Diversity of Water Frogs Pelophylax spp. in Turkey: Do Mating Vocalizations Mirror Nominal Taxon Delimitation? †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations and Sampling
2.2. Call Records and Analyses
2.3. Statistical Procedures
3. Results
3.1. Comparison of Advertisement Calls
3.2. Comparison of Release Calls
3.2.1. Type 1 Calls
3.2.2. Type 2 Calls
4. Discussion
4.1. Validation of Call Identification
4.2. Acoustic Premating Barriers and Taxon Delimitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiorano, L.; Amori, G.; Capula, M.; Falcucci, A.; Masi, M.; Montemaggiori, A.; Pottier, J.; Psomas, A.; Rondinini, C.; Russo, D.; et al. Threats from Climate Change to Terrestrial Vertebrate Hotspots in Europe. PLoS ONE 2013, 8, e74989. [Google Scholar] [CrossRef] [PubMed]
- Karataş, A.; Filiz, H.; Erciyas-Yavuz, K.; Özeren, S.C.; Tok, C.V. The vertebrate biodiversity of Turkey. In Biodiversity, Conservation and Sustainability in Asia: Volume 1: Prospects and Challenges in West Asia and Caucasus; Öztürk, M., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 175–274. [Google Scholar] [CrossRef]
- Eken, G.; Isfendiyaroğlu, S.; Yeniyurt, C.; Erkol, I.L.; Karataş, A.; Ataol, M. Identifying key biodiversity areas in Turkey: A multi-taxon approach. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2016, 12, 181–190. [Google Scholar] [CrossRef]
- Yaşar, Ç.; Çiçek, K.; Mulder, J.; Tok, C. The distribution and biogeography of amphibians and reptiles in Turkey. North-West. J. Zool. 2021, 17, 232–275. [Google Scholar]
- Başkale, E.; Kaya, U. Richness and Distribution of Amphibian Species in Relation to Ecological Variables in Western Aegean Region of Turkey. Ekoloji 2009, 18, 25–31. [Google Scholar] [CrossRef]
- Ambarlı, D.; Zeydanlı, U.S.; Balkız, Ö.; Aslan, S.; Karaçetin, E.; Sözen, M.; Ilgaz, Ç.; Gürsoy Ergen, A.; Lise, Y.; Demirbaş Çağlayan, S.; et al. An overview of biodiversity and conservation status of steppes of the Anatolian Biogeographical Region. Biodivers. Conserv. 2016, 25, 2491–2519. [Google Scholar] [CrossRef]
- Erismis, U.C.; Konuk, M.; Yoldas, T.; Agyar, P.; Yumuk, D.; Korcan, S.E. Survey of Turkey’s endemic amphibians for chytrid fungus Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2014, 111, 153–157. [Google Scholar] [CrossRef]
- Çiçek, K.; Ayaz, D.; Afsar, M.; Bayrakci, Y.; Peksen, C.A.; Cumhuriyet, O.; Ismail, I.B.; Yenmis, M.; Ustundag, E.; Tok, C.V.; et al. Unsustainable harvest of water frogs in southern Turkey for the European market. Oryx 2021, 55, 364–372. [Google Scholar] [CrossRef]
- Başkale, E.; Kaya, U. Decline of the Levantine Frog, Pelophylax bedriagae Camerano, 1882, in the western Aegean Region of Turkey: Changes in population size and implications for conservation (Amphibia: Ranidae). Zool. Middle East 2012, 57, 69–76. [Google Scholar] [CrossRef]
- Bodenheimer, F.S. Introduction into the knowledge of the Amphibia and Reptilia of Turkey. Rev. Fac. Sci. Univ. Istanb. 1944, 9, 1–78. [Google Scholar]
- Basoglu, M.; Özeti, N. Türkiye Amfibileri; Ege Üniversitesi Fen Fakültesi Kitaplar Serisi: Izmir, Turkey, 1973; Volume 50, pp. 1–155. [Google Scholar]
- Schneider, H.; Sinsch, U.; Nevo, E. The lake frogs in Israel represent a new species. Zool. Anz. 1992, 228, 97–106. [Google Scholar]
- Schneider, H.; Sinsch, U. Mating call variation in lake frogs referred to as Rana ridibunda Pallas, 1771. Taxonomic implications. Z. Für Zool. Syst. Und Evol. 1992, 30, 297–315. [Google Scholar] [CrossRef]
- Schneider, H.; Sinsch, U. Taxonomic reassessment of Middle Eastern water frogs: Bioacoustic variation among populations considered as Rana ridibunda, R. bedriagae or R. levantina. J. Zool. Syst. Evol. Res. 1999, 37, 57–66. [Google Scholar] [CrossRef]
- Berger, L.; Uzzell, T.; Hotz, H.J. Postzygotic reproductive isolation between Mendelian species of European water frogs. Zool. Pol. 1994, 39, 209–242. [Google Scholar]
- Bülbül, U.; Matsui, M.; Kutrup, B.; Eto, K. Taxonomic Relationships among Turkish Water Frogs as Revealed by Phylogenetic Analyses using mtDNA Gene Sequences. Zool. Sci. 2011, 12, 930–936. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference; Version 6.1; Electronic Database; American Museum of Natural History: New York, NY, USA, 2023; Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 16 April 2023).
