PK/PD Analysis by Nonlinear Mixed-Effects Modeling of a Marbofloxacin Dose Regimen for Treatment of Goat Mastitis Produced by Coagulase-Negative Staphylococci
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
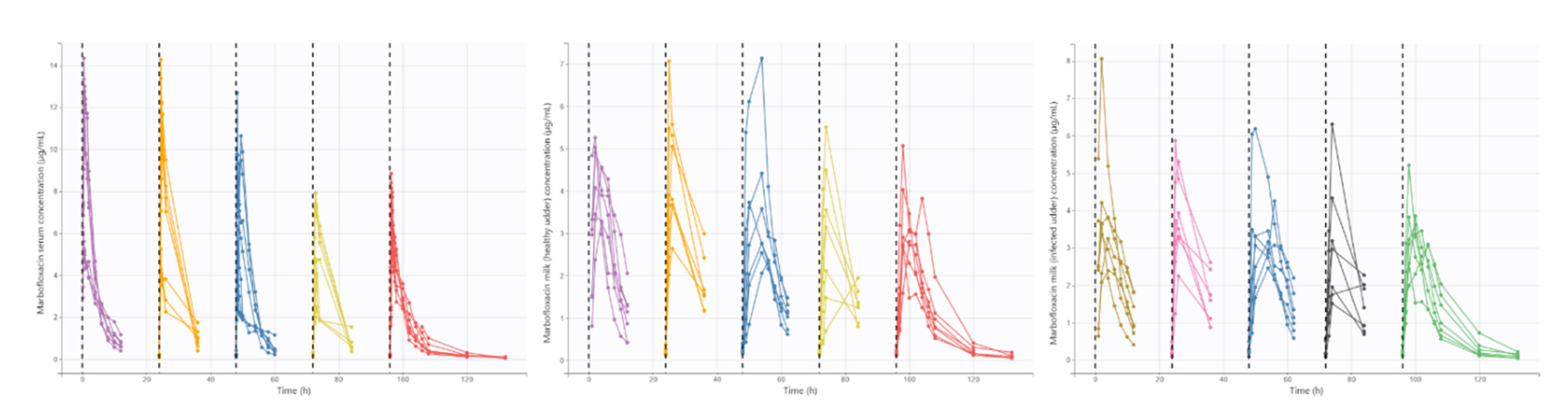
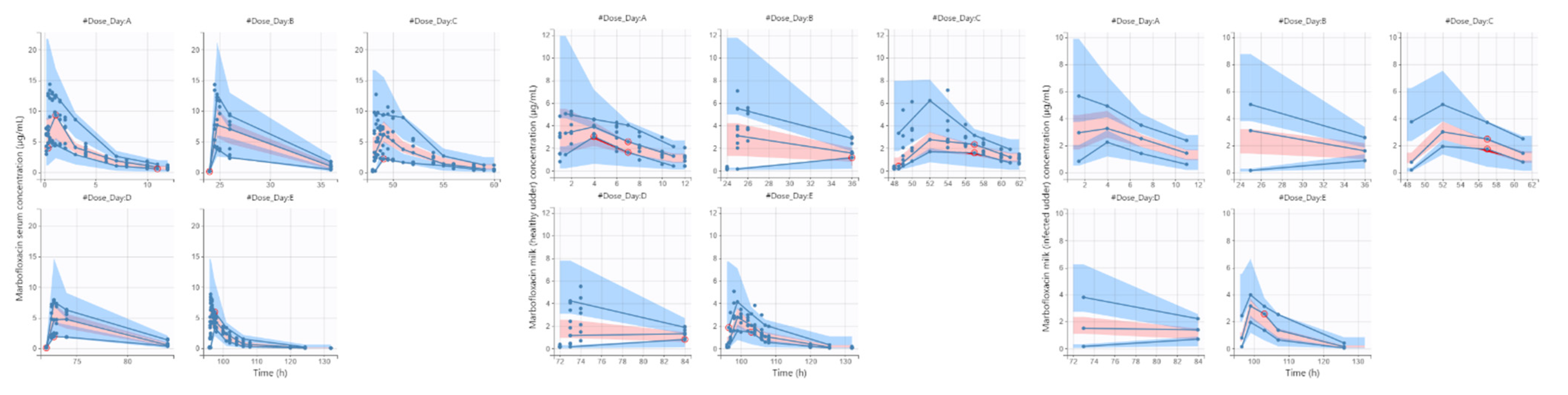
2.1. Animals, Treatments and Samples
2.2. Marbofloxacin Determination
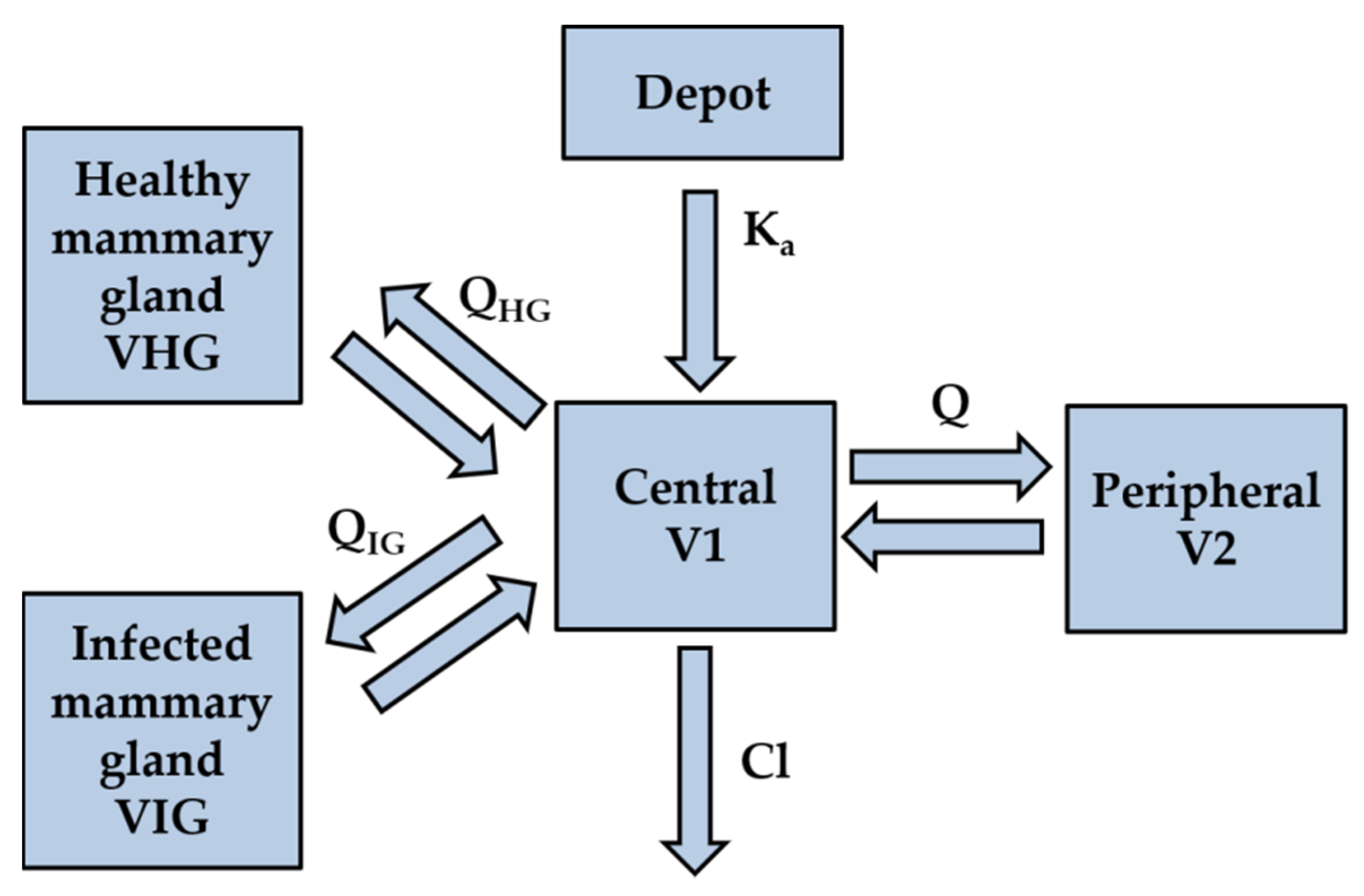
2.3. Pharmacokinetic Modeling and Simulation
2.4. Time Kill Curves Assays
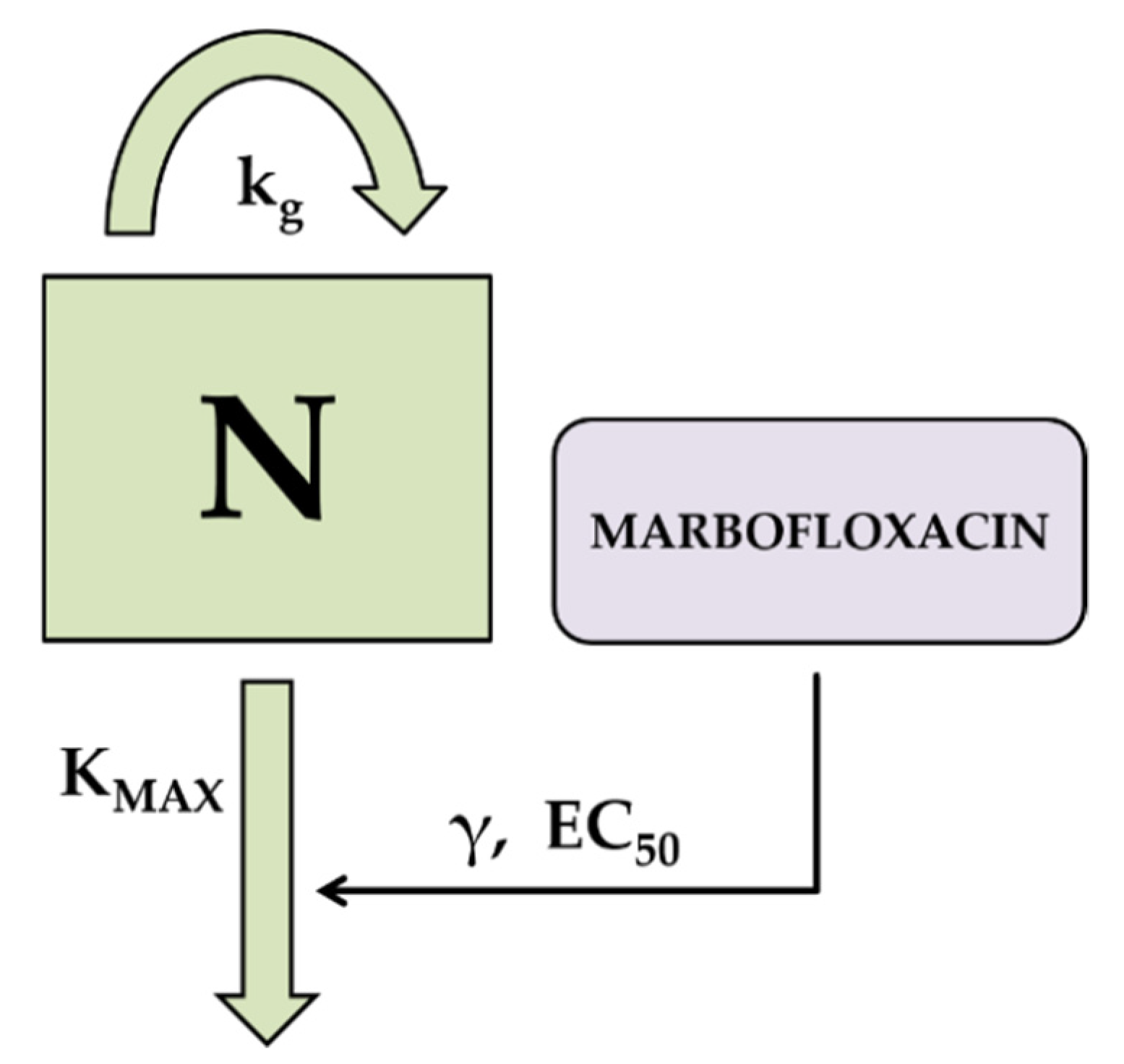
2.5. Pharmacodynamic Modeling and Simulation
2.6. Determination of the PK/PD Cutoff Values
