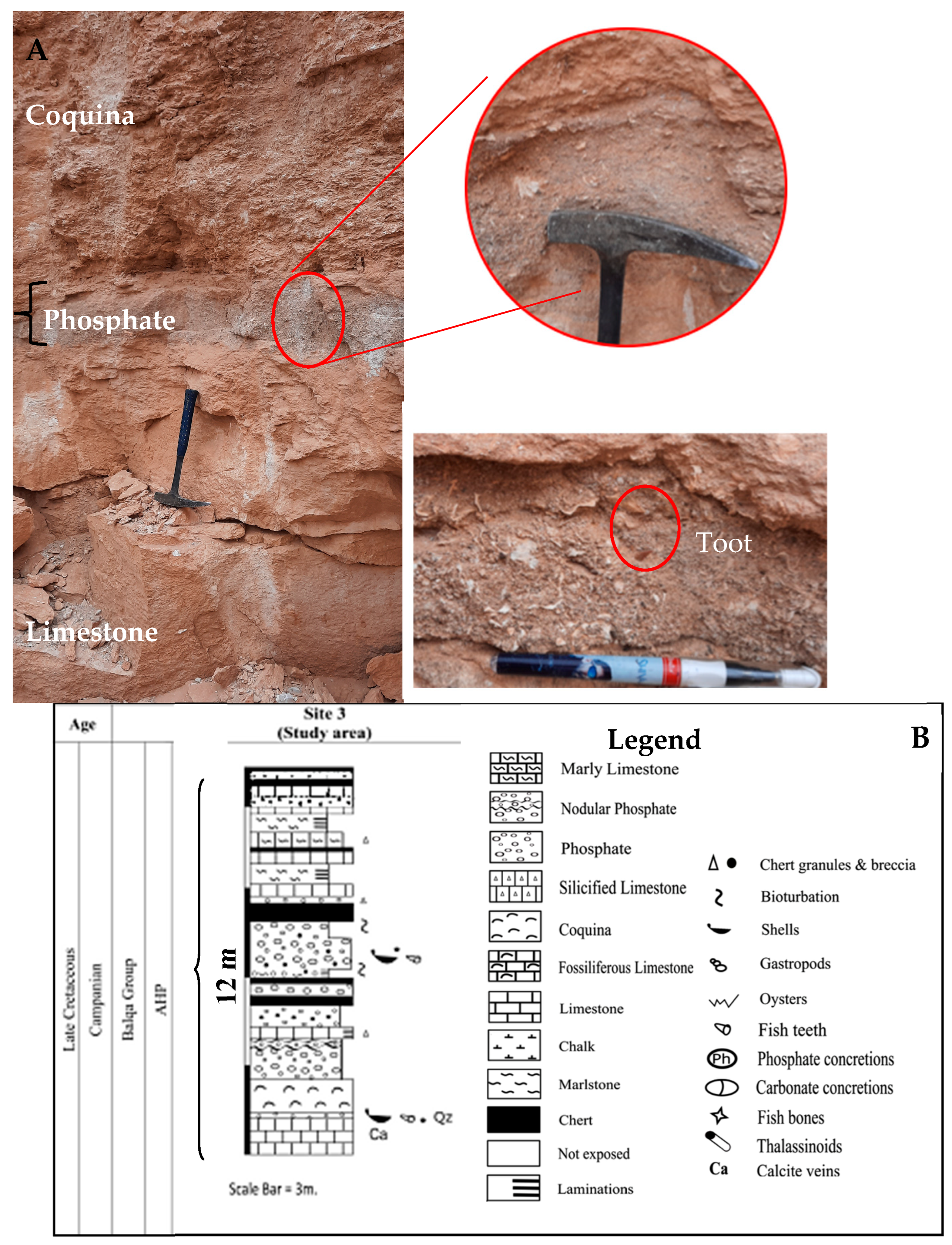
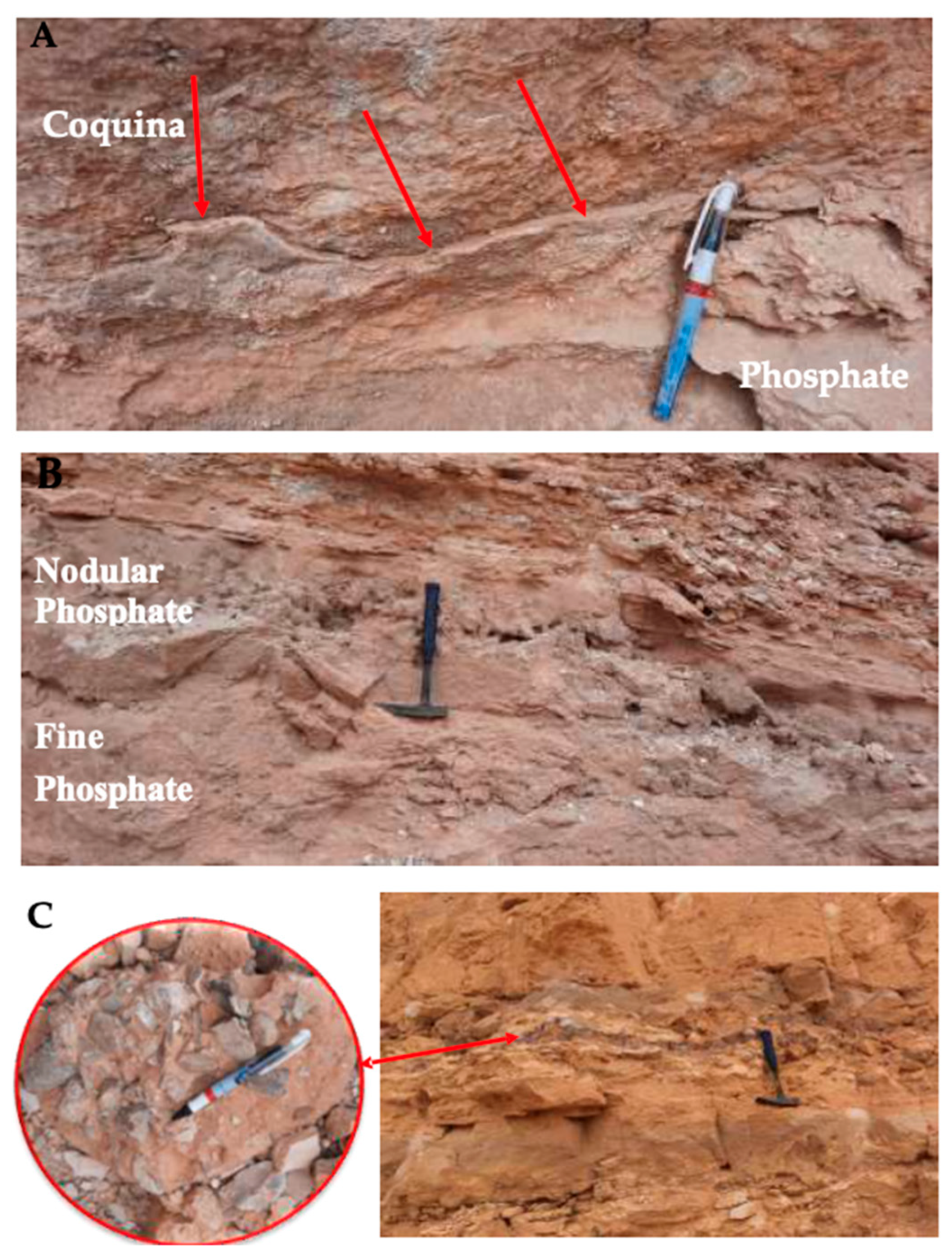
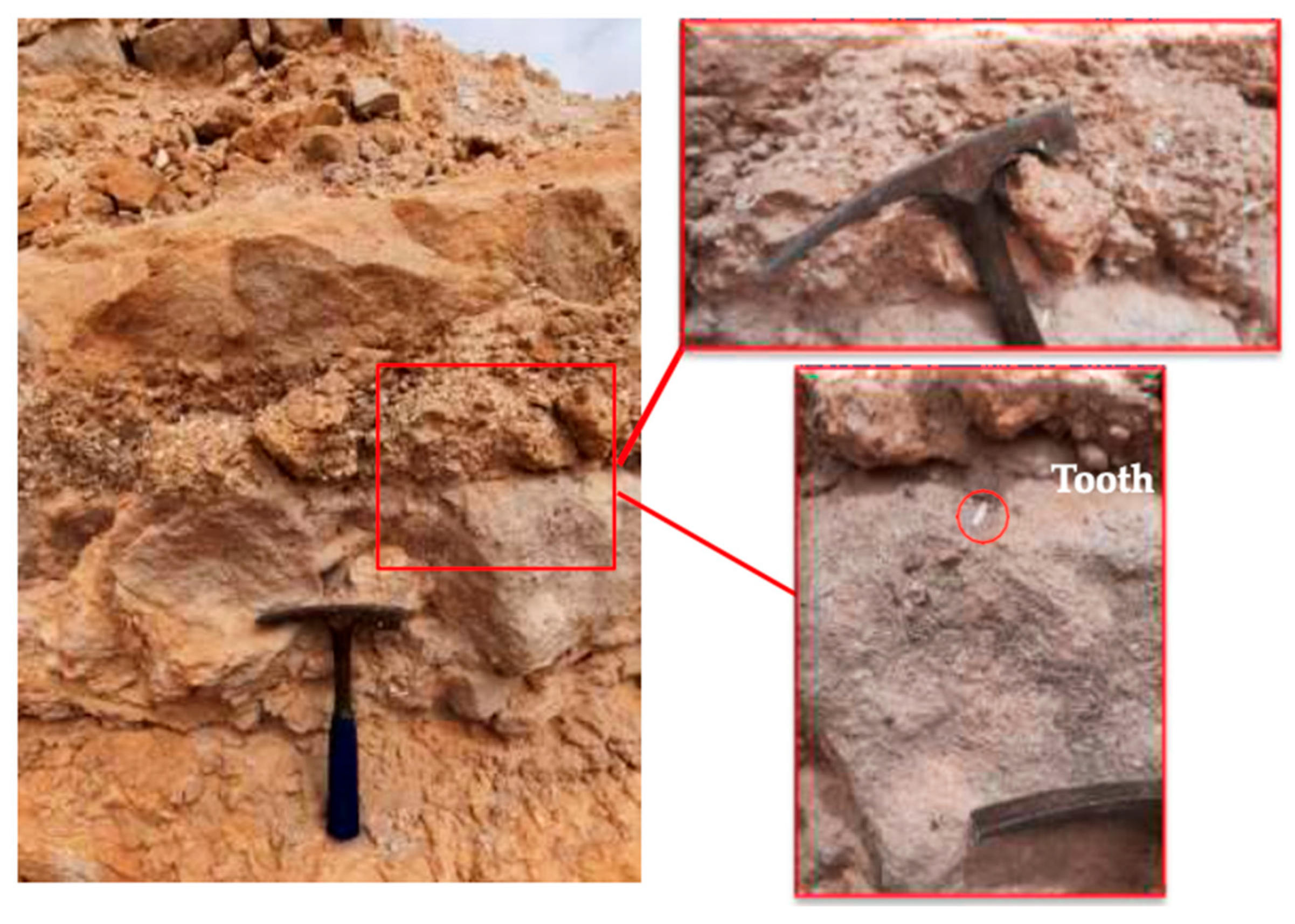
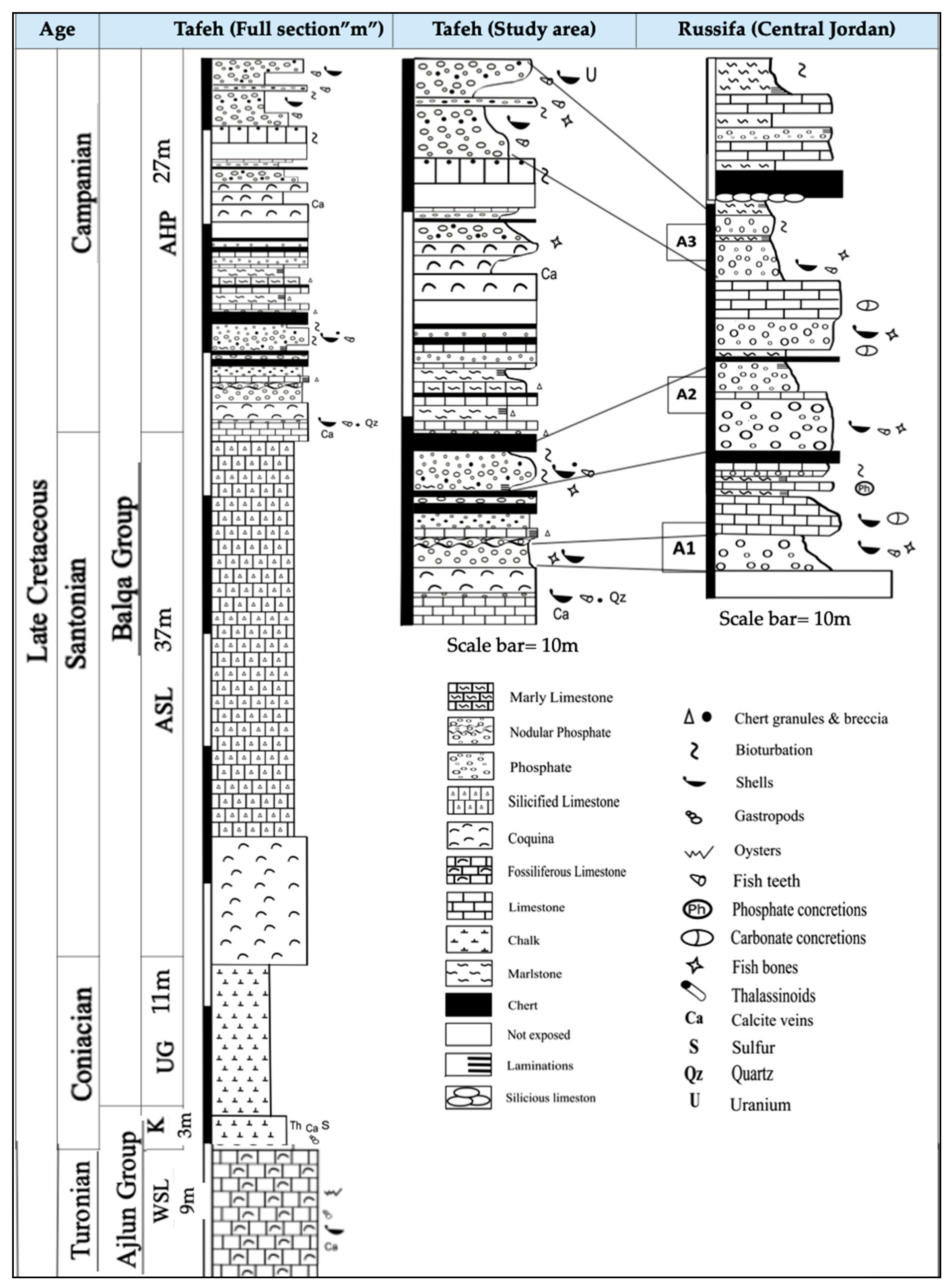
2.1. Geological Settings and Field Observations
2.2. Laboratory Works
3. Results
3.1. Geochemistry
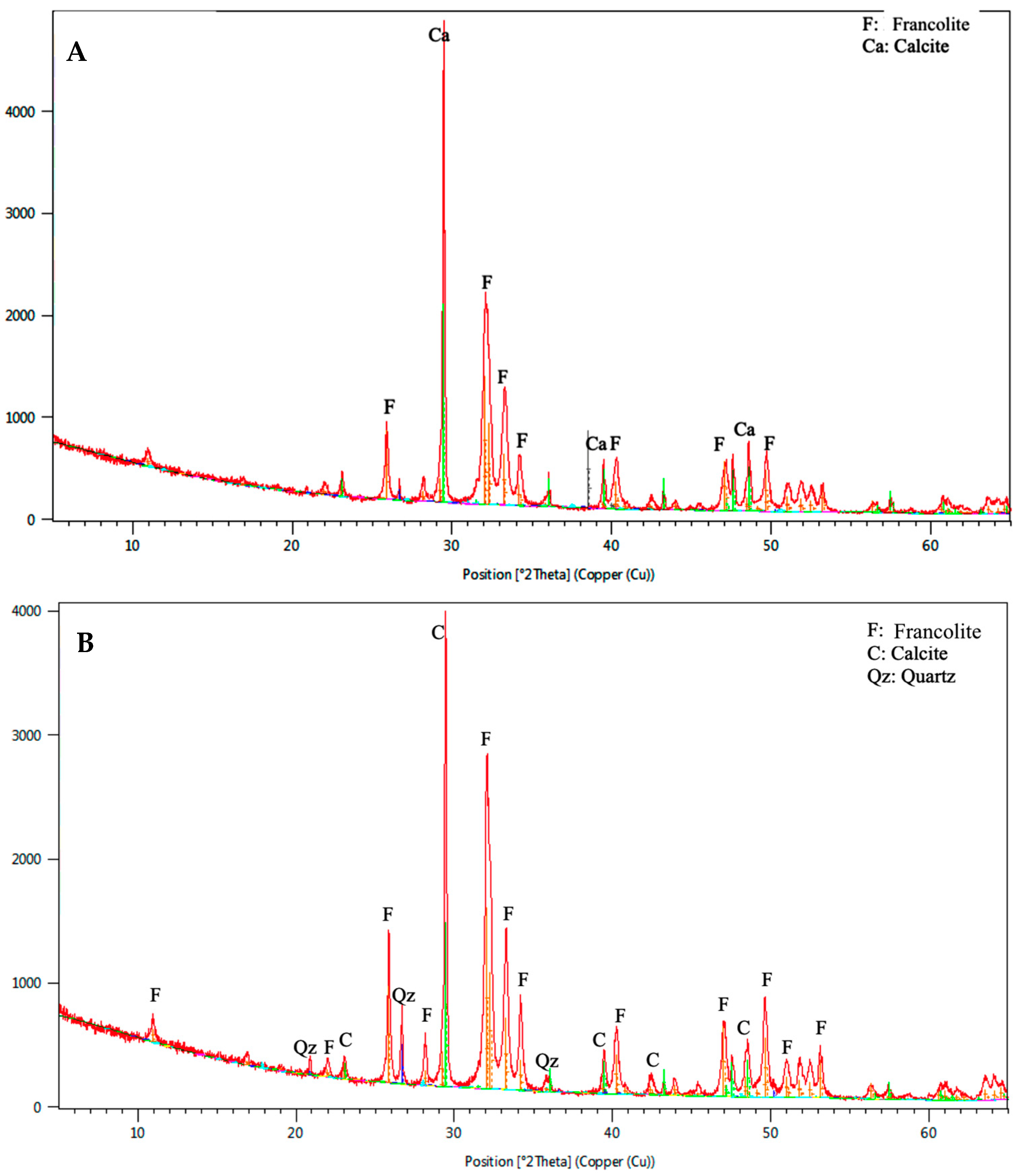
3.2. Mineralogical Study
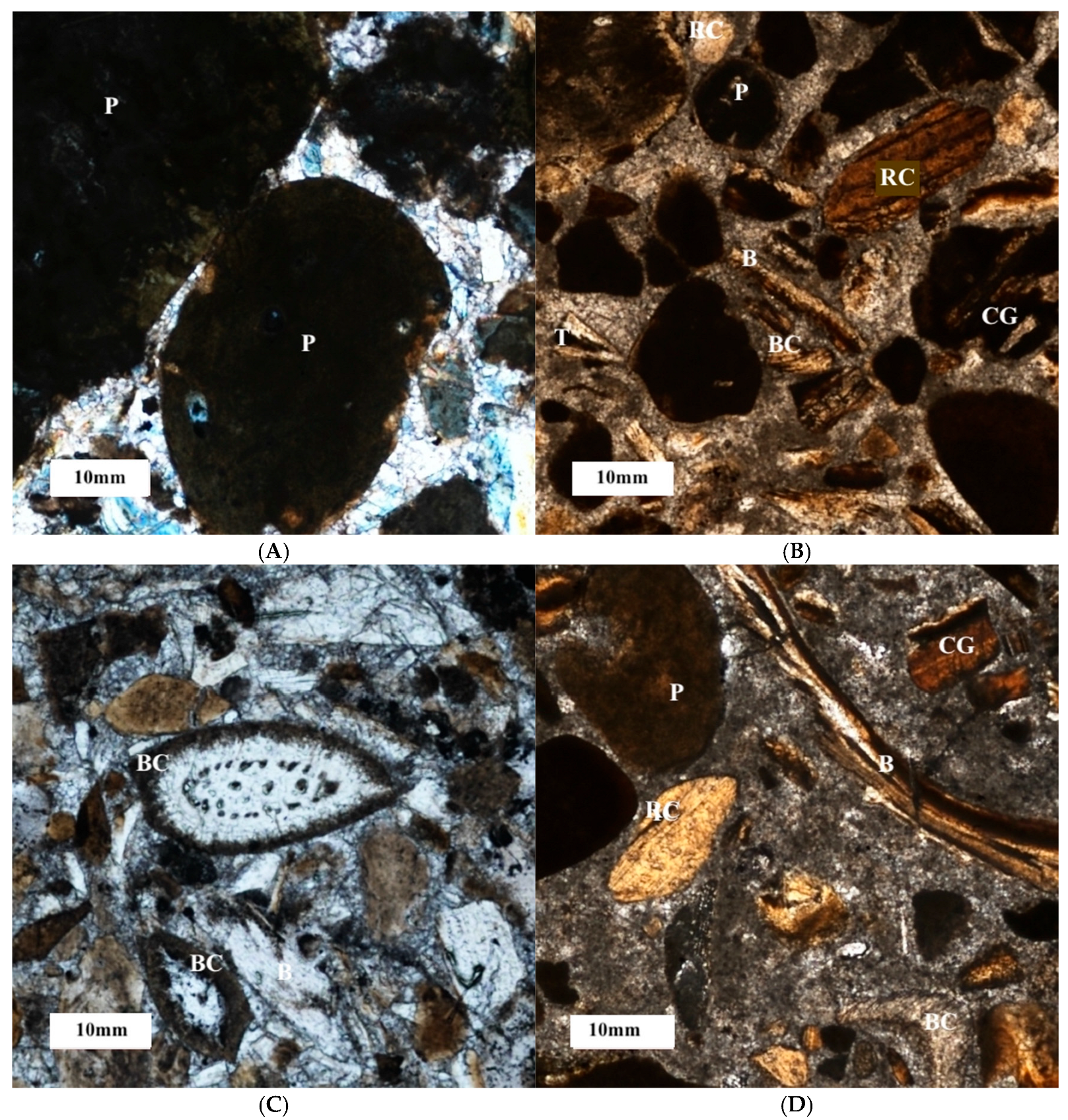
3.3. Petrographic Description
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, P.J. Volume 1: Proterozoic and Cambrian phosphorites. In Phosphate Deposits of the World; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Föllmi, K.B. The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits. Earth-Sci. Rev. 1996, 40, 55–124. [Google Scholar] [CrossRef]
- Glenn, C.R.; Föllmi, K.B.; Riggs, S.R.; Baturin, G.N.; Grimm, K.A.; Trappe, J.; Abed, A.M.; Galli-Oliver, C.; Garrison, R.E.; Ilyan, A.V. Phosphorus and phosphorites: Sedimentology and Environments of Formation. Eclogae Geol. Helv. 1994, 87, 747–788. [Google Scholar]
- US Geological Survey (USGS). Mineral Commodity Summaries: Phosphate Rock. U.S. Department of the Interior, 2022. Available online: https://www.sciencebase.gov/catalog/item/6197ccbed34eb622f692ee1c (accessed on 7 September 2025). [CrossRef]