- Sinsch, U.; Schneider, H. Taxonomic reassessment of Middle Eastern water frogs: Morphological variation among populations considered as Rana ridibunda, R. bedriagae or R. levantina. J. Zool. Syst. Evol. Res. 1999, 37, 67–74. [Google Scholar] [CrossRef]
- Arikan, H. On a new form of Rana ridibunda (Anura, Ranidae) from Turkey. Istanb. Üniv. Fen Fak. Biyol. Der. 1988, 53, 81–87. [Google Scholar]
- Atatür, K.M.; Arikan, H.; Mermer, A. A taxonomical investigation on Rana ridibunda Palla (Anura, Ranidae) populations from the Lakes District-Anatolia. Istanb. Üniversitesi Fen. Fakültesi Biyol. Derg. 1990, 54, 79–83. [Google Scholar]
- Alpagut, N.; Falakali, B. Karyotype analysis of two Rana ridibunda (Ranidae: Anura) populations in Turkey. Isr. J. Zool. 1995, 41, 523–531. [Google Scholar]
- Budak, A.; Tok, C.V.; Ayaz, D. On specimens of Rana ridibunda Pallas, 1771 (Anura: Ranidae) collected from Isikli Lake (Civril-Denizli). Turk. J. Zool. 2000, 24, 135–137. [Google Scholar]
- Kaya, U.; Cevik, E.; Erismis, U.C. New distributional records for Rana bedriagae caralitana in Anatolia. Turk. J. Zool. 2002, 26, 381–383. [Google Scholar]
- Tosunoğlu, M.; Ayaz, D.; Göçmen, B. On Specimens of Rana ridibunda Pallas, 1771 (Anura: Ranidae) Collected from Yağmapınar (Karapinar-Konya). Anadolu Üniversitesi Bilim Ve Teknol. Derg. 2005, 6, 55–59. [Google Scholar]
- Ayaz, D.; Tok, C.V.; Mermer, A.; Tosunoğlu, M.; Afsar, M.; Çiçek, K. A New Locality for Rana ridibunda caralitana Arıkan, 1988 (Anura: Ranidae) in the Central Anatolia. Su Ürünleri Derg. 2006, 23, 181–183. [Google Scholar]
