Pelt Biting as a Practical Indicator of Social and Environment Stress in Farmed Red Deer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hypotheses
2.2. Pelt Biting
2.3. Data and Animals
2.4. Hierarchy Rank
2.5. Heat Stress Index
2.6. Statistical Analysis
3. Results
3.1. Pelt Biting Description
3.2. Hypotheses Testing
4. Discussion
Hierarchical Rank, Age, Body Weight, Date of Birth and Heat Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowell, T.E. The Concept of Social Dominance. Behav. Biol. 1974, 11, 131–154. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Albon, S.D.; Guinness, F.E. Maternal Dominance, Breeding Success and Birth Sex-Ratios in Red Deer. Nature 1984, 308, 358–360. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Albon, S.D.; Guinness, F.E. Great Expectations: Dominance, Breeding Success and Offspring Sex Ratios in Red Deer. Anim. Behav. 1986, 34, 460–471. [Google Scholar] [CrossRef]
- Barrette, C.; Vandal, D. Social Rank, Dominance, Antler Size, and Access to Food in Snow-Bound Wild Woodland Caribou. Behaviour 1986, 97, 118–146. [Google Scholar] [CrossRef] [Green Version]
- Bon, R.; Badia, J.; Maublanc, M.L.; Recarte, J.M. Social Grouping Dynamics of Mouflon (Ovis Ammon) During Rut. Z. Saugetierkd. Int. J. Mamm. Biol. 1993, 58, 294–301. [Google Scholar]
- Cote, S.D. Dominance Hierarchies in Female Mountain Goats: Stability, Aggressiveness and Determinants of Rank. Behaviour 2000, 137, 1541–1566. [Google Scholar] [CrossRef]
- Murch, P.; Tovey, C.; Chase, I. Two’s Company, Three’s a Crowd: Differences in Dominance Relationships in Isolated Versus Socially Embedded Pairs of Fish. Behaviour 2003, 140, 1193–1217. [Google Scholar] [CrossRef] [Green Version]
- Huntingford, F.A. Animal Conflict; Chapman & Hall Animal Behaviour Series; Springer: New York, NY, USA, 1987; ISBN 978-94-010-9008-7. [Google Scholar]
- Houpt, K.A.; Law, K.; Martinisi, V. Dominance Hierarchies in Domestic Horses. Appl. Anim. Ethol. 1978, 4, 273–283. [Google Scholar] [CrossRef]
- Leonard, F.C.; O’Connell, J.; O’Farrell, K. Effect of Different Housing Conditions on Behaviour and Foot Lesions in Friesian Heifers. Vet. Rec. 1994, 134, 490–494. [Google Scholar] [CrossRef]