- Abed, A.M. The eastern Mediterranean phosphorite giants: An interplay between tectonics and upwelling. GeoArabia 2013, 18, 67–94. [Google Scholar] [CrossRef]
- Mikbel, S.; Abed, A.M. Discovery of large phosphate deposits in north-west Jordan. Ann. Société Géologique Belg. 1985, 108, 169–174. [Google Scholar]
- Abed, A.M. On the genesis of the phosphorite–chert association of the Amman Formation in the Tel es Sur area, Ruseifa, Jordan. Sci. Géologiques Bull. Mémoires 1989, 42, 141–153. [Google Scholar] [CrossRef]
- Abed, A.M.; Amireh, B.S. Sedimentology, geochemistry, economic potential and palaeogeography of an Upper Cretaceous phosphorite belt in the southeastern desert of Jordan. Cretac. Res. 1999, 20, 119–133. [Google Scholar] [CrossRef]
- Pufahl, P.K.; Grimm, K.A.; Abed, A.M.; Sadaqah, R.M. Upper Cretaceous 11. (Campanian) phosphorites in Jordan: Implications for the formation of a south Tethyan phosphorite giant. Sediment. Geol. 2003, 161, 175–205. [Google Scholar] [CrossRef]
- Al-Shereideh, S.A.K.; Tarawneh, K.; El-Radaideh, N.; Saqqa, W.; Alnawafleh, H. Geology and mineralogy of Cabal Kabid phosphorite deposits, southeastern Jordan. Jordan J. Earth Environ. Sci. 2010, 3, 27–40. [Google Scholar]
- Al-Shereideh, S.; Saqqa, W.; El-Radaideh, N.; Tarawneh, K.; Alnawafleh, H. Calcitization and bulk chemistry of phosphate grains from the upper part of the Amman Formation, southeast Irbid, Jordan. J. Geogr. Geol. 2013, 5, 16–28. [Google Scholar] [CrossRef]
- Abu-Hamatteh, Z.S. Geology, geochemistry and are characteristics of the Jordanian phosphates. Cent. Eur. Geol. 2007, 50, 283–295. [Google Scholar] [CrossRef]
- Alnimrat, R.M.; Alqudah, M.; Al-Shereideh, S.; Alsaleh, A.R.S. Geochemical and petrological analyses of the phosphorite deposits (Amman/Al Hisa Formation) from northwest Jordan, and their insights for paleoenvironment. Arab. J. Geosci. 2022, 15, 1259. [Google Scholar] [CrossRef]
- Sadaqah, R.M.; Abed, A.M.; Grimm, K.A.; Pufahl, P.K. Oxygen and carbon isotopes in Jordanian phosphorites and associated fossils. J. Asian Earth Sci. 2007, 29, 803–812. [Google Scholar] [CrossRef]
