Integrative High-Throughput Screening and Microscopic Evidence Implicates Microsporidia as a Potential Pathogen of “Pus Crab” in the Mud Crab (Scylla paramamosain)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Hematoxylin–Eosin, Wright–Giemsa, and Masson Staining Analysis
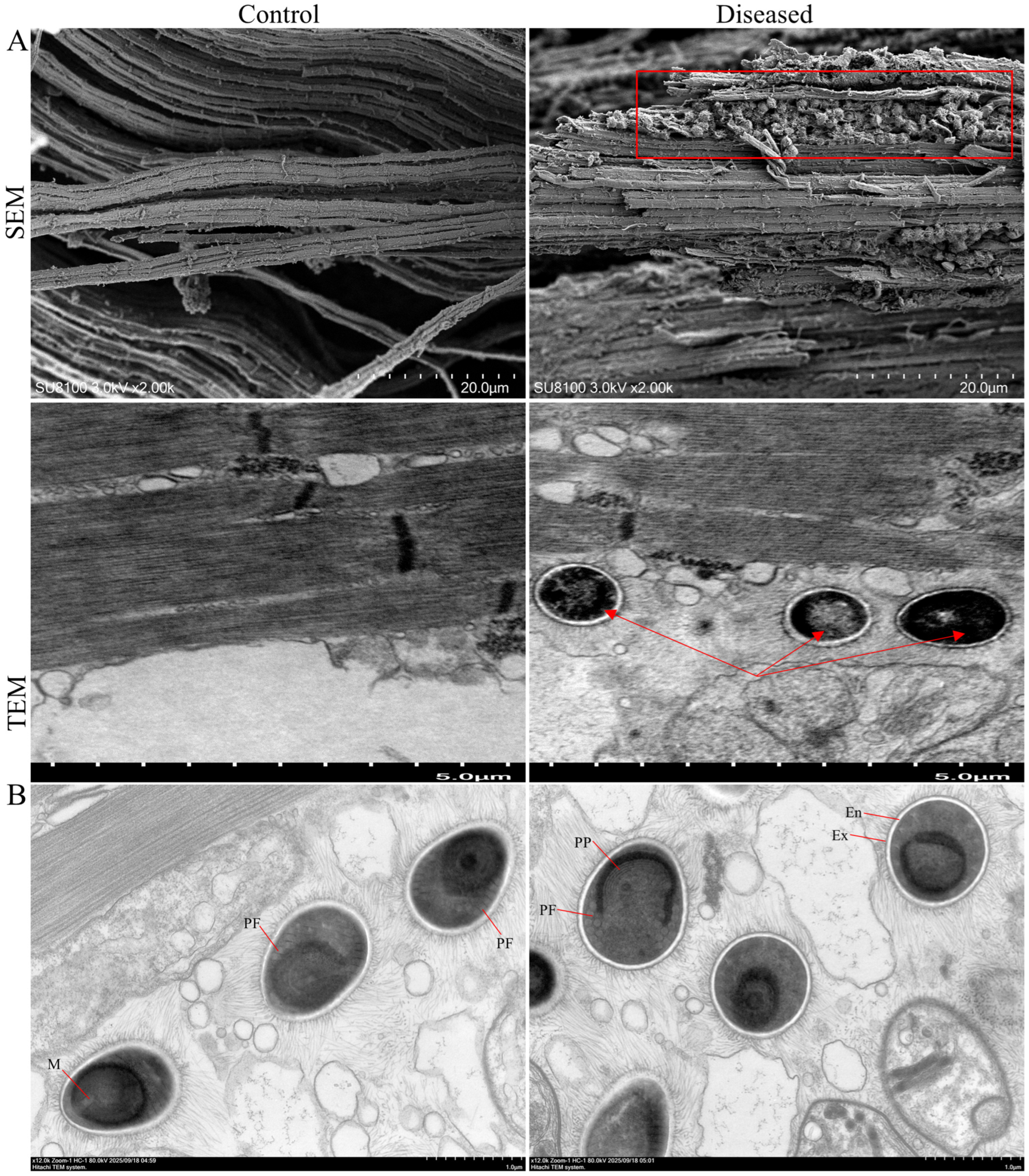
2.3. Electron Microscope Analysis
2.4. DNA Extraction and Illumina High-Throughput Sequencing
2.5. Metagenomic De Novo Assembly, Gene Prediction, and Functional Annotation
2.6. Isolation and Regression Infection of Microsporidia
2.7. Statistical Analysis
3. Results
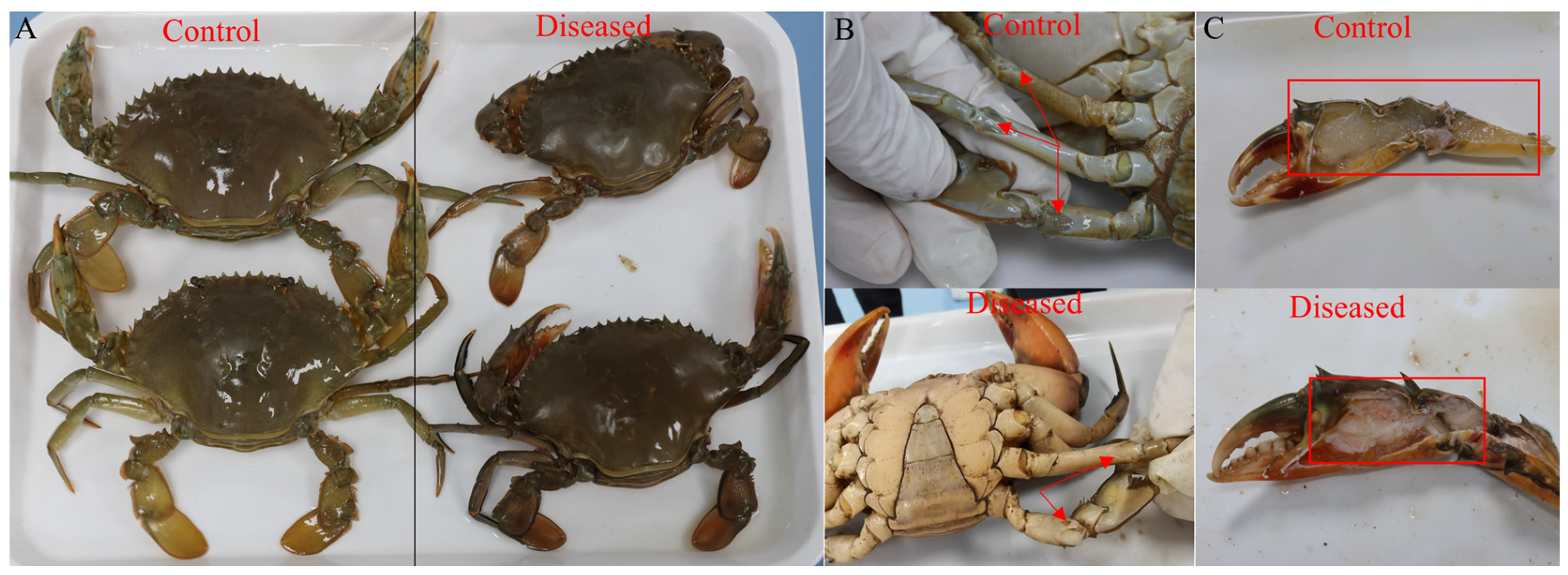
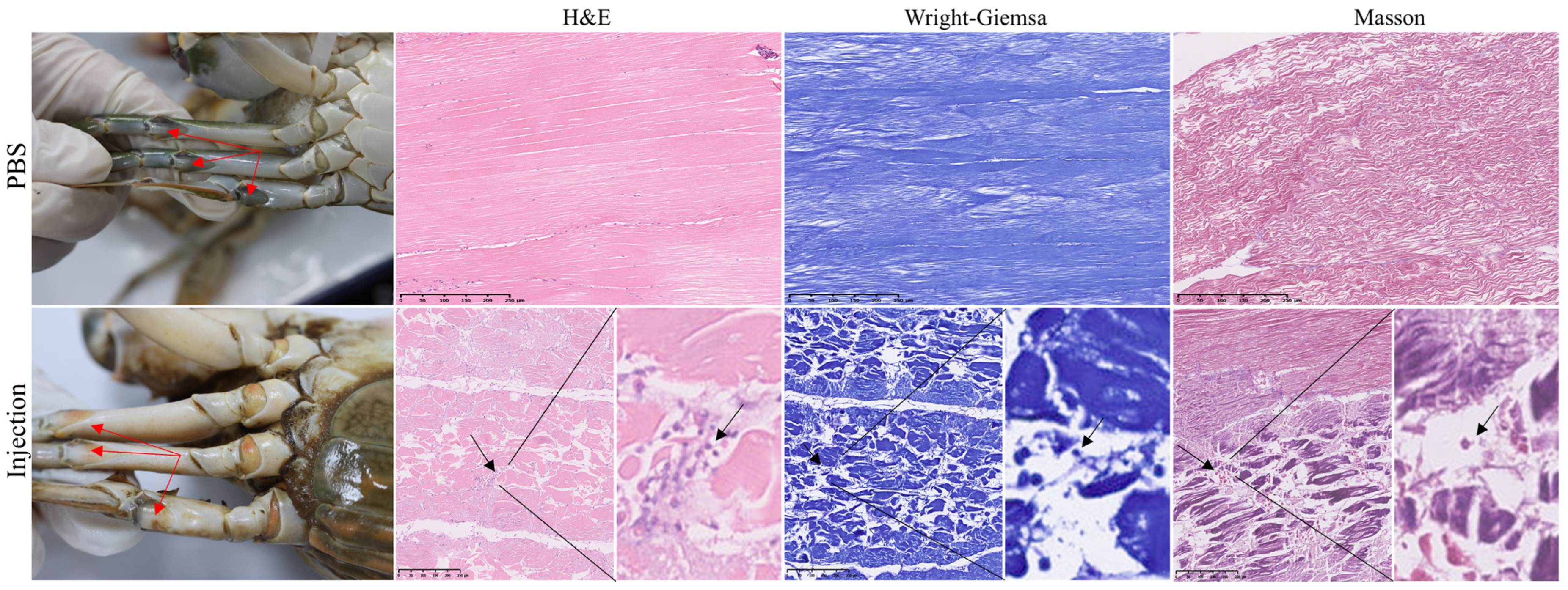
3.1. Histopathological Changes of Muscle
3.2. Metagenomic Data Output
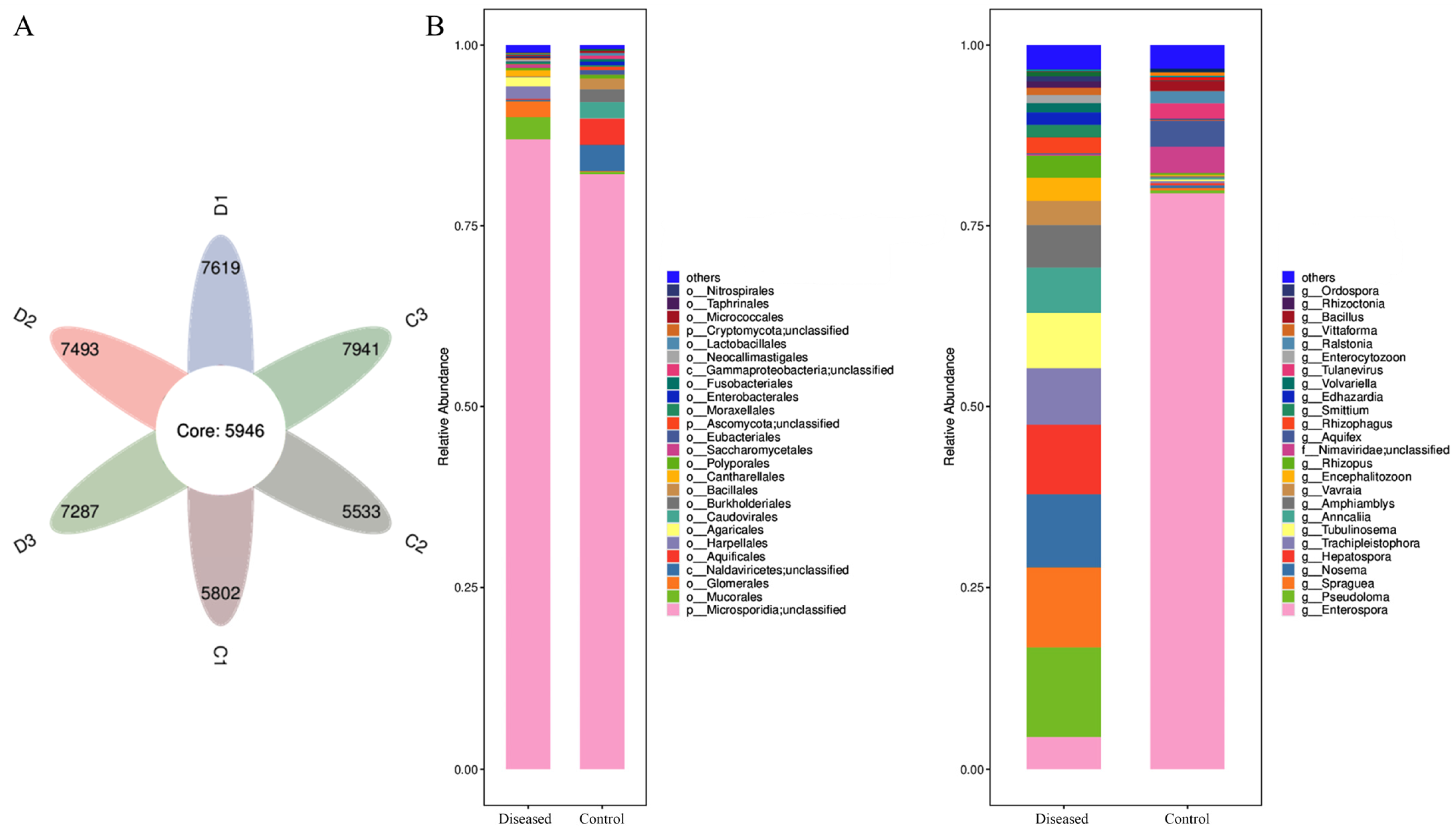
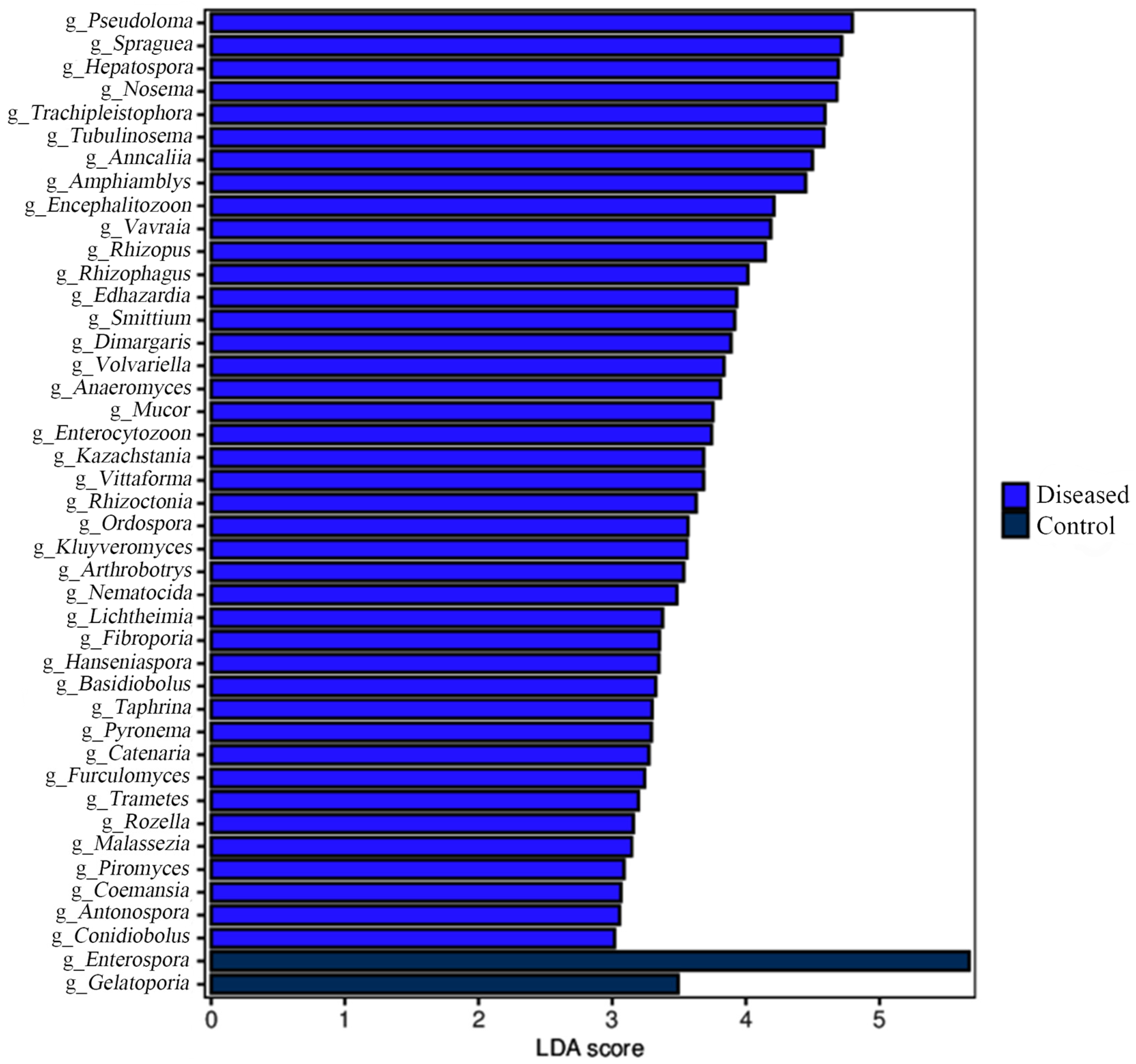
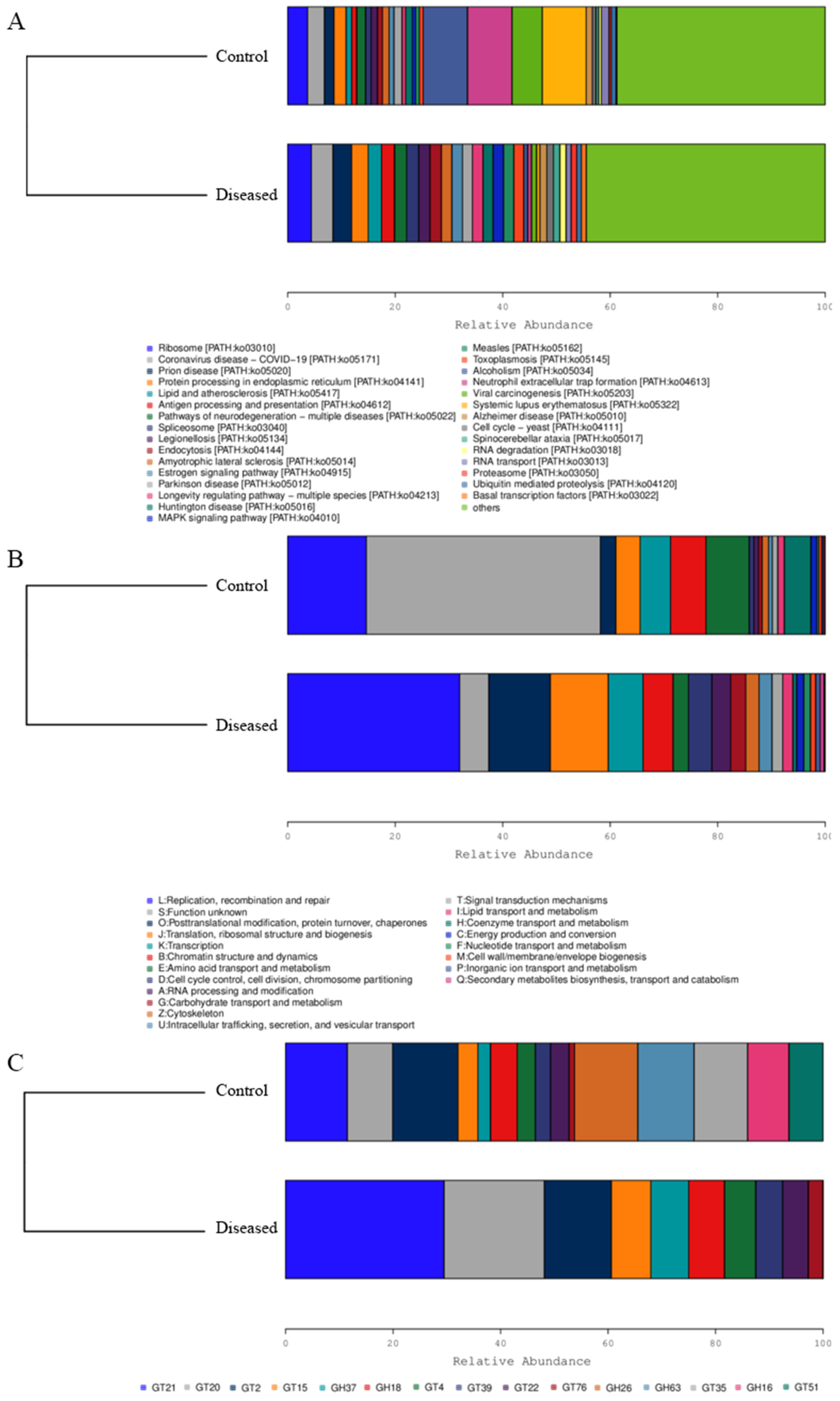
3.3. Muscle Microbial Composition Analysis
3.4. Functional Analysis
3.5. Pathogenicity Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xin, Z.Z.; Zhang, X.T.; Zhu, Z.Q.; Chen, J.Y.; Zhang, J.Y. Comparative transcriptomic analysis provided insights into the local molecular responses of swimming crabs, Portunus trituberculatus and mud crabs, Scylla paramamosain to the infection of Ameson portunus (Microsporidia). Aquaculture 2023, 576, 739885. [Google Scholar] [CrossRef]
- Meng, X.H.; Jang, I.K.; Seo, H.C.; Cho, Y.R. White spot syndrome virus quantification in blue crab Portunus trituberculatus hatchery-produced larvae and wild populations by TaqMan real-time PCR, with an emphasis on the relationship between viral infection and crab health. Aquaculture 2009, 291, 18–22. [Google Scholar] [CrossRef]