2.7. PK/PD Analysis of Marbofloxacin against Coagulase Negative Staphylococci Isolated from Goat Mastitis
2.8. Statistical Analysis
3. Results
3.1. Pharmacokinetic Modeling and Simulation
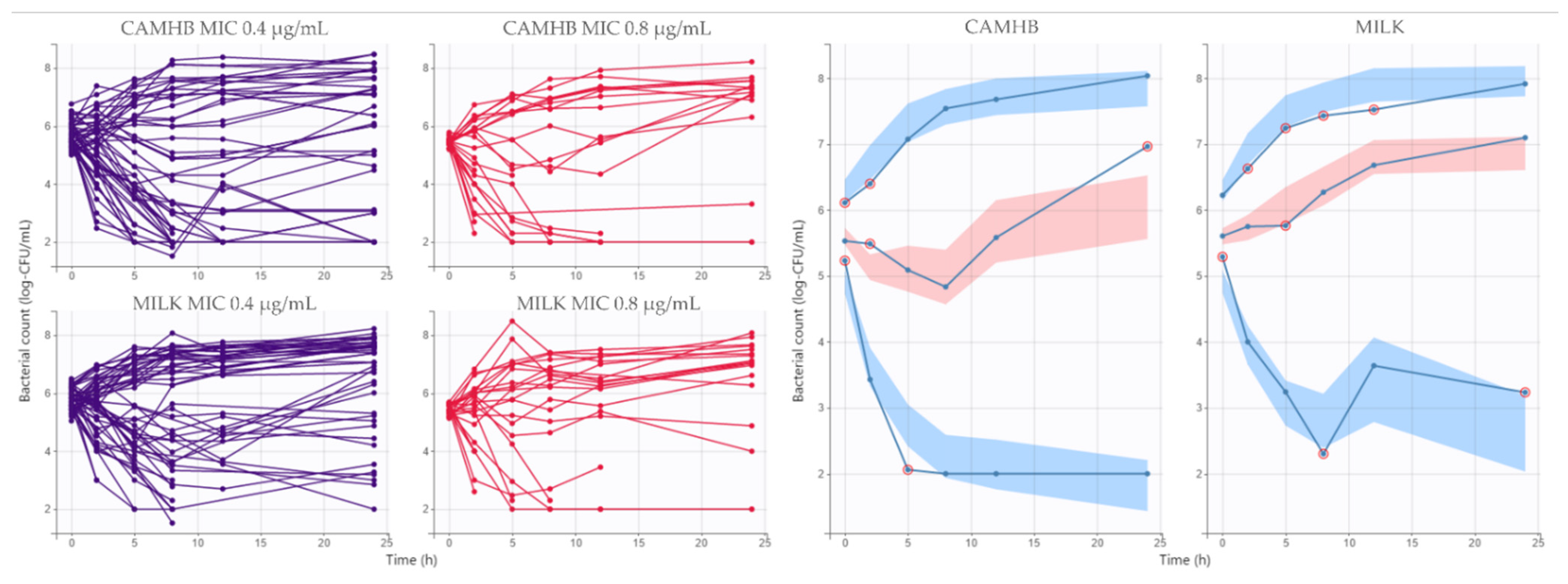
3.2. Pharmacodynamic Modeling and Simulation
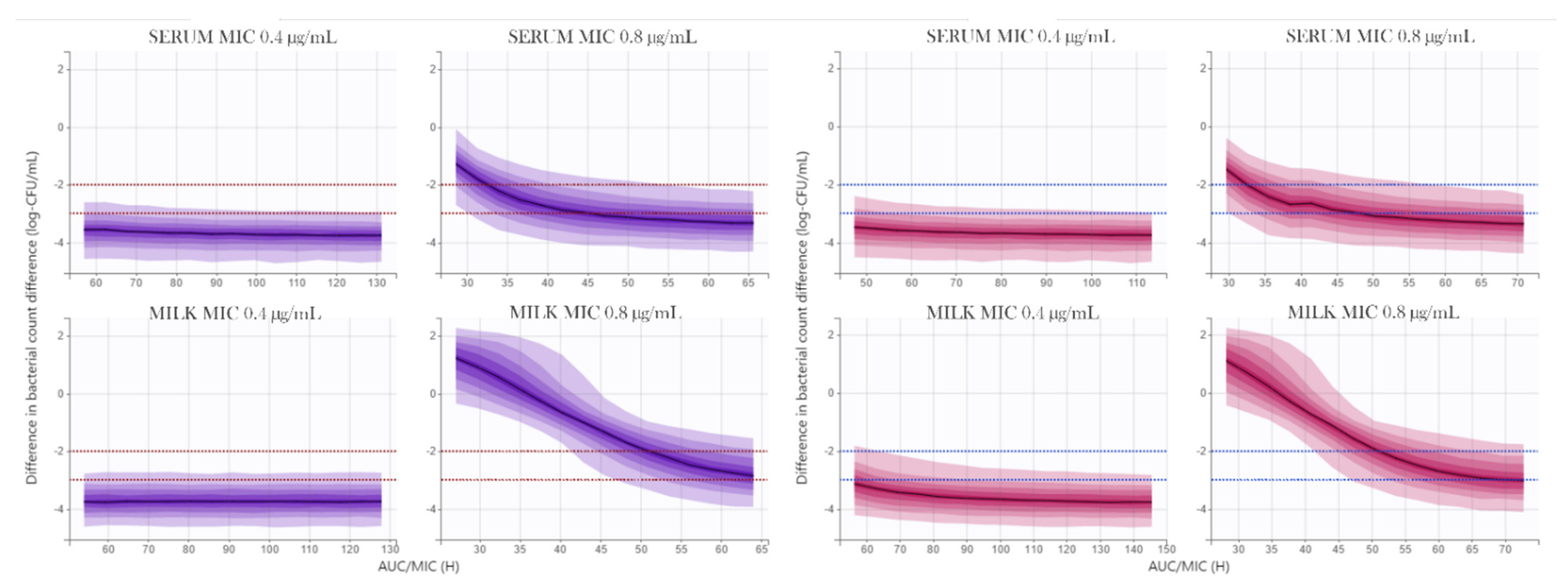
3.3. Determination of Marbofloxacin PK/PD Cutoff Values
3.4. PK/PD Analysis of Marbofloxacin against Coagulase Negative Staphylococci Isolated from Goat Mastitis
4. Discussion
4.1. Pharmacokinetic Modeling and Simulation
4.2. Pharmacodynamic Modeling and Simulation
4.3. Determination of Marbofloxacin PK/PD Cutoff Values