- Alqudah, M.; Abu-Jaber, N.; Al-Rawabdeh, A.; Al-Tamimi, M. Paleoenvironmental study of the Late Cretaceous–Eocene Tethyan Sea associated with phosphorite deposits in Jordan. Appl. Sci. 2023, 13, 1568. [Google Scholar] [CrossRef]
- Basha, S. On the Tertiary phosphate rocks of the Risha area, NE Jordan. Dirasat 1987, 14, 211–227. [Google Scholar]
- Abed, A.M.; Jaber, O.; Alkuisi, M.; Sadaqah, R. Rare earth elements and uranium geochemistry in the Al-Kora phosphorite province, Late Cretaceous, northwestern Jordan. Arab. J. Geosci. 2016, 9, 187. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Al-Zughoul, K.; Muhtaseb, K. New occurrence of potential phosphate resource in northeast Jordan. Arab. J. Geosci. 2016, 9, 497. [Google Scholar] [CrossRef]
- Abu Qudaira, M. The Geology of Zarqa Area, Bulletin 58; Natural Resources Authority: Amman, Jordan, 2004; 47p. [Google Scholar]
- Abed, A.M.; Abu Murry, O.S. Rare earth element geochemistry of the Jordanian Upper Cretaceous Phosphorites. Arab. Gulf J. Sci. Res. 1997, 15, 41–158. [Google Scholar]
- Ryszko, U.; Rusek, P.; Kołodyńska, D. Quality of phosphate rocks from various deposits used in wet phosphoric acid and P-fertilizer production. Materials 2023, 16, 793. [Google Scholar] [CrossRef] [PubMed]