- Zhang, J.; Small, H.J.; Li, M.; Huang, Q.; Hu, L.J.; Xue, Q.; Wang, J.Y.; Li, C.W. The parasitic dinoflagellate Hematodinium perezi suppresses the innate immunity of Portunus trituberculatus via exosomal miRNAs. Aquaculture 2025, 609, 742827. [Google Scholar] [CrossRef]
- Fang, L.X.; Huang, Z.Y.; Fan, L.M.; Hu, G.D.; Qiu, L.P.; Song, C.; Chen, J.Z. Health risks associated with sulfonamide and quinolone residues in cultured Chinese mitten crab (Eriocheir sinensis) in China. Mar. Pollut. Bull. 2021, 165, 112184. [Google Scholar] [CrossRef]
- Li, C.W.; Li, M.; Huang, Q. The parasitic dinoflagellate Hematodinium infects marine crustaceans. Mar. Life Sci. Technol. 2021, 3, 313–325. [Google Scholar] [CrossRef]
- Jiang, H.B.; Bao, J.; Cao, G.N.; Xing, Y.N.; Feng, C.C.; Hu, Q.B.; Li, X.D.; Chen, Q.J. Experimental Transmission of the Yeast, Metschnikowia bicuspidata, in the Chinese Mitten Crab, Eriocheir sinensis. J. Fungi 2022, 8, 210. [Google Scholar] [CrossRef]
- Xiao, L.F.; Chen, C.; Liang, Y.J.; Wu, K.; Wen, X.B. Ammonia-nitrogen stress affects immune regulation via TNFα in yellow catfish (Pelteobagrus fulvidraco). Aquaculture 2024, 583, 740593. [Google Scholar] [CrossRef]
- Dissasa, G.; Lemma, B.; Mamo, H. Isolation and identification of major bacteria from three Ethiopian rift valley lakes live and processed fish, and water samples: Implications in sanitary system of fish products. BMC Vet. Res. 2022, 18, 439. [Google Scholar] [CrossRef]
- Pavloudi, C.; Tsertou, M.I.; Antonopoulou, E.; Katharios, P. Investigation of systemic granulomatosis in cultured meagre, Argyrosomus regius, using clinical metagenomics. Aquaculture 2023, 567, 739249. [Google Scholar] [CrossRef]