4.4. PK/PD Analysis of Marbofloxacin against Coagulase Negative Staphylococci Isolated from Goat Mastitis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergonier, D.; de Crémoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Rev. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef] [Green Version]
- Contreras, A.; Sierra, D.; Sánchez, A.; Corrales, J.C.; Marco, J.C.; Paape, M.J.; Gonzalo, C. Mastitis in small ruminants. Small Rumin. Res. 2007, 68, 145–153. [Google Scholar] [CrossRef]
- Papich, M.G. Pharmacokinetic-pharmacodynamic (PK-PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Vet. Microbiol. 2014, 171, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Asín-Prieto, E.; Rodríguez-Gascón, A.; Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. Rev. J. Infect. Chemother. 2015, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Huang, L.; Hao, H.; Sanders, P.; Yuan, Z. Application of PK/PD Modeling in Veterinary Field: Dose Optimization and Drug Resistance Prediction. Rev. Biomed. Res. Int. 2016, 2016, 5465678. [Google Scholar] [CrossRef]
- de Velde, F.; Mouton, J.W.; de Winter, B.C.M.; van Gelder, T.; Koch, B.C.P. Clinical applications of population pharmacokinetic models of antibiotics: Challenges and perspectives. Rev. Pharmacol. Res. 2018, 134, 280–288. [Google Scholar] [CrossRef]
- Toutain, P.L.; Pelligand, L.; Lees, P.; Bousquet-Mélou, A.; Ferran, A.A.; Turnidge, J.D. The pharmacokinetic/pharmacodynamic paradigm for antimicrobial drugs in veterinary medicine: Recent advances and critical appraisal. Vet. Pharmacol. Ther. 2021, 44, 172–200. [Google Scholar] [CrossRef] [PubMed]
- EMA. Committee for Veterinary Medicinal Products. Marbofloxacin (Extension to All Food Producing Species). Summary Report. 2009. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014864.pdf (accessed on 30 May 2021).