- Akin, C.; Bilgin, M.; Bilgin, C.C. Discordance between ventral colour and mtDNA haplotype in the water frog Rana (ridibunda) caralitana, 1988 Arikan. Amphib.-Reptil. 2010, 31, 9–20. [Google Scholar] [CrossRef]
- Akin, C.; Bilgin, C.C.; Beerli, P.; Westaway, R.; Ohst, T.; Litvinchuk, S.N.; Uzzell, T.; Bilgin, M.; Hotz, H.; Guex, G.-D.; et al. Phylogeographic patterns of genetic diversity in eastern Mediterranean water frogs were determined by geological processes and climate change in the Late Cenozoic. J. Biogeogr. 2010, 37, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Jdeidi, T.; Bilgin, C.C.; Kence, M. New localities extend the range of Rana bedriagae caralitana Arikan, 1988 (Anura: Ranidae) further west and suggest specific status. Turk. J. Zool. 2001, 25, 153–158. [Google Scholar]
- Plötner, J.; Ohst, T.; Böhme, W.; Schreiber, R. Divergence in mitochondrial DNA of Near Eastern water frogs with special reference to the systematic status of Cypriote and Anatolian populations (Anura, Ranidae). Amphib.-Reptil. 2001, 22, 397–412. [Google Scholar] [CrossRef]
- Alpagut-Keskin, N.; Falakali-Mutaf, B. Rod-Shaped Bivalents indicate new assemblage among Anatolian Water Frog Populations. Amphib.-Reptil. 2006, 27, 47–53. [Google Scholar]
- Jdeidi, T.; Bilgin, C.C.; Kence, M. Morphometric and bioacoustic studies in the Water Frog (Rana ridibunda) complex in Turkey. In Proceedings of the Sixth International Congress of Vertebrate Morphology, Jena, Germany, 21–26 July 2001; pp. 245–246. [Google Scholar]
- Schneider, H.; Sinsch, U.; Sofianidou, T. The water frogs of Greece: Bioacoustic evidence for a new species. Z. Für Zool. Syst. Und Evol. 1993, 31, 47–63. [Google Scholar] [CrossRef]