- Handelsman, J.; Rondon, M.R.; Brady, S.F.; Clardy, J.; Goodman, R.M. Molecular biological access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 1998, 5, R245–R249. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Buratto, V.M.; Tenfen, H.; Owatari, M.S.; Lapa, K.R. Moving bed biofilm reactor for Pimelodus maculatus reared in RAS: Start-up maturation, bioreactor microbiome and nitrogen removal. Water Biol. Secur. 2024, 3, 100251. [Google Scholar] [CrossRef]
- Deng, B.N.; Gao, Z.M.; Ru, X.S.; Tong, H.Y.; Liang, W.K.; Eeckhaut, I.; Zhang, L.B.; Xu, J.L. Metagenomic insights into the energy metabolism and immune variation of sea cucumber Apostichopus japonicus during reproduction. Aquaculture 2024, 579, 740125. [Google Scholar] [CrossRef]
- Han, B.; Pan, G.Q.; Weiss, L.M. Microsporidiosis in Humans. Clin. Microbiol. Rev. 2021, 34, e0001020. [Google Scholar] [CrossRef]
- Hirt, R.P.; Logsdon, J.M.; Healy, B.; Dorey, M.W.; Doolittle, W.F.; Embley, T.M. Microsporidia are related to Fungi: Evidence from the largest subunit of RNA polymerase II and other proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 580–585. [Google Scholar] [CrossRef]
- Sprague, V.; Becnel, J.J. Note on the Name–Author–Date Combination for the Taxon MICROSPORIDIES Balbiani, 1882, When Ranked as a Phylum. J. Invertebr. Pathol. 1998, 71, 91–94. [Google Scholar] [CrossRef]
- Cali, A.; Takvorian, P.M. Developmental Morphology and Life Cycles of the Microsporidia. In Microsporidia: Pathogens of Opportunity; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 71–133. [Google Scholar] [CrossRef]