- Abed, A.M.; Al-Agha, M.R. Petrography, geochemistry and origin of the NW Jordan phosphorites. J. Geol. Soc. 1989, 146, 499–506. [Google Scholar] [CrossRef]
- Abed, A.M.; Sadaqah, R. Role of Upper Cretaceous oyster bioherms in the deposition and accumulation of high-grade phosphorites in central Jordan. J. Sediment. Res. 1998, 68, 1009–1020. [Google Scholar] [CrossRef]
- Powell, J.H.; Moh’d, B.K. Early diagenesis of Late Cretaceous chalk–chert–phosphorite hardgrounds in Jordan: Implications for sedimentation on a Coniacian–Campanian pelagic ramp. GeoArabia 2012, 17, 17–38. [Google Scholar] [CrossRef]
- Khaled, H.; Abed, A.M. Petrography and geochemistry of Esh-Shidiya phosphates. Dirasat 1982, 9, 81–102. [Google Scholar]


| Elements (wt.%) | Study Area (Al-Tafeh) | Jordanian Phosphorites [20] | Djebel Onk (Algiers) [21] | Khneifiss (Syria) [21] | Bou Craa (Morocco) [21] |
|---|---|---|---|---|---|
| CaO | 52.78 | 33.90–53.90 | 53.20 | 53.40 | 48.40 |
| P2O5 | 24.32 | 27.09–34.34 | 29.00 | 28.70 | 29.40 |
| SiO2 | 2.29 | 0.88–26.23 | 2.93 | 8.67 | 2.66 |
| Al2O3 | 0.21 | 0.08–2.12 | 0.51 | 0.33 | 0.44 |