- Young, A.L.; Richard, A.F.; Aiello, L.C. Female Dominance and Maternal Investment in Strepsirhine Primates. Am. Nat. 1990, 135, 473–488. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H. The Functions of Antlers. Behaviour 1982, 79, 108–124. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J.; Garcia, A.J.; Cappelli, J.; Landete-Castillejos, T.; Serrano, M.P.; Gallego, L. Heat Stress Reduces Growth Rate of Red Deer Calf: Climate Warming Implications. PLoS ONE 2020, 15, e0233809. [Google Scholar] [CrossRef]
- Pavitt, A.T.; Walling, C.A.; Möstl, E.; Pemberton, J.M.; Kruuk, L.E.B. Cortisol but Not Testosterone Is Repeatable and Varies with Reproductive Effort in Wild Red Deer Stags. Gen. Comp. Endocrinol. 2015, 222, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013; pp. 1–1535. [Google Scholar]
- Spiers, D.E.; Spain, J.N.; Sampson, J.D.; Rhoads, R.P. Use of Physiological Parameters to Predict Milk Yield and Feed Intake in Heat-Stressed Dairy Cows. J. Therm. Biol. 2004, 29, 759–764. [Google Scholar] [CrossRef]
- Fernandes, J.N.; Hemsworth, P.H.; Coleman, G.J.; Tilbrook, A.J. Costs and Benefits of Improving Farm Animal Welfare. Agriculture 2021, 11, 104. [Google Scholar] [CrossRef]
- Peden, R.S.E.; Turner, S.P.; Camerlink, I.; Akaichi, F. An Estimation of the Financial Consequences of Reducing Pig Aggression. PLoS ONE 2021, 16, e0250556. [Google Scholar] [CrossRef]
- Ceacero, F.; Landete-Castillejos, T.; García, A.J.; Estévez, J.A.; Gallego, L. Kinship Discrimination and Effects on Social Rank and Aggressiveness Levels in Iberian Red Deer Hinds. Ethology 2007, 113, 1133–1140. [Google Scholar] [CrossRef]
- Hall, M.J. Social Organisation in an Enclosed Group of Red Deer (Cervus elaphus L.) on Rhum. Z. Für Tierpsychol. 1983, 61, 273–292. [Google Scholar] [CrossRef]
- Hollingdale, E.; Pérez-Barbería, F.J.; Walker, D.M. Inferring Symmetric and Asymmetric Interactions between Animals and Groups from Positional Data. PLoS ONE 2018, 13, e0208202. [Google Scholar] [CrossRef]
- Hansen, B.B.; Aanes, R.; Sæther, B.-E. Feeding-Crater Selection by High-Arctic Reindeer Facing Ice-Blocked Pastures. Can. J. Zool. 2010, 88, 170–177. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour. An Introductory Guide, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Walker, D.M.; Carmeli, C.; Pérez-Barbería, F.J.; Small, M.; Pérez-Fernández, E. Inferring Networks from Multivariate Symbolic Time Series to Unravel Behavioural Interactions among Animals. Anim. Behav. 2010, 79, 351–359. [Google Scholar] [CrossRef]