- Chayaburakul, K.; Nash, G.; Pratanpipat, P.; Sriurairatana, S.; Withyachumnarnkul, B. Multiple pathogens found in growth-retarded black tiger shrimp Penaeus monodon cultivated in Thailand. Dis. Aquat. Organ. 2004, 60, 89–96. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.C.; Fu, G.h.; Zhao, S.; Chen, Y.G.; Wang, H.; Chen, T.T.; Zhou, J.F.; Fang, W.H. Morphology and phylogeny of Ameson portunus n. sp. (Microsporidia) infecting the swimming crab Portunus trituberculatus from China. Eur. J. Protistol. 2017, 61, 122–136. [Google Scholar] [CrossRef]
- Huang, W.F.; Tsai, S.J.; Lo, C.F.; Soichi, Y.; Wang, C.H. The novel organization and complete sequence of the ribosomal RNA gene of Nosema bombycis. Fungal Genet. Biol. 2004, 41, 473–481. [Google Scholar] [CrossRef]
- Wei, J.H.; Fei, Z.H.; Pan, G.Q.; Weiss, L.M.; Zhou, Z.Y. Current Therapy and Therapeutic Targets for Microsporidiosis. Front. Microbiol. 2022, 13, 835390. [Google Scholar] [CrossRef]
- Aldama-Cano, D.J.; Sanguanrut, P.; Munkongwongsiri, N.; Ibarra-Gámez, J.C.; Itsathitphaisarn, O.; Vanichviriyakit, R.; Flegel, T.W.; Sritunyalucksana, K.; Thitamadee, S. Bioassay for spore polar tube extrusion of shrimp Enterocytozoon hepatopenaei (EHP). Aquaculture 2018, 490, 156–161. [Google Scholar] [CrossRef]
- Zhang, X.T.; Xin, Z.Z.; Xue, S.J.; Tang, S.L.; Zhang, J.Y. First report of Ameson portunus (microsporidia) infection in earthen pond-cultured mud crab, Scylla paramamosain (Decapoda: Portunidae) in China, causing mass morbidity. J. Fish Dis. 2023, 46, 715–721. [Google Scholar] [CrossRef]
- Bojko, J.; Reinke, A.W.; Stentiford, G.D.; Williams, B.; Rogers, M.S.J.; Bass, D. Microsporidia: A new taxonomic, evolutionary, and ecological synthesis. Trends Parasitol. 2022, 38, 642–659. [Google Scholar] [CrossRef] [PubMed]