| Fe2O3 | 0.18 | 0.07–0.72 | 0.64 | 0.21 | 0.55 |
| MgO | 0.31 | 0.18–0.61 | 1.31 | 0.34 | 1.01 |
| Na2O | 0.32 | 0.03–1.59 | 1.54 | 0.61 | 1.09 |
| K2O | 0.02 | 0.01–0.18 | 0.20 | 0.10 | 0.22 |
| F | 3.73 | 2.68–4.38 | 3.12 | 3.53 | 3.47 |
| Cl | 0.073 | 0.07 | 0.012 | 0.064 | 0.015 |
| Elements (mg/g) | Study Area (Al-Tafeh) | Jordanian Phosphorites [23] | Djebel Onk (Algiers) [21] | Khneifiss (Syria) [21] | Bou Craa (Morocco) [21] |
|---|---|---|---|---|---|
| Ni | 0.019 | 0.018 | 0.015 | 0.024 | 0.018 |
| Cu | 0.016 | 0.019 | 0.011 | 0.027 | 0.011 |
| Zn | 0.099 | 0.149 | 0.161 | 0.030 | 0.152 |
| Cd | 0.025 | 0.011 | 0.014 | 0.008 | 0.145 |
| U | 0.045 | 0.090 | 0.041 | 0.072 | 0.035 |
| CaO | P2O5 | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | CO2 | Cl | Ni | Cu | Zn | Cd | U | Y | La | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | 1 | ||||||||||||||||
| P2O5 | −0.9003 | 1 | |||||||||||||||
| SiO2 | −0.5152 | 0.2193 | 1 | ||||||||||||||
| Al2O3 | −0.3301 | 0.1825 | −0.0228 | 1 | |||||||||||||
| Fe2O3 | −0.4013 | 0.2395 | 0.1242 | 0.9749 | 1 | ||||||||||||
| MgO | −0.4900 | 0.4038 | −0.2330 | 0.7191 | 0.6194 | 1 | |||||||||||