- Volodin, I.A.; Sibiryakova, O.V.; Vasilieva, N.A.; Volodina, E.V.; Matrosova, V.A.; Garcia, A.J.; Pérez-Barbería, F.J.; Gallego, L.; Landete-Castillejos, T. Old and Young Female Voices: Effects of Body Weight, Condition and Social Discomfort on the Vocal Aging in Red Deer Hinds (Cervus Elaphus). Behaviour 2018, 155, 915–939. [Google Scholar] [CrossRef] [Green Version]
- Carranza, J.; Pérez-Barbería, J.; Mateos, C.; Alarcos, S.; Torres-Porras, J.; Pérez-González, J.; Sánchez-Prieto, C.B.; Valencia, J.; Castillo, L.; de la Peña, E.; et al. Social Environment Modulates Investment in Sex Trait versus Lifespan: Red Deer Produce Bigger Antlers When Facing More Rivalry. Sci. Rep. 2020, 10, 9234. [Google Scholar] [CrossRef]
- Sackett, G.; Holm, R.; Davis, A.; Fahrenbuch, C. Prematurity and Low Birth Weight in Pigtail Macaques: Incidence, Prediction and Effects of Infant Development; Japan Science Press: Nagoya, Japan, 1975. [Google Scholar]
- Resko, J.A. Androgen Secretion by the Fetal and Neonatal Rhesus Monkey. Endocrinology 1970, 87, 680–687. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Schmid, V.S.; de Vries, H. Finding a Dominance Order Most Consistent with a Linear Hierarchy: An Improved Algorithm for the I&SI Method. Anim. Behav. 2013, 86, 1097–1105. [Google Scholar] [CrossRef]
- Davis, S.; Mader, T. Adjustments for Wind Speed and Solar Radiation to the Temperature-Humidity Index. Neb. Beef Cattle Rep. 2003, 224, 49–51. [Google Scholar]
- Mader, T.; Davis, M.S.; Brown-Brandl, T. Environmental Factors Influencing Heat Stress in Feedlot Cattle. Fac. Pap. Publ. Anim. Sci. Univ. Neb. Linc. 2006, 84, 712–719. [Google Scholar]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, v. 3.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Murtaugh, P.A. In Defense of p Values. Ecology 2014, 95, 611–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsova, A.; Brockhoff, B.; Christensen, R.H.B. LmerTest: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Baayen, R.H.; Davidson, D.J.; Bates, D.M. Mixed-Effects Modeling with Crossed Random Effects for Subjects and Items. J. Mem. Lang. 2008, 59, 390–412. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Schielzeth, H. A General and Simple Method for Obtaining R2 from Generalized Linear Mixed-Effects Models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Wickham, H. Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bouissou, M.F.; Boissy, A.; Neindre, P.L.; Veissier, I. The social behaviour of cattle. In Social Behaviour in Farm Animals; Keeling, L.J., Gonyou, H.W., Eds.; CABI: Wallingford, UK, 2001; pp. 113–145. ISBN 978-0-85199-397-3. [Google Scholar]