- Seabkongseng, T.; Limkul, S.; Sriphuttha, C.; Phiwthong, T.; Aunkam, P.; Suwannathit, R.; Jaree, P.; Somboonwiwat, K.; Tittabutr, P.; Teaumroong, N.; et al. Supplementation of Bacillus velezensis S141 in feed as a probiotic enhances growth performance, pathogenic tolerances, and immune system in shrimp. Aquaculture 2025, 604, 742448. [Google Scholar] [CrossRef]
- Sumithra, T.G.; Gayathri, S.; Krupesha Sharma, S.R.; Ebeneezar, S.; Anikuttan, K.K.; Sajina, K.A.; Narasimapallavan, G.I.; Reshma, K.J.; Vishnu, R.; Tamilmani, G.; et al. Metagenomic signatures of transportation stress in the early life stages of cobia (Rachycentron canadum) to aid in mitigation strategies. Aquaculture 2022, 559, 738407. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Pang, Y.Y.; Song, X.Z.; Zhou, N.; Wang, J.; He, L.; Lv, J.H.; Song, Y.M.; Cheng, Y.X.; et al. The protective effects of melatonin on oxidative damage and the immune system of the Chinese mitten crab (Eriocheir sinensis) exposed to deltamethrin. Sci. Total Environ. 2019, 653, 1426–1434. [Google Scholar] [CrossRef]
- Cao, Y.W.; Zhang, L.T.; Yang, Y.; Li, J.Y.; Luan, X.Q.; Xia, X.L.; Gu, W.; Du, J.; Bi, K.R.; Wang, L.; et al. Proteome and gut microbiota analysis of Chinese mitten crab (Eriocheir sinensis) in response to Hepatospora eriocheir infection. Aquaculture 2024, 582, 740572. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, W.H.; Zhou, J.F.; Li, X.C.; Liu, Q. The pathogenic and pathological analysis of “microsporidiasis in the muscle of Exopalaemon carinicauda”. J. Shanghai Ocean. Univ. 2013, 22, 726–733. [Google Scholar]