- Waxman, S.; Rodríguez, C.; González, F.; De Vicente, M.L.; San Andrés, M.I.; San Andrés, M.D. Pharmacokinetic behavior of marbofloxacin after intravenous and intramuscular administrations in adult goats. J. Vet. Pharmacol. Ther. 2001, 24, 375–378. [Google Scholar] [CrossRef]
- Waxman, S.; San Andrés, M.D.; González, F.; De Lucas, J.J.; San Andrés, M.I.; Rodríguez, C. Influence of Escherichia coli endotoxin-induced fever on the pharmacokinetic behavior of marbofloxacin after intravenous administration in goats. J. Vet. Pharmacol. Ther. 2003, 26, 65–69. [Google Scholar] [CrossRef]
- Waxman, S.; San Andrés, M.D.; González, F.; San Andrés, M.I.; De Lucas, J.J.; Rodríguez, C. Age-related changes in the pharmacokinetics of marbofloxacin after intravenous administration in goats. J. Vet. Pharmacol. Ther. 2004, 27, 31–35. [Google Scholar] [CrossRef]
- Dova, S.W.; San Andrés, M.D.; González, F.; San Andrés, M.I.; De Lucas, J.J.; Rodríguez, C. Pharmacokinetic behavior and pharmacokinetic/pharmacodynamic integration of marbofloxacin after subcutaneous administration in goats. Vet. J. 2007, 174, 425–427. [Google Scholar] [CrossRef]
- Sidhu, P.K.; Landoni, M.F.; Aliabadi, F.S.; Lees, P. Pharmacokinetic and pharmacodynamic modelling of marbofloxacin administered alone and in combination with tolfenamic acid in goats. Vet. J. 2010, 184, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Sidhu, P.K.; Lonare, M.K.; Kaur, R.; Dumka, V.K.; Rampal, S. Pharmacokinetic-pharmacodynamic integration of marbofloxacin after single and repeated intravenous administration in goats. Res. Vet. Sci. 2018, 121, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Sidhu, P.K.; Saini, S.P.S.; Rampal, S. Pharmacokinetic-pharmacodynamic relationship of marbofloxacin for Escherichia coli and Pasturella multocida following repeated intramuscular administration in goats. J. Vet. Pharmacol. Ther. 2019, 42, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Lorenzutti, A.M.; Litterio, N.J.; Himelfarb, M.A.; Zarazaga, M.D.P.; San Andrés, M.I.; De Lucas, J.J. Pharmacokinetics, milk penetration and PK/PD analysis by Monte Carlo simulation of marbofloxacin, after intravenous and intramuscular administration to lactating goats. J. Vet. Pharmacol. Ther. 2017, 40, 629–640. [Google Scholar] [CrossRef]
- Fernández-Varón, E.; García-Romero, E.; Serrano-Rodríguez, J.M.; Cárceles, C.M.; García-Galán, A.; Cárceles-García, C.; Fernández, R.; Muñoz, C.; De la Fe, C. PK/PD Analysis of Marbofloxacin by Monte Carlo Simulation against Mycoplasma agalactiae in Plasma and Milk of Lactating Goats after IV, SC and SC-Long Acting Formulations Administration. Animals 2021, 12, 1104. [Google Scholar] [CrossRef]
- Singh, V.; Ram, M.; Roy, B.K.; Singh, K.K. Effect of Induced Mastitis on Disposition Kinetics of Gatifloxacin Following Intravenous Administration in Goats. JBABM 2010, 2, 044–047. [Google Scholar] [CrossRef]
- Mould, D.R.; Upton, R.N. Basic concepts in population modeling, simulation, and model-based drug development. CPT Pharmacomet. Syst. Pharmacol. 2012, 1, e6. [Google Scholar] [CrossRef]
- Owen, J.S.; Fiedler-Kelly, J. Introduction to Population Pharmacokinetic/Pharmacodynamic Analysis with Nonlinear Mixed Effects Models; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Lavielle, M. Mixed Effects Models for the Population Approach: Models, Tasks, Methods and Tools; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Nielsen, E.I.; Friberg, L.E. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Rev. Pharmacol. Rev. 2013, 65, 1053–1090. [Google Scholar] [CrossRef] [Green Version]
- Oliver, S.P.; Gonzalez, R.N.; Hogan, J.S.; Jayarao, B.M.; Owens, W.E. Microbiological Procedures for the Diagnosis of Bovine Udder Infection and Determination of Milk Quality, 4th ed.; National Mastitis Council: Verona, WI, USA, 2004; Available online: http://www.nmconline.org/wp-content/uploads/2016/09/Procedures-for-Collecting-Milk-Samples.pdf (accessed on 24 July 2021).