- Hurnik, J.F. Dictionary of Farm Animal Behavior, 2nd ed.; Iowa State University Press: Ames, IA, USA, 1995; ISBN 978-0-8138-2464-2. [Google Scholar]
- Rousing, T.; Wemelsfelder, F. Qualitative Assessment of Social Behaviour of Dairy Cows Housed in Loose Housing Systems. Appl. Anim. Behav. Sci. 2006, 101, 40–53. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J.; Small, M.; Hooper, R.J.; Aldezabal, A.; Soriguer-Escofet, R.; Bakken, G.S.; Gordon, I.J. State-Space Modelling of the Drivers of Movement Behaviour in Sympatric Species. PLoS ONE 2015, 10, e0142707. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Barbería, F.J.; Robertson, E.; Soriguer, R.; Aldezabal, A.; Mendizabal, M.; Perez-Fernandez, E. Why Do Polygynous Ungulates Segregate in Space? Testing the Activity-Budget Hypothesis in Soay Sheep. Ecol. Monogr. 2007, 77, 631–647. [Google Scholar] [CrossRef] [Green Version]
- Miranda-de la Lama, G.C.; Sepúlveda, W.S.; Montaldo, H.H.; María, G.A.; Galindo, F. Social Strategies Associated with Identity Profiles in Dairy Goats. Appl. Anim. Behav. Sci. 2011, 134, 48–55. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J.; Carranza, J.; Sánchez-Prieto, C. Wear Fast, Die Young: More Worn Teeth and Shorter Lives in Iberian Compared to Scottish Red Deer. PLoS ONE 2015, 10, e0134788. [Google Scholar] [CrossRef]
- Senft, R.L.; Coughenour, M.B.; Bailey, D.W.; Rittenhouse, L.R.; Sala, O.E.; Swift, D.M. Large Herbivore Foraging and Ecological Hierarchies. Bioscience 1987, 37, 789. [Google Scholar] [CrossRef]
- Schaefer, J.A.; Messier, F. Winter Foraging by Muskoxen—A Hierarchical Approach to Patch Residence Time and Cratering Behavior. Oecologia 1995, 104, 39–44. [Google Scholar] [CrossRef]
- Appleby, M.C. The Consequences and Causes of High Social Rank in Red Deer Stags. Behaviour 1982, 80, 259–273. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Guinness, F.E.; Albon, S.D. Red Deer: Behaviour and Ecology of Two Sexes; University of Chicago Press: Chicago, IL, USA, 1982. [Google Scholar]
- Thouless, C.R.; Guinness, F.E. Conflict Between Red Deer Hinds—The Winner Always Wins. Anim. Behav. 1986, 34, 1166–1171. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J.; Yearsley, J.M. Sexual Selection for Fighting Skills as a Driver of Sexual Segregation in Polygynous Ungulates: An Evolutionary Model. Anim. Behav. 2010, 80, 745–755. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Mattiello, S. The Importance of Social Behaviour for Goat Welfare in Livestock Farming. Small Rumin. Res. 2010, 90, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Thouless, C.R. Feeding Competition between Grazing Red Deer Hinds. Anim. Behav. 1990, 40, 105–111. [Google Scholar] [CrossRef]