- Twomey, E.; Mayer, M.; Summers, K. Intraspecific Call Variation in the Mimic Poison Frog Ranitomeya imitator. Herpetologica 2015, 71, 252–259. [Google Scholar] [CrossRef]
- Stewart, K.A.; Austin, J.D.; Zamudio, K.R.; Lougheed, S.C. Contact zone dynamics during early stages of speciation in a chorus frog (Pseudacris crucifer). Heredity 2016, 116, 239–247. [Google Scholar] [CrossRef]
- Weaver, S.J.; Callaghan, C.T.; Rowley, J.J.L. Anuran accents: Continental-scale citizen science data reveal spatial and temporal patterns of call variability. Ecol. Evol. 2020, 10, 12115–12128. [Google Scholar] [CrossRef]
- Leary, C.J. Comparison between Release Vocalizations Emitted during Artificial and Conspecific Amplexus in Bufo americanus. Copeia 1999, 1999, 506–508. [Google Scholar] [CrossRef]
- Di Tada, I.E.; Martino, A.; Sinsch, U. Release vocalizations in neotropical toads (Bufo): Ecological constraints and phylogenetic implications. J. Zool. Syst. Evol. Res. 2001, 39, 13–23. [Google Scholar] [CrossRef]
- Di Tada, I.E.; Sinsch, U. Geographical variation of advertisement and release calls in toads referred to as Rhinella diptycha (Anura: Bufonidae). Salamandra 2023, 59, 96–101. [Google Scholar]
- Köhler, J.; Jansen, M.; Rodríguez, A.; Kok, P.J.R.; Toledo, L.F.; Emmrich, M.; Glaw, F.; Haddad, C.F.B.; Rödel, M.-O.; Vences, M. The use of bioacoustics in anuran taxonomy: Theory, terminology, methods and recommendations for best practice. Zootaxa 2017, 4251, 124. [Google Scholar] [CrossRef]
- Schneider, H.; Egiasarjan, E. The structure of the calls of lake frogs (Rana ridibunda: Amphibia) in the Terra Typica Restricta. Zool. Anz. 1991, 227, 121–135. [Google Scholar]