| Na2O | −0.8857 | 0.8705 | 0.0937 | 0.3839 | 0.3696 | 0.7426 | 1 | ||||||||||
| K2O | −0.2882 | 0.1636 | −0.0805 | 0.9834 | 0.9657 | 0.7156 | 0.3527 | 1 | |||||||||
| CO2 | 0.4826 | −0.3808 | −0.0981 | −0.5089 | −0.5023 | −0.5384 | −0.5095 | −0.5782 | 1 | ||||||||
| Cl | 0.2213 | −0.1942 | −0.3448 | −0.2224 | −0.3721 | 0.0431 | 0.0424 | −0.3351 | 0.3549 | 1 | |||||||
| Ni | 0.6860 | −0.7110 | −0.4425 | 0.2900 | 0.1726 | 0.1025 | −0.5499 | 0.2919 | 0.2795 | 0.0923 | 1 | ||||||
| Cu | −0.0493 | 0.2276 | −0.1067 | −0.5189 | −0.5630 | −0.1604 | 0.1259 | −0.6149 | 0.7027 | 0.5260 | −0.1214 | 1 | |||||
| Zn | −0.3786 | 0.3732 | 0.6182 | −0.5483 | −0.3821 | −0.5129 | 0.0897 | −0.5833 | 0.3586 | −0.1412 | −0.7214 | 0.4226 | 1 | ||||
| Cd | 0.5615 | −0.6588 | −0.1130 | −0.2599 | −0.2374 | −0.2556 | −0.5514 | −0.1328 | −0.0633 | −0.2668 | 0.2301 | −0.5202 | −0.1500 | 1 | |||
| U | −0.3981 | 0.4257 | 0.6263 | −0.3689 | −0.2004 | −0.4926 | 0.0643 | −0.4264 | 0.4202 | −0.2324 | −0.5650 | 0.4610 | 0.9254 | −0.3817 | 1 | ||
| Y | −0.8364 | 0.7115 | 0.1594 | 0.4664 | 0.4551 | 0.7811 | 0.9479 | 0.4395 | −0.5863 | 0.0614 | −0.5103 | −0.0707 | 0.0297 | −0.3555 | −0.0607 | 1 | |