- Bartoŝ, L. Dominance and Aggression in Various Sized Groups of Red Deer Stags. Aggress. Behav. 1986, 12, 175–182. [Google Scholar] [CrossRef]
- Fisher, R.A. The Genetical Theory of Natural Selection; Clarendon Press: Oxford, UK, 1930. [Google Scholar]
- Mulley, R.C. CHAPTER 129—Reproductive Management of Fallow Deer. In Current Therapy in Large Animal Theriogenology, 2nd ed.; Youngquist, R.S., Threlfall, W.R., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2007; pp. 952–965. ISBN 978-0-7216-9323-1. [Google Scholar]
- Couchman, R.C. Deer farming in Australia. In Animal Production in Australia; Livestock Library: Joondalup, Australia, 1980; pp. 196–208. [Google Scholar]
- Hirotani, A. Social-Organization of Reindeer (Rangifer Tarandus), with Special Reference to Relationships among Females. Can. J. Zool. Rev. Can. Zool. 1990, 68, 743–749. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J.; Arroyo-González, I.; García, A.J.; Serrano, M.P.; Gallego, L.; Landete-Castillejos, T. Water Sprinkling as a Tool for Heat Abatement in Farmed Iberian Red Deer: Effects on Calf Growth and Behaviour. PLoS ONE 2021, 16, e0249540. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.L.; Moir, C.E. A Note on the Effect of Birth Date on the Performance of Suckled Red Deer Calves and Their Dams on Low-Ground Pasture. Anim. Sci. 1987, 44, 330–332. [Google Scholar] [CrossRef]
- Aikens, E.O.; Dwinnell, S.P.H.; LaSharr, T.N.; Jakopak, R.P.; Fralick, G.L.; Randall, J.; Kaiser, R.; Thonhoff, M.; Kauffman, M.J.; Monteith, K.L. Migration Distance and Maternal Resource Allocation Determine Timing of Birth in a Large Herbivore. Ecology 2021, 102, e03334. [Google Scholar] [CrossRef]
- Braza, F.; Sanjose, C.; Blom, A. Birth Measurements, Parturition Dates, and Progeny Sex-Ratio of Dama- Dama in Donana, Spain. J. Mammal. 1988, 69, 607–610. [Google Scholar] [CrossRef]