- Weisskopf, E.; Guidi, M.; Fischer, C.J.; Graz, M.B.; Beaufils, E.; Nguyen, K.A.; Harari, M.M.; Rouiller, S.; Rothenburger, S.; Gaucherand, S.; et al. A population pharmacokinetic model for escitalopram and its major metabolite in depressive patients during the perinatal period: Prediction of infant drug exposure through breast milk. Multicenter Study. Br. J. Clin. Pharmacol. 2020, 86, 1642–1653. [Google Scholar] [CrossRef]
- Mould, D.R.; Upton, R.N. Basic concepts in population modeling, simulation, and model-based drug development-part 2, introduction to pharmacokinetic modeling methods. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e38. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; CLSI Document M100-S23; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Melchior, M.B.; Fink-Gremmels, J.; Gaastra, W. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Jia, H.; Miyanaga, K.; Tanji, Y. Effect of milk on antibacterial activity of tetracycline against Escherichia coli and Staphylococcus aureus isolated from bovine mastitis. Appl. Microbiol. Biotechnol. 2009, 84, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, S.; Tulkens, P.M.; Van Bambeke, F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.; Grégoire, N.; Couet, W.; Bulitta, J.B. Distinguishing Antimicrobial Models with Different Resistance Mechanisms via Population Pharmacodynamic Modeling. PLoS Comput. Biol. 2016, 11, e1004782. [Google Scholar] [CrossRef] [Green Version]
- Schmith, V.D.; Foss, J.F. Effects of inflammation on pharmacokinetics/pharmacodynamics: Increasing recognition of its contribution to variability in response. Clin. Pharmacol. Ther. 2008, 83, 809–811. [Google Scholar] [CrossRef]
- Renton, K.W. Regulation of drug metabolism and disposition during inflammation and infection. Rev. Expert Opin. Drug Metab. Toxicol. 2005, 1, 629–640. [Google Scholar] [CrossRef]
- Aitken, A.E.; Richardson, T.A.; Morgan, E.T. Regulation of drug-metabolizing enzymes and transporters in inflammation. Rev. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 123–149. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Teng, S.; Piquette-Miller, M. Regulation of drug transporters during infection and inflammation. Rev. Mol. Interv. 2007, 7, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.T.; Goralski, K.B.; Piquette-Miller, M.; Renton, K.W.; Robertson, G.R.; Chaluvadi, M.R.; Charles, K.A.; Clarke, S.J.; Kacevska, M.; Liddle, C.; et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab. Dispos. 2008, 36, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Merino, G.; Alvarez, A.I.; Pulido, M.M.; Molina, A.J.; Schinkel, A.H.; Prieto, J.G. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab. Dispos. 2006, 34, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Pulido, M.M.; Molina, A.J.; Merino, G.; Mendoza, G.; Prieto, J.G.; Álvarez, A.I. Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): Influence of flavonoids and role in milk secretion in sheep. J. Vet. Pharmacol. Ther. 2006, 29, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Schrickx, J.A.; Fink-Gremmels, J. Implications of ABC transporters on the disposition of typical veterinary medicinal products. Eur. J. Pharmacol. 2008, 585, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Luo, J.; Wu, N.; Matand, K.; Zhang, L.J.; Han, X.F.; Yang, B.J. Cloning, sequence and functional analysis of goat ATP-binding cassette transporter G2 (ABCG2). Mol. Biotechnol. 2008, 39, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Real, R.; Egido, E.; Pérez, M.; González-Lobato, L.; Barrera, B.; Prieto, J.G.; Alvarez, A.I.; Merino, G. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the secretion of danofloxacin into milk: Interaction with ivermectin. J. Vet. Pharmacol. Ther. 2011, 34, 313–321. [Google Scholar] [CrossRef]
- Mealey, K.L. ABCG2 transporter: Therapeutic and physiologic implications in veterinary species. Rev. J. Vet. Pharmacol. Ther. 2012, 35, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Otero, J.A.; Barrera, B.; Prieto, J.G.; Merino, G.; Alvarez, A.I. Inhibition of ABCG2/BCRP transporter by soy isoflavones genistein and daidzein: Effect on plasma and milk levels of danofloxacin in sheep. Vet. J. 2013, 196, 203–208. [Google Scholar] [CrossRef]
- de los Campos, G.; Gianola, D.; Boettcher, P.; Moroni, P. A structural equation model for describing relationships between somatic cell score and milk yield in dairy goats. J. Anim. Sci. 2006, 84, 2934–2941. [Google Scholar] [CrossRef]
- McDougall, S.; Supré, K.; De Vliegher, S.; Haesebrouck, F.; Hussein, H.; Clausen, L.; Prosser, C. Diagnosis and treatment of subclinical mastitis in early lactation in dairy goats. J. Dairy Sci. 2010, 93, 4710–4721. [Google Scholar] [CrossRef] [Green Version]
- Vetoquinol. Marbocyl. Marbocyl 10%. Summary of Product Characteristics. Available online: http://mri.cts-mrp.eu/download/FR_V_0107_001_FinalSPC.pdf (accessed on 24 March 2021).
- EMA. Committee for Veterinary Medicinal Products: Marbofloxacin; Summary Report; Committee for Veterinary Medicinal Products: London, UK, 2000; Available online: https://www.ema.europa.eu/en/documents/mrl-report/marbofloxacin-summary-report-2-committee-veterinary-medicinal-products_en.pdf (accessed on 22 July 2021).