- Schneider, H. Calls and reproductive behaviour of the water frogs of Damascus, Syria (Amphibia: Anura: Rana bedriagae Camerano, 1882). Zool. Middle East 1997, 15, 51–66. [Google Scholar] [CrossRef]
- Schneider, H. Calls of the Levantine frog, Rana bedriagae, at Birket Ata, Israel (Amphibia: Anura). Zool. Middle East 1999, 19, 101–116. [Google Scholar] [CrossRef]
- Pesarakloo, A.; Najibzadeh, M.; Rastegar-Pouyani, N.; Rastegar-Pouyani, E. Taxonomic survey of water frog populations of Pelophylax bedriagae (Anura: Ranidae) in western Iran: A morphometric and bioacoustic approach. Biologia 2018, 73, 673–681. [Google Scholar] [CrossRef]
- Schneider, H.; Brzoska, J. Die Befreiungsrufe der mitteleuropäischen Wasserfrösche. Zool. Anz. 1981, 206, 189–202. [Google Scholar]
- Zamfirescu, Ş. Comparison between water frogs (Rana esculenta complex) release calls. An. Ştiinţifice Ale Univ. “Alexandru Ioan Cuza” Din Iaşi S. Biol. Anim. 2002, 48, 182–194. [Google Scholar]
- Chernyshov, K. Variability of advertisement calls and release calls of green frogs in the Moscow oblast, Russia. In Proceedings of the Herpetologia Petropolitana–12th Ordinary General Meeting of the Societas Europaea Herpetologica, Saint-Petersburg, Russia, 12–16 August 2003; pp. 20–26. [Google Scholar]
- Fedorova, A.; Shabanov, D. Differences in release calls of the hybrid water frog Pelophylax esculentus and its parental species Pelophylax ridibundus (Anura: Ranidae) in Ukraine. Biologia 2023, 78, 497–504. [Google Scholar] [CrossRef]
- Wahl, M. Untersuchungen zur Bio-Akustik des Wasserfrosches Rana esculenta (L.). Oecologia 1969, 3, 14–55. [Google Scholar] [CrossRef] [PubMed]