- Landete-Castillejos, T.; García, A.; Gomez, J.A.; Berruga, M.I.; Gallego, L. Effects of Birth Date and Order in Lactation Performance of Iberian Red Deer (Cervus Elaphus Hispanicus). J. Dairy Sci. 2005, 88, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Landete-Castillejos, T.; Garcia, A.; Gallego, L. Calf Growth in Captive Iberian Red Deer (Cervus Elaphus Hispanicus): Effects of Birth Date and Hind Milk Production and Composition. J. Anim. Sci. 2001, 79, 1085–1092. [Google Scholar] [CrossRef]
- Carrión, D.; García, A.J.; Gaspar-López, E.; Landete-Castillejos, T.; Gallego, L. Development of Body Condition in Hinds of Iberian Red Deer during Gestation and Its Effects on Calf Birth Weight and Milk Production. J. Exp. Zool. Part. Ecol. Genet. Physiol. 2008, 309, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Appleby, M.C. Social Rank and Food Access in Red Deer Stags. Behaviour 1980, 74, 294–309. [Google Scholar] [CrossRef]
- Maertens, W.; Vangeyte, J.; Baert, J.; Jantuan, A.; Mertens, K.; Campeneere, S.D.; Pluk, A.; Opsomer, G.; Weyenberg, S.; Nuffel, A.V. Development of a Real Time Cow Gait Tracking and Analysing Tool to Assess Lameness Using a Pressure Sensitive Walkway: The GAITWISE System. Biosyst. Eng. 2011, 110, 29–39. [Google Scholar] [CrossRef]
- Nilsson, M.; Herlin, A.H.; Ardö, H.; Guzhva, O.; Åström, K.; Bergsten, C. Development of Automatic Surveillance of Animal Behaviour and Welfare Using Image Analysis and Machine Learned Segmentation Technique. Animal 2015, 9, 1859–1865. [Google Scholar] [CrossRef] [Green Version]
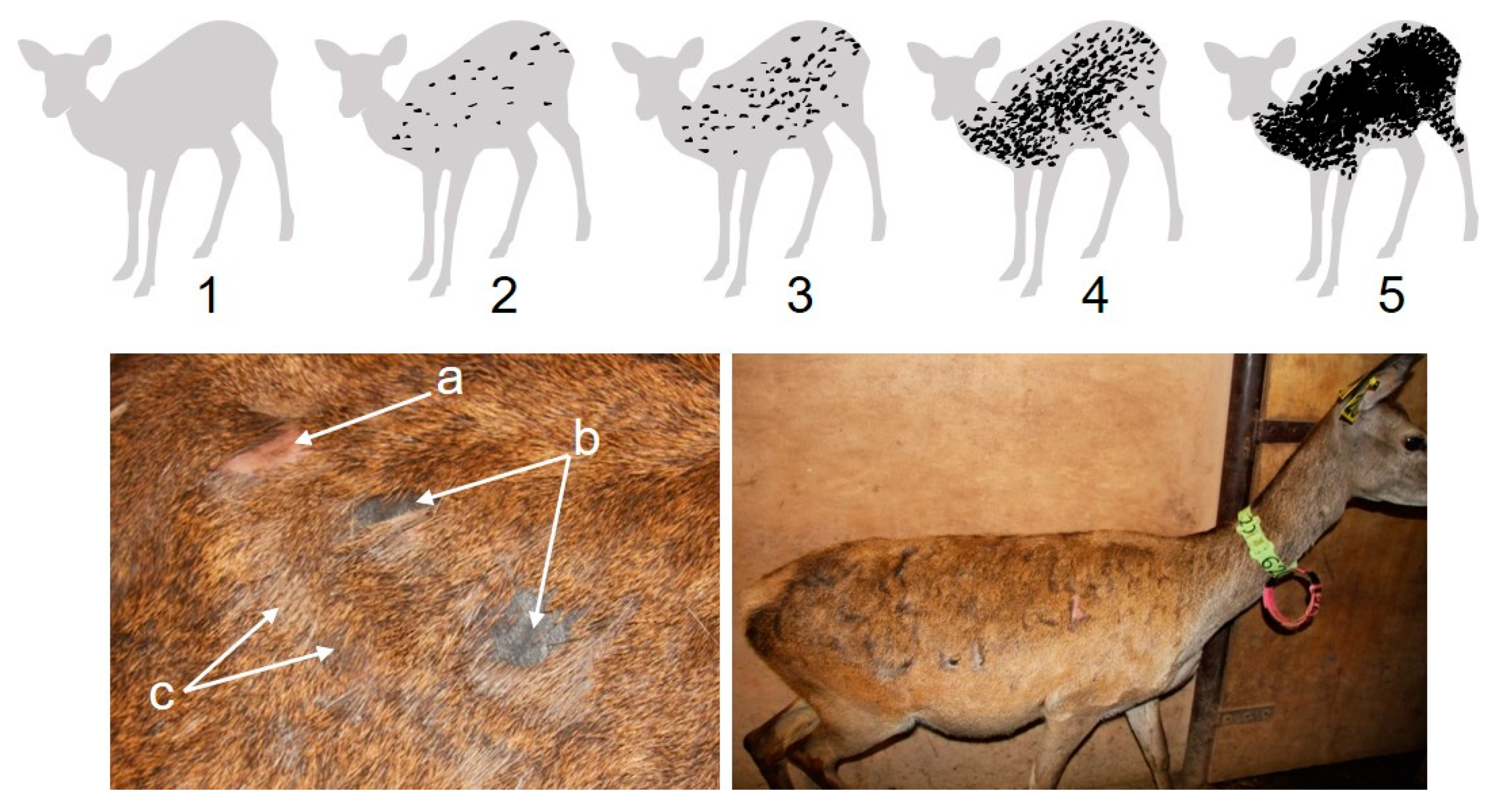
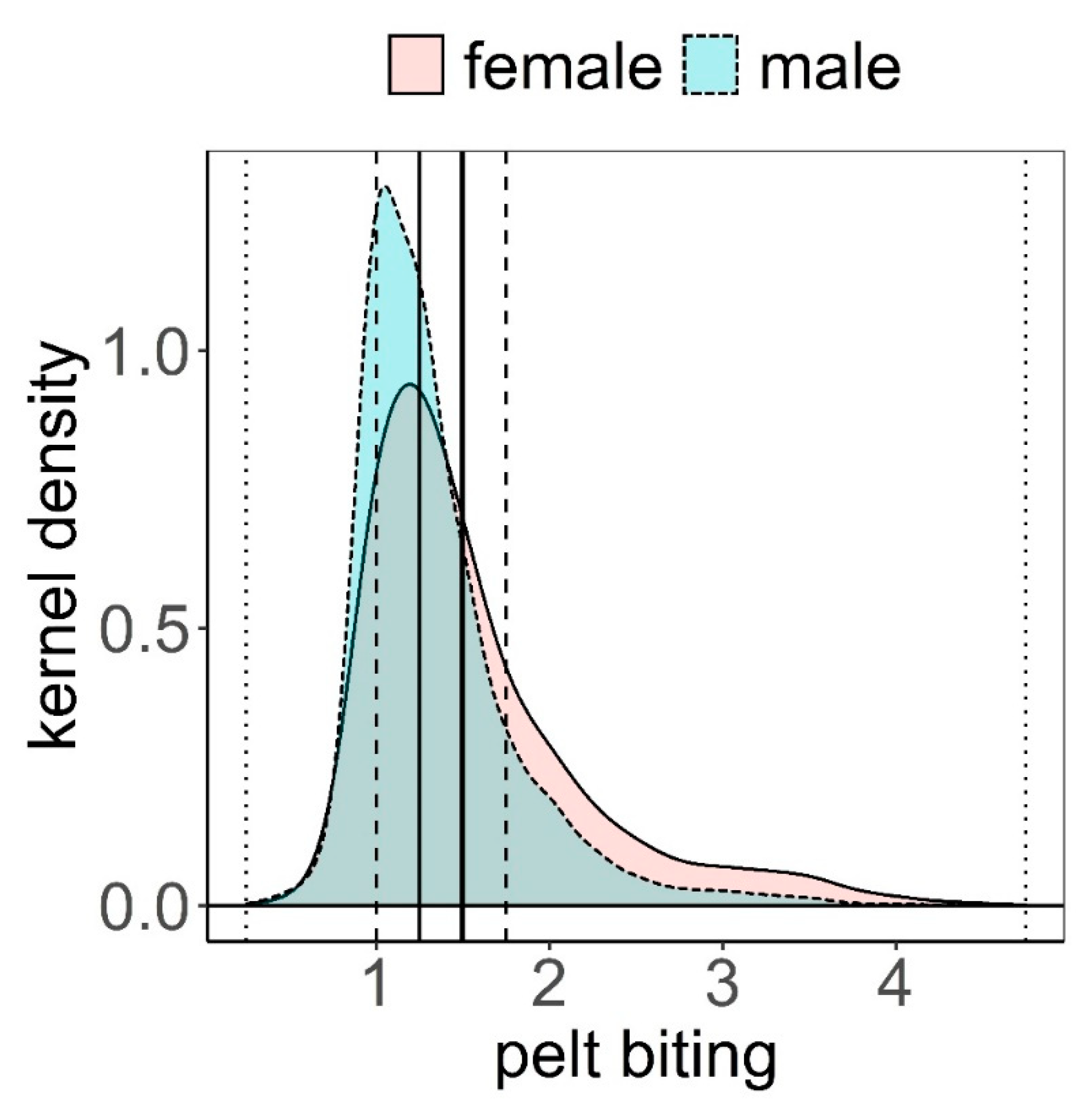
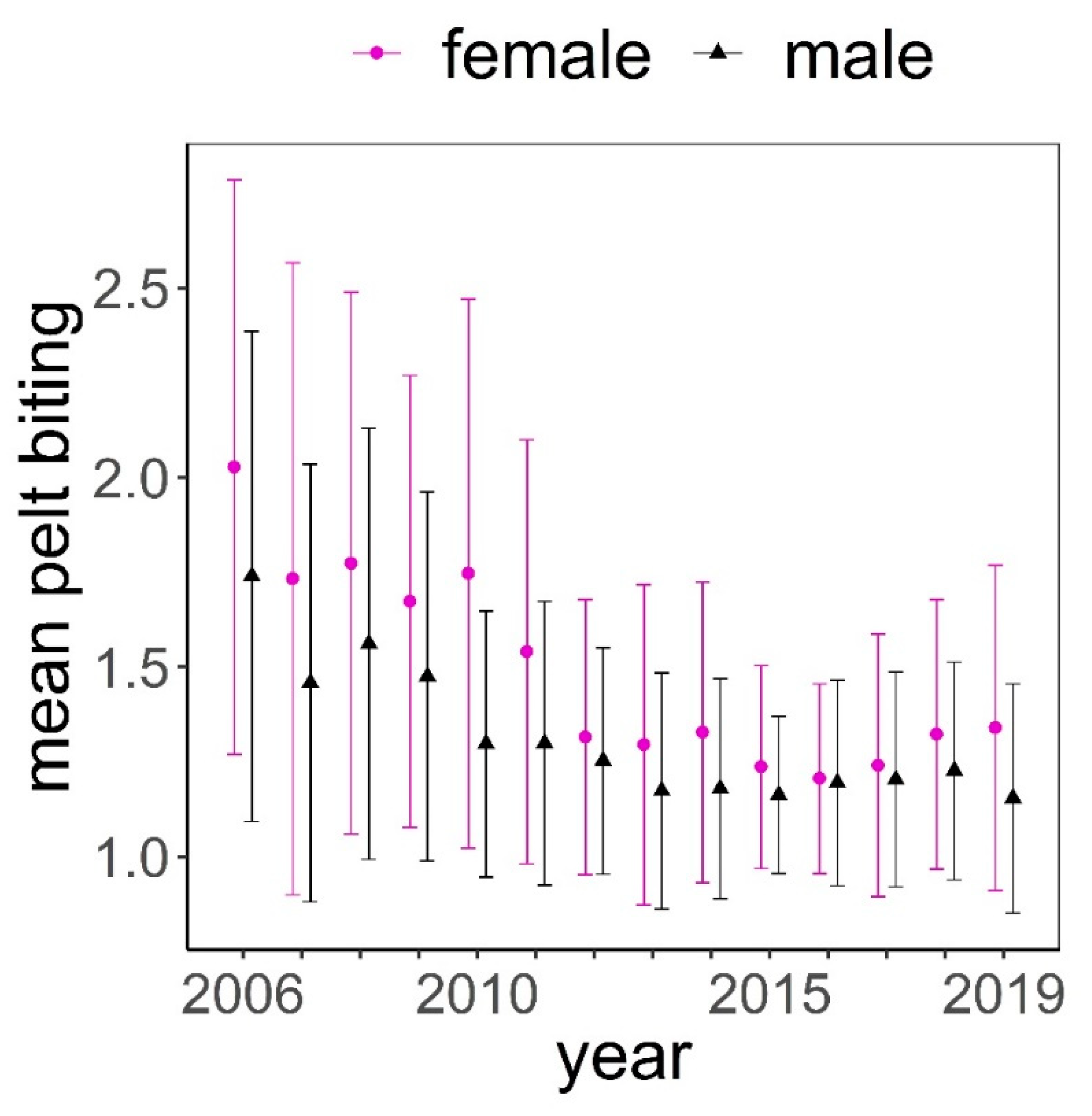
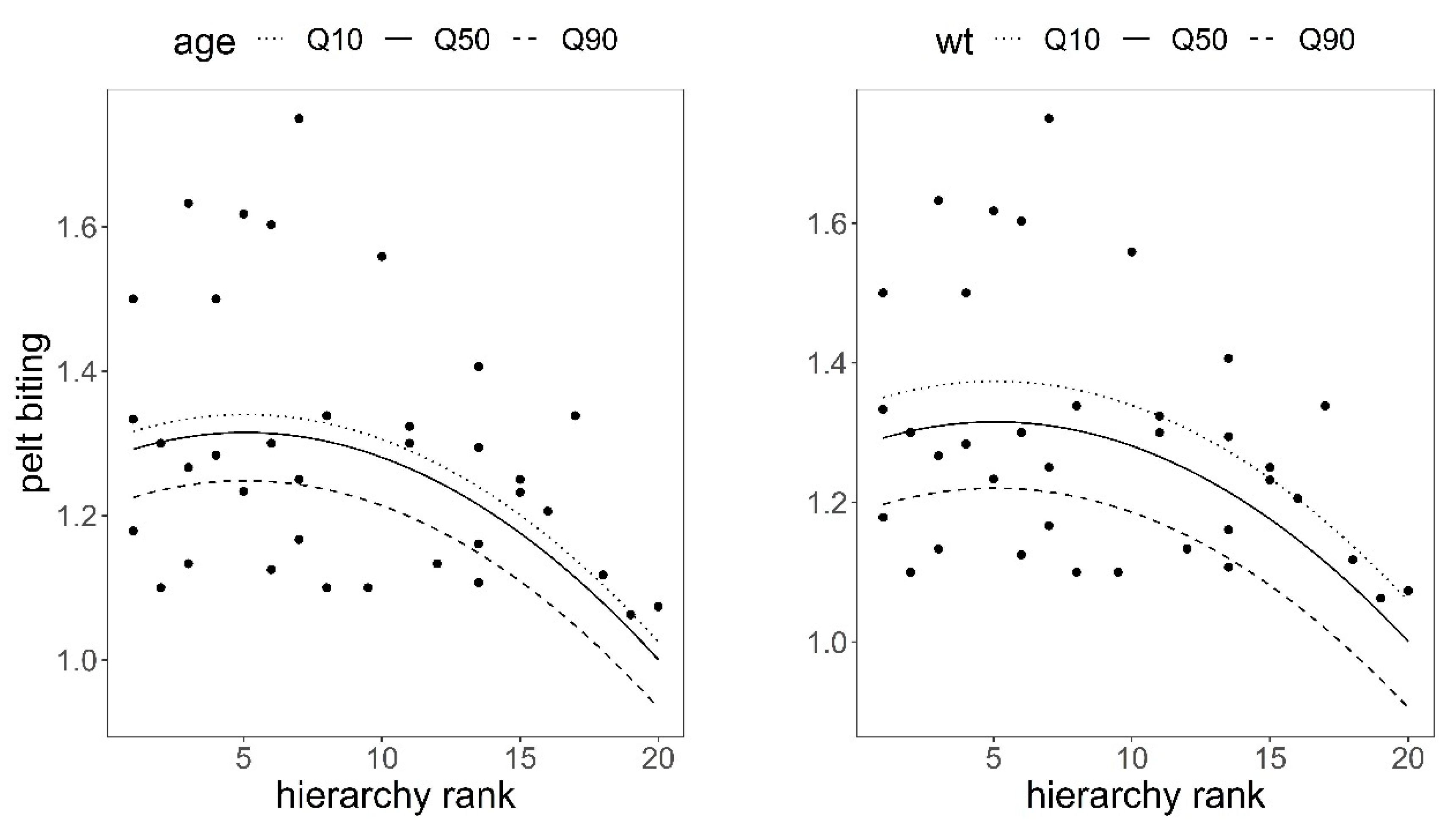
| Group of Hypotheses | Terms | Hypotheses/Predictions |
|---|---|---|
| Life history traits related to hierarchy | Hierarchical rank | H1. Individuals of lower hierarchical rank suffer more pelt biting than individuals of higher hierarchical rank. |
| Body weight | H2. Lighter individuals suffer more pelt biting than heavier individuals. | |
| Age | H3. Younger individuals are more likely to suffer pelt biting from older individuals. | |
| Sex | H4. Males are more likely to suffer pelt biting than females due to their higher intra-sexual interactions to achieve competitive skills for access to mating opportunities. H5. Pregnant hinds carrying female foetuses are more frequently attacked by conspecifics than those pregnant with sons, and so suffering more pelt biting. | |
| Physical environment stress | Meteorological index of heat stress | H6. Heat stress conditions promote aggressions between animals within social units: animals exhibit more pelt biting during periods of high heat stress. |