- Boakye, D.W.; Jaroenlak, P.; Prachumwat, A.; Williams, T.A.; Bateman, K.S.; Itsathitphaisarn, O.; Sritunyalucksana, K.; Paszkiewicz, K.H.; Moore, K.A.; Stentiford, G.D.; et al. Decay of the glycolytic pathway and adaptation to intranuclear parasitism within Enterocytozoonidae microsporidia. Environ. Microbiol. 2017, 19, 2077–2089. [Google Scholar] [CrossRef]
- Ding, Z.F. Lipid metabolism disorders contribute to the pathogenesis of Hepatospora eriocheir in the crab Eriocheir sinensis. J. Fish Dis. 2021, 44, 305–313. [Google Scholar] [CrossRef]
- Huang, Z.S.; Aweya, J.J.; Zhu, C.H.; Tran, N.T.; Hong, Y.J.; Li, S.K.; Yao, D.F.; Zhang, Y.L. Modulation of Crustacean Innate Immune Response by Amino Acids and Their Metabolites: Inferences From Other Species. Front. Immunol. 2020, 11, 574721. [Google Scholar] [CrossRef]
- Chen, S.J.; Huang, L.J.; Liu, B.D.; Duan, H.M.; Li, Z.; Liu, Y.F.; Li, H.; Fu, X.; Lin, J.C.; Xu, Y.L.; et al. Dynamic changes in butyrate levels regulate satellite cell homeostasis by preventing spontaneous activation during aging. Sci. China Life Sci. 2024, 67, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.; Leis, E.M.; Richard, J.C.; Standish, I.F.; Bojko, J.; Weinzinger, J.; Waller, D.L. Hirsutonosema embarrassi n. gen. n. sp. (Phylum Microsporidia) in the Ovary of Mucket (Actinonaias ligamentina), Plain Pocketbook (Lampsilis cardium), and Fatmucket (Lampsilis siliquoidea) (Unionidae) from the Embarrass River, Wisconsin, USA. Parasitologia 2024, 4, 184–198. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Lv, Q.; Liao, H.J.; Xie, Z.K.; Hong, L.Y.; Qi, L.; Pan, G.Q.; Long, M.X.; Zhou, Z.Y. The microsporidian polar tube: Origin, structure, composition, function, and application. Parasit. Vectors 2023, 16, 305. [Google Scholar] [CrossRef]