- Burbrink, F.T.; Crother, B.I.; Murray, C.M.; Smith, B.T.; Ruane, S.; Myers, E.A.; Pyron, R.A. Empirical and philosophical problems with the subspecies rank. Ecol. Evol. 2022, 12, e9069. [Google Scholar] [CrossRef]
- Kıraç, A.; Gidiş, M.; Mert, A.; Başkale, E. Climate change and the fate of endemic Beysehir Frog, Pelophylax caralitanus. Amphib. Reptile Conserv. 2022, 16, 76–85. [Google Scholar]
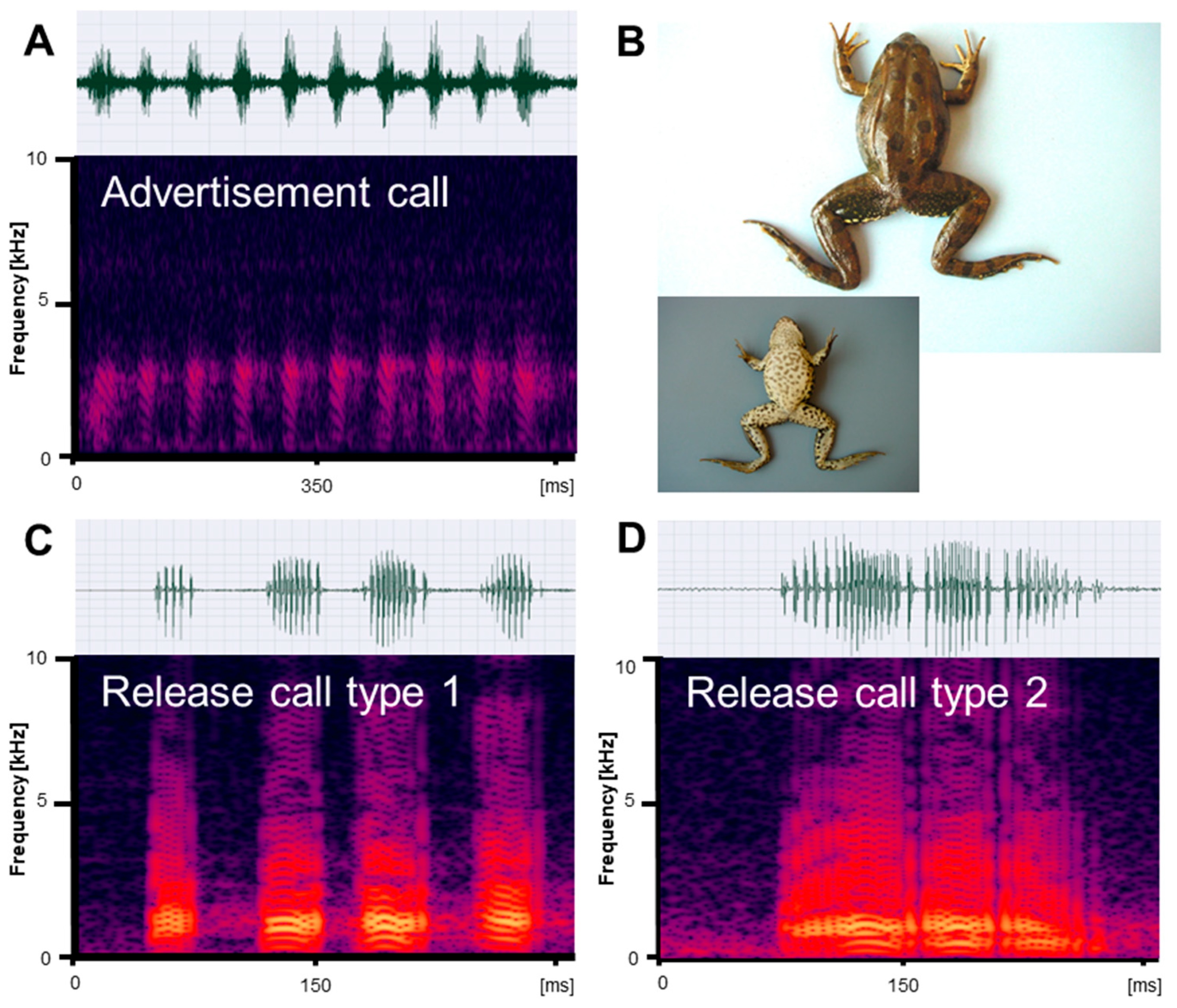


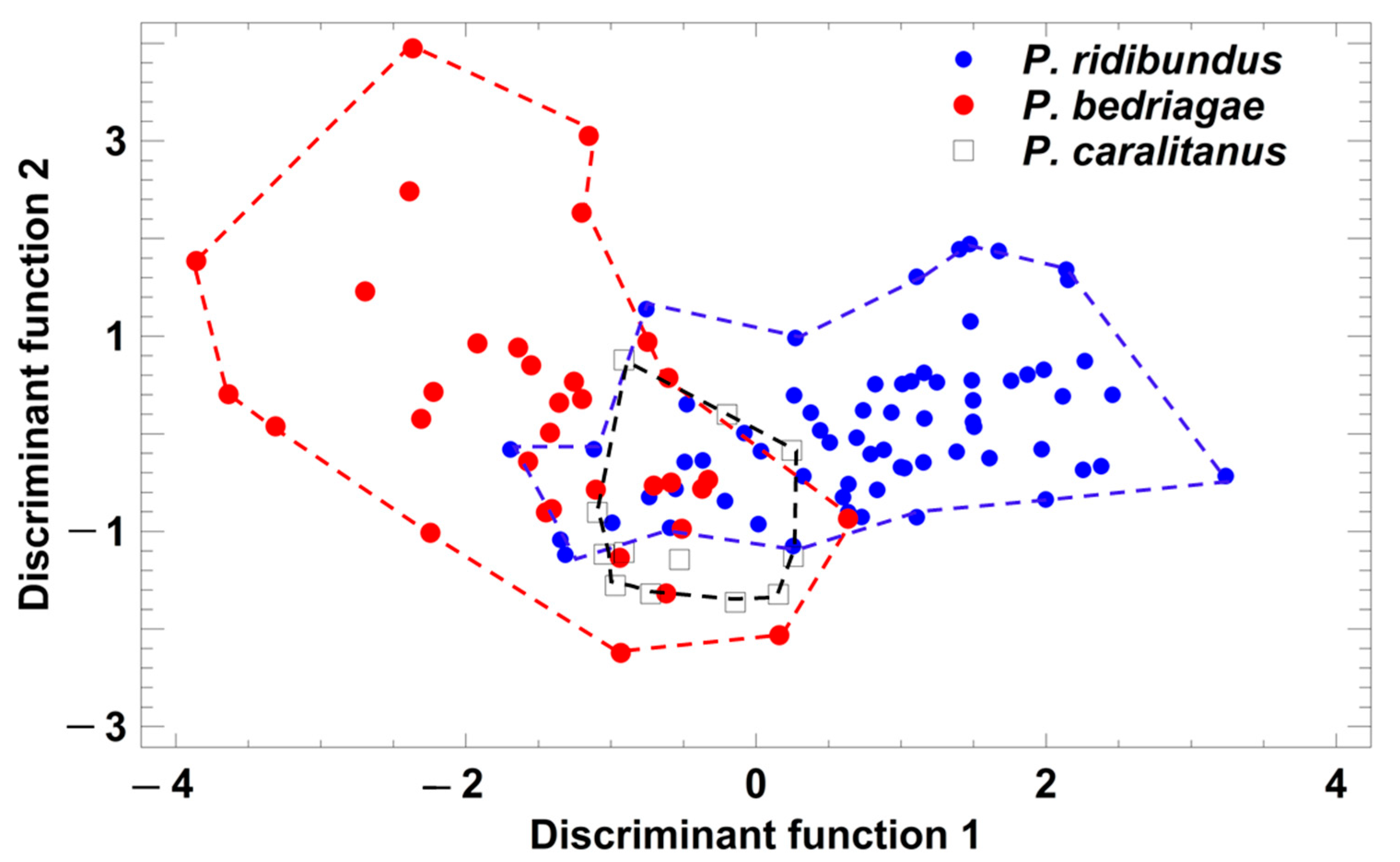
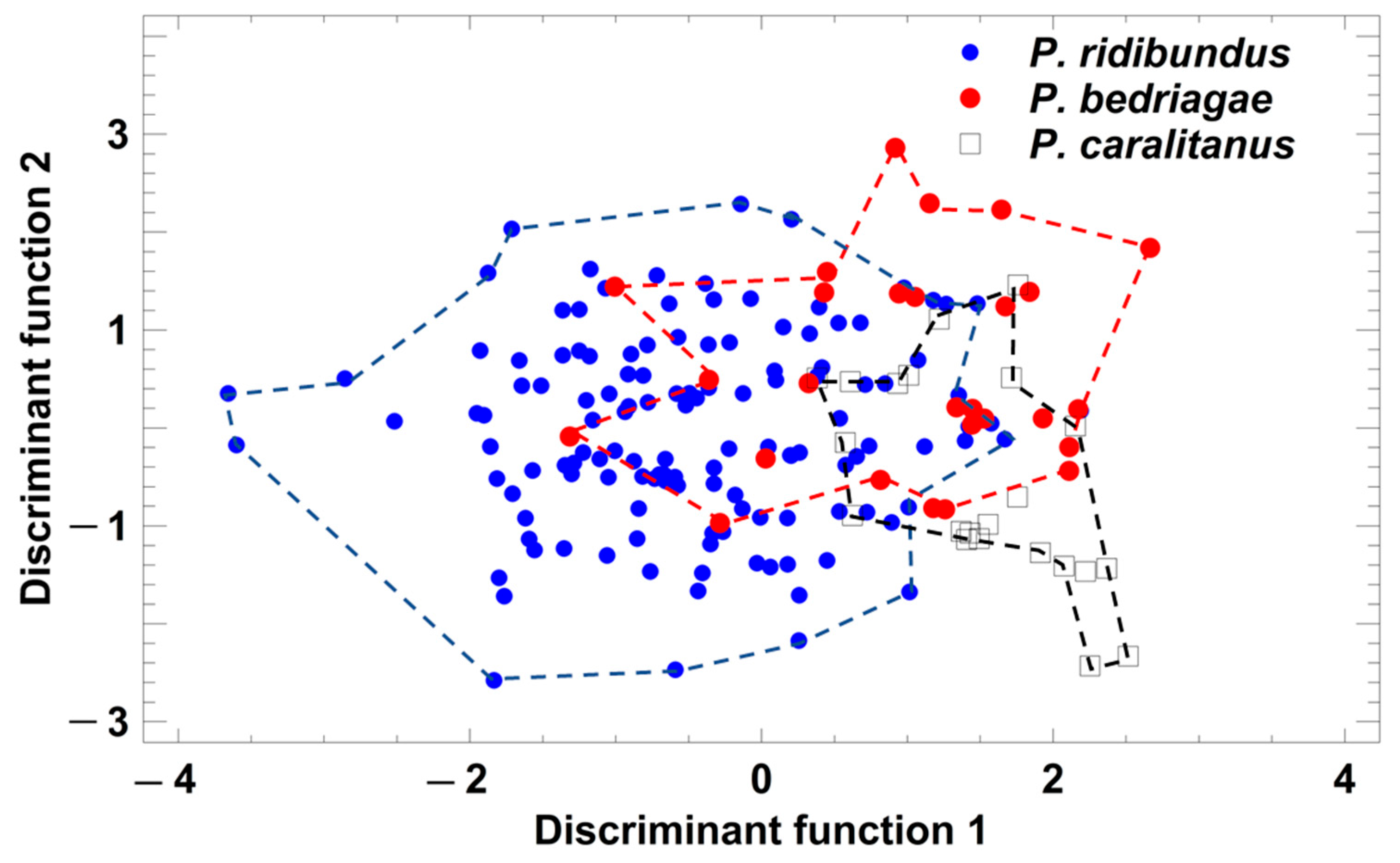

| Call Parameter | P. ridibundus | P. bedriagae | P. caralitanus | Significance |
|---|---|---|---|---|
| Call duration [ms] | 609 ± 17 | 625 ± 24 | 680 ± 41 | p = 0.2734 |
| Pulse groups per call [N] | 7.0 ± 0.2 a | 8.1 ± 0.3 b | 8.5 ± 0.4 b | p = 0.0003 |
| Pulse group duration [ms] | 58.8 ± 0.9 a | 44.1 ± 1.2 c | 53.0 ± 2.1 b | p < 0.0001 |
| Pulse group interval [ms] | 38.6 ± 1.1 a | 45.1 ± 1.1 b | 41.2 ± 1.7 b | p = 0.0070 |
| Pulse group repetition rate [N/s] | 11.6 ± 0.2 a | 13.1 ± 0.3 b | 12.8 ± 2.7 b | p = 0.0002 |
| Pulses per pulse group [N] | 20.1 ± 0.3 a | 12.2 ± 0.5 b | 13.0 ± 0.8 b | p < 0.0001 |
| Pulse repetition rate per pulse group [N/s] | 343 ± 7 a | 287 ± 9 b | 244 ± 16 c | p < 0.0001 |
| Dominant frequency [Hz] | 2209 ± 51 | 2183 ± 61 | 1983 ± 92 | p = 0.4632 |
| Call Parameter | P. ridibundus | P. bedriagae | P. caralitanus | Significance |
|---|---|---|---|---|
| Call duration [ms] | 248 ± 7 | 254 ± 16 | 213 ± 19 | p = 0.5727 |
| Pulse groups per call [N] | 3.8 ± 0.1 a | 4.4 ± 0.2 b | 3.2 ± 0.2 a | p = 0.0005 |
| Pulse group duration [ms] | 45.3 ± 1.0 b | 40.2 ± 2.2 a | 46.8 ± 2.8 b | p = 0.0148 |