| La | −0.8190 | 0.6791 | 0.1442 | 0.6378 | 0.6270 | 0.8524 | 0.9196 | 0.6162 | −0.6367 | −0.0458 | −0.3775 | −0.1855 | −0.0926 | −0.3627 | −0.1243 | 0.9768 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Slaty, F.; Ibrahim, K.M.; Amjad, M.; Muhtaseb, M. Characterization of Newly Discovered Phosphorite Deposits in Al-Tafeh, Jordan. Geosciences 2025, 15, 433. https://doi.org/10.3390/geosciences15110433
Al-Slaty F, Ibrahim KM, Amjad M, Muhtaseb M. Characterization of Newly Discovered Phosphorite Deposits in Al-Tafeh, Jordan. Geosciences. 2025; 15(11):433. https://doi.org/10.3390/geosciences15110433
Chicago/Turabian StyleAl-Slaty, Faten, Khalil M. Ibrahim, Madlin Amjad, and Mohammad Muhtaseb. 2025. "Characterization of Newly Discovered Phosphorite Deposits in Al-Tafeh, Jordan" Geosciences 15, no. 11: 433. https://doi.org/10.3390/geosciences15110433
APA StyleAl-Slaty, F., Ibrahim, K. M., Amjad, M., & Muhtaseb, M. (2025). Characterization of Newly Discovered Phosphorite Deposits in Al-Tafeh, Jordan. Geosciences, 15(11), 433. https://doi.org/10.3390/geosciences15110433