| Year | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| females | n | 137 | 178 | 193 | 205 | 158 | 126 | 79 | 29 | 38 | 40 | 51 | 61 | 61 | 56 |
| records | 1800 | 3389 | 2915 | 3321 | 3245 | 2614 | 1665 | 709 | 874 | 1390 | 1188 | 1453 | 1613 | 1210 | |
| males | n | 128 | 97 | 103 | 103 | 47 | 70 | 65 | 31 | 38 | 30 | 38 | 47 | 49 | 35 |
| records | 1125 | 1470 | 1066 | 1002 | 846 | 1149 | 954 | 566 | 510 | 717 | 687 | 1035 | 1018 | 627 |
| Random Effects | n | SD | ||||
|---|---|---|---|---|---|---|
| hind ID (intercept) | 22 | 0.142 | ||||
| year (intercept) | 2 | 0.112 | ||||
| residual | 0.087 | |||||
| no. observations | 37 | |||||
| Fixed effects | Coefficients of Polynomial Functions | |||||
| Degree | Estimate | SE | df | t-Value | p | |
| (intercept) | — | 1.817 | 0.2361 | 14.7 | 7.696 | <0.001 |
| age | 1 | −0.008 | 0.0044 | 31.0 | −1.888 | 0.068 |
| body weight | 1 | −0.005 | 0.0018 | 31.1 | −2.766 | 0.009 |
| hierarchical rank | 1 | −0.495 | 0.1451 | 30.9 | −3.409 | 0.002 |
| hierarchical rank | 2 | −0.257 | 0.0918 | 30.5 | −2.803 | 0.009 |
| R2LMM(m) = 0.436 | ||||||
| R2LMM(c) = 0.848 | ||||||
| Random Effects | n | SD | ||||
|---|---|---|---|---|---|---|
| ID (intercept) | 510 | 0.269 | ||||
| year (intercept) | 14 | 0.255 | ||||
| residual | 0.426 | |||||
| no. observations | 8154 | |||||
| Fixed effects | Coefficients of Polynomial Functions | |||||
| Degree | Estimate | SE | df | t-Value | p | |
| (intercept) | — | 1.35 | 0.073 | 15.8 | 18.68 | <0.001 |
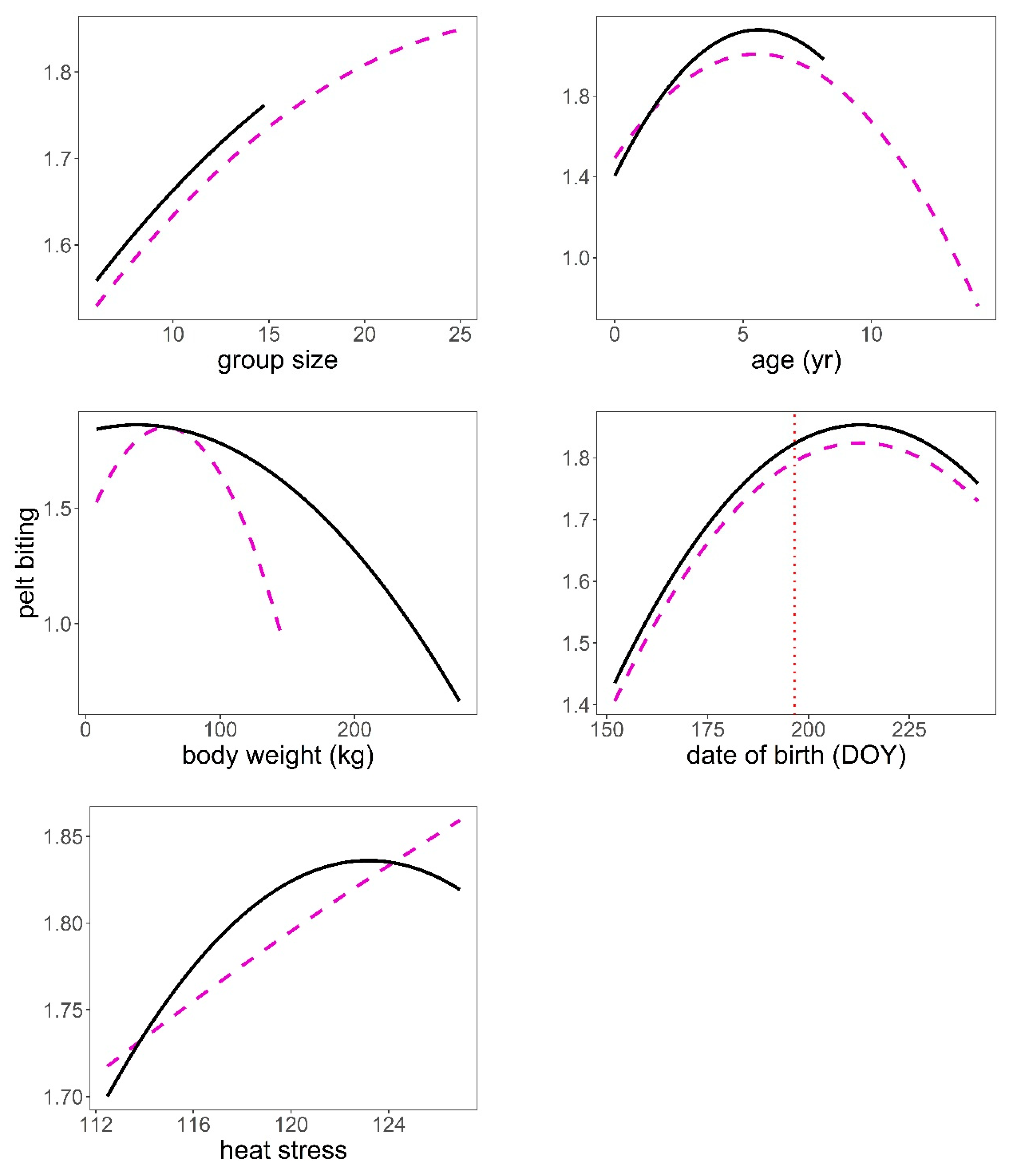
| group size | 1 | 8.36 | 0.981 | 5066.6 | 8.521 | <0.001 |
| group size | 2 | −3.22 | 0.653 | 7952.5 | −4.926 | <0.001 |
| age | 1 | 9.85 | 1.479 | 4840.4 | 6.664 | <0.001 |
| age | 2 | −11.85 | 0.894 | 8129.2 | −13.259 | <0.001 |
| sex (male) | — | 0.18 | 0.038 | 1315.1 | 4.897 | <0.001 |
| date of birth | 1 | 8.49 | 0.492 | 7716.6 | 17.261 | <0.001 |
| date of birth | 2 | −6.37 | 0.541 | 7599.9 | −11.773 | <0.001 |
| weight | 1 | −35.75 | 3.745 | 7901.0 | −9.544 | <0.001 |
| weight | 2 | −35.64 | 2.735 | 8098.5 | −13.033 | <0.001 |
| heat stress | 1 | 3.84 | 0.698 | 7671.0 | 5.499 | <0.001 |
| heat stress | 2 | −0.12 | 0.632 | 7627.3 | −0.185 | 0.853 |
| age × sex (male) | 1 | 4.30 | 3.966 | 7930.4 | 1.084 | 0.279 |
| age × sex (male) | 2 | −4.34 | 3.628 | 8060.8 | −1.196 | 0.232 |
| weight × sex (male) | 1 | 26.04 | 4.507 | 7623.1 | 5.777 | <0.001 |