| Pulse group interval [ms] | 25.9 ± 0.9 a | 21.6 ± 1.9 a | 28.7 ± 2.5 b | p = 0.0041 |
| Pulse group repetition rate [N/s] | 16.9 ± 0.4 | 18.6 ± 0.9 | 11.4 ± 1.1 | p = 0.0746 |
| Pulses per call [N] | 7.5 ± 0.1 | 6.8 ± 0.3 | 7.4 ± 0.4 | p = 0.0504 |
| Pulse repetition rate per pulse group [N/s] | 183.5 ± 3.0 b | 178.0 ± 6.7 b | 145.8 ± 8.5 a | p = 0.0185 |
| Dominant frequency [Hz] | 895 ± 10 a | 978 ± 21 b | 998 ± 27 b | p < 0.0001 |
| Call Parameter | P. ridibundus | P. bedriagae | P. caralitanus | Significance |
|---|---|---|---|---|
| Call duration [ms] | 214 ± 12 a | 247 ± 5 b | 243 ± 3 b | p = 0.0040 |
| Pulse groups per call [N] | 1 | 1 | 1 | - |
| Pulses per call [N] | 23.6 ± 1.3 b | 13.0 ± 0.5 a | 19.0 ± 0.3 b | p < 0.0001 |
| Pulse repetition rate [N/s] | 120.0 ± 5.1 c | 52.7 ± 1.9 a | 82.7 ± 1.2 b | p < 0.0001 |
| Dominant frequency [Hz] | 867 ± 32 a | 842 ± 12 a | 742 ± 2 b | p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinsch, U.; Werding, S.; Kaya, U. Diversity of Water Frogs Pelophylax spp. in Turkey: Do Mating Vocalizations Mirror Nominal Taxon Delimitation? Animals 2023, 13, 1725. https://doi.org/10.3390/ani13111725
Sinsch U, Werding S, Kaya U. Diversity of Water Frogs Pelophylax spp. in Turkey: Do Mating Vocalizations Mirror Nominal Taxon Delimitation? Animals. 2023; 13(11):1725. https://doi.org/10.3390/ani13111725
Chicago/Turabian StyleSinsch, Ulrich, Stefan Werding, and Uğur Kaya. 2023. "Diversity of Water Frogs Pelophylax spp. in Turkey: Do Mating Vocalizations Mirror Nominal Taxon Delimitation?" Animals 13, no. 11: 1725. https://doi.org/10.3390/ani13111725