- Schmidt, S.F.; Rohm, M.; Herzig, S.; Diaz, M.B. Cancer Cachexia: More Than Skeletal Muscle Wasting. Trends Cancer 2018, 4, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chu, L.L.; Du, J.L.; Nie, Z.J.; Cao, L.P.; Gao, J.C.; Xu, G.C. Oxidative stress and immune response of hepatopancreas in Chinese mitten crab Eriocheir sinensis under lipopolysaccharide challenge. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 263, 109495. [Google Scholar] [CrossRef]
- Wei, Y.B.; Pei, S.B.; Huang, Y.R.; Yao, K.; Yu, J.J.; Yue, R.M.; Wu, H.; Xiao, J.; Feng, H. UBE2J1 suppresses interferon signaling by facilitating the ubiquitination and degradation of IRF7. Aquaculture 2025, 595, 741640. [Google Scholar] [CrossRef]
- Han, B.; Weiss Louis, M. Microsporidia: Obligate Intracellular Pathogens Within the Fungal Kingdom. Microbiol. Spectr. 2017, 5, 10-1128. [Google Scholar] [CrossRef]
- Gantt, S.M.; Myung, J.M.; Briones, M.R.; Li, W.D.; Corey, E.J.; Omura, S.; Nussenzweig, V.; Sinnis, P. Proteasome Inhibitors Block Development of Plasmodium spp. Antimicrob. Agents Chemother. 1998, 42, 2731–2738. [Google Scholar] [CrossRef]
- Peuvel, I.; Peyret, P.; Méténier, G.; Vivarès, C.P.; Delbac, F. The microsporidian polar tube: Evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi. Mol. Biochem. Parasitol. 2002, 122, 69–80. [Google Scholar] [CrossRef]

| Index | Control | Diseased |
|---|---|---|
| Total reads | 41,398,079 ± 2,284,177.55 | 41,160,876 ± 3,143,707.62 |
| Host reads | 39,271,391 ± 2,147,092.00 b | 28,848,690 ± 2,279,256.00 a |
| Clean reads | 2,126,688 ± 149,976.30 a | 12,312,187 ± 913,305.30 b |