| weight × sex (male) | 2 | 29.54 | 2.892 | 8094.0 | 10.214 | <0.001 |
| heat stress × sex (male) | 1 | −0.91 | 0.925 | 7880.7 | −0.986 | 0.324 |
| heat stress × sex (male) | 2 | −1.73 | 0.907 | 7745.8 | −1.908 | 0.056 |
| R2LMM(m) = 0.159 | ||||||
| R2LMM(c) = 0.520 | ||||||
| Random Effects | n | SD | ||||
|---|---|---|---|---|---|---|
| calf ID (intercept) | 581 | 0.212 | ||||
| hind ID (intercept) | 156 | 0.325 | ||||
| year (intercept) | 14 | 0.253 | ||||
| residual | 0.430 | |||||
| no. observations | 8071 | |||||
| Fixed Effects | Coefficients of Polynomial Functions | |||||
| Degree | Estimate | SE | df | t-Value | p | |
| (intercept) | — | 1.49 | 0.076 | 16.0 | 19.658 | <0.001 |
| hind age | 1 | −2.00 | 2.313 | 246.9 | −0.866 | 0.387 |
| hind age | 2 | 2.18 | 1.222 | 744.6 | 1.781 | 0.075 |
| hind age | 3 | 0.37 | 0.990 | 729.2 | 0.374 | 0.709 |
| hind weight | 1 | −23.60 | 1.100 | 5656.9 | −21.442 | <0.001 |
| hind weight | 2 | 7.20 | 0.604 | 7770.5 | 11.921 | <0.001 |
| hind weight | 3 | −1.14 | 0.537 | 8032.4 | −2.117 | 0.034 |
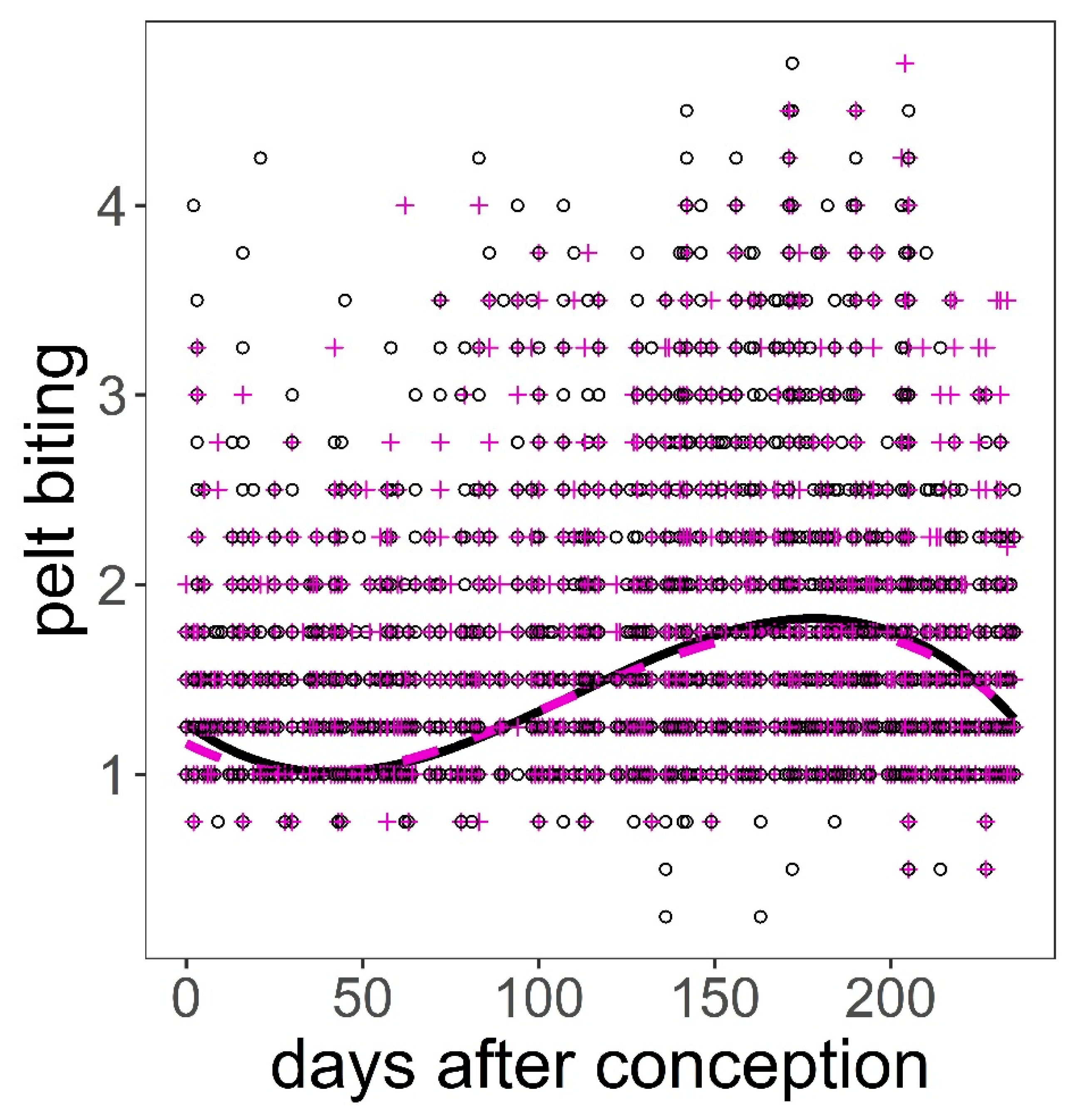
| conception day | 1 | 19.78 | 0.796 | 7811.5 | 24.854 | <0.001 |
| conception day | 2 | −8.56 | 0.663 | 7743.2 | −12.907 | <0.001 |
| conception day | 3 | −12.08 | 0.644 | 7604.9 | −18.753 | <0.001 |
| sex (male) | — | 0.02 | 0.023 | 430.3 | 0.984 | 0.326 |
| conception day × sex (male) | 1 | −0.18 | 0.885 | 7627.7 | −0.207 | 0.836 |
| conception day × sex (male) | 2 | 0.56 | 0.875 | 7533.9 | 0.644 | 0.520 |
| conception day × sex (male) | 3 | −2.33 | 0.870 | 7497.1 | −2.679 | 0.007 |
| R2LMM(m) = 0.236 | ||||||
| R2LMM(c) = 0.646 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Barbería, F.J.; García, A.J.; López-Quintanilla, M.; Landete-Castillejos, T. Pelt Biting as a Practical Indicator of Social and Environment Stress in Farmed Red Deer. Animals 2021, 11, 3134. https://doi.org/10.3390/ani11113134
Pérez-Barbería FJ, García AJ, López-Quintanilla M, Landete-Castillejos T. Pelt Biting as a Practical Indicator of Social and Environment Stress in Farmed Red Deer. Animals. 2021; 11(11):3134. https://doi.org/10.3390/ani11113134
Chicago/Turabian StylePérez-Barbería, Francisco Javier, Andrés José García, María López-Quintanilla, and Tomás Landete-Castillejos. 2021. "Pelt Biting as a Practical Indicator of Social and Environment Stress in Farmed Red Deer" Animals 11, no. 11: 3134. https://doi.org/10.3390/ani11113134