| ORF number | 8555 ± 1296.61 a | 11,485 ± 273.00 b |
| Sample | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|
| Archaea | ||||||
| Control | 2 | 4 | 4 | 4 | 4 | 5 |
| Diseased | 5 | 7 | 7 | 7 | 7 | 9 |
| Bacteria | ||||||
| Control | 10 | 18 | 30 | 46 | 69 | 86 |
| Diseased | 12 | 18 | 31 | 40 | 48 | 54 |
| Fungi | ||||||
| Control | 8 | 18 | 19 | 35 | 43 | 56 |
| Diseased | 8 | 21 | 22 | 38 | 46 | 60 |
| Virus | ||||||
| Control | 3 | 3 | 3 | 3 | 6 | 12 |
| Diseased | 4 | 4 | 4 | 4 | 5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Liang, Y.; Hao, S.; Wu, K. Integrative High-Throughput Screening and Microscopic Evidence Implicates Microsporidia as a Potential Pathogen of “Pus Crab” in the Mud Crab (Scylla paramamosain). Animals 2025, 15, 3463. https://doi.org/10.3390/ani15233463
Xiao L, Liang Y, Hao S, Wu K. Integrative High-Throughput Screening and Microscopic Evidence Implicates Microsporidia as a Potential Pathogen of “Pus Crab” in the Mud Crab (Scylla paramamosain). Animals. 2025; 15(23):3463. https://doi.org/10.3390/ani15233463
Chicago/Turabian StyleXiao, Lanfei, Yongjun Liang, Shuangli Hao, and Kun Wu. 2025. "Integrative High-Throughput Screening and Microscopic Evidence Implicates Microsporidia as a Potential Pathogen of “Pus Crab” in the Mud Crab (Scylla paramamosain)" Animals 15, no. 23: 3463. https://doi.org/10.3390/ani15233463
APA StyleXiao, L., Liang, Y., Hao, S., & Wu, K. (2025). Integrative High-Throughput Screening and Microscopic Evidence Implicates Microsporidia as a Potential Pathogen of “Pus Crab” in the Mud Crab (Scylla paramamosain). Animals, 15(23), 3463. https://doi.org/10.3390/ani15233463