- Beyene, T. Veterinary Drug Residues in Food-animal Products: Its Risk Factors and Potential Effects on Public Health. J. Vet. Sci. Technol. 2016, 7, 285. [Google Scholar] [CrossRef]
- Louhi, M.; Inkinen, K.; Myllys, V.; Sandholm, M. Relevance of sensitivity testings (MIC) of S. aureus to predict the antibacterial action in milk. Zent. Vet. B 1992, 39, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Pyörälä, S. Mastitis-causing Escherichia coli: Serum sensitivity and susceptibility to selected antibacterials in milk. J. Dairy Sci. 1996, 79, 76–82. [Google Scholar] [CrossRef]
- Mouton, J.W.; Vinks, A.A. Pharmacokinetic/pharmacodynamic modelling of antibacterials in vitro and in vivo using bacterial growth and kill kinetics: The minimum inhibitory concentration versus stationary concentration. Comp. Study Clin. Pharm. 2005, 44, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Pharmacokinetics and pharmacodynamics of antimicrobials. Rev. Clin. Infect. Dis. 2007, 45 (Suppl. 1), S89–S95. [Google Scholar] [CrossRef] [Green Version]
- Lister, P.D. Pharmacodynamics of gatifloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model: Impact of area under the curve/MIC ratios on eradication. Antimicrob. Agents Chemother. 2002, 46, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drusano, G.L.; Fregeau, C.; Liu, W.; Brown, D.L.; Louie, A. Impact of burden on granulocyte clearance of bacteria in a mouse thigh infection model. Antimicrob. Agents Chemother. 2010, 54, 4368–4372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drusano, G.L.; Vanscoy, B.; Liu, W.; Fikes, S.; Brown, D.; Louie, A. Saturability of granulocyte kill of Pseudomonas aeruginosa in a murine model of pneumonia. Antimicrob. Agents Chemother. 2011, 55, 2693–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drusano, G.L.; Liu, W.; Fikes, S.; Cirz, R.; Robbins, N.; Kurhanewicz, S.; Rodriquez, J.; Brown, D.; Baluya, D.; Louie, A. Interaction of drug- and granulocyte-mediated killing of Pseudomonas aeruginosa in a murine pneumonia model. J. Infect. Dis. 2014, 210, 1319–1324. [Google Scholar] [CrossRef]
- Drusano, G.L.; Liu, W.; Brown, D.L.; Rice, L.B.; Louie, A. Impact of short-course quinolone therapy on susceptible and resistant populations of Staphylococcus aureus. J. Infect. Dis. 2009, 199, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Ledesma, K.R.; Chang, K.Y.; Hou, J.G.; Prince, R.A.; Tam, V.H. Pharmacodynamics of moxifloxacin against a high inoculum of Escherichia coli in an in vitro infection model. J. Antimicrob. Chemother. 2009, 64, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Bhagunde, P.; Chang, K.Y.; Singh, R.; Singh, V.; Garey, K.W.; Nikolaou, M.; Tam, V.H. Mathematical modeling to characterize the inoculum effect. Antimicrob. Agents Chemother. 2010, 54, 4739–4743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, E.I.; Khan, D.D.; Cao, S.; Lustig, U.; Hughes, D.; Andersson, D.I.; Friberg, L.E. Can a pharmacokinetic/pharmacodynamic (PKPD) model be predictive across bacterial densities and strains? External evaluation of a PKPD model describing longitudinal in vitro data. J. Antimicrob. Chemother. 2017, 72, 3108–3116. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Healthy Gland | Infected Gland | p-Value |
|---|---|---|---|
| Milk production (L/day) | 0.26 ± 0.15 | 0.12 ± 0.15 | 0.045 |
| SCC (×103)/mL | 541 ± 126 | 1389 ± 173 | 0.0002 |
| pH | 6.57 ± 0.03 | 6.72 ± 0.04 | 0.0001 |
| Estimates (RSE; %) | IIV (RSE; %) | IOV (RSE; %) | Shrinkage (%) | |
|---|---|---|---|---|
| Parameter estimates | ||||
| Kapop (h−1) | 10.8 (14.9) | 0.253 (34.2) | 0.24 (18.8) | 44.8 |
| Clpop (mL·Kg−1·h−1) | 0.237 (10.1) | 0.235 (27.8) | 0.0758 (25) | −0.19 |
| V1pop (L·Kg−1) | 0.953 (22.3) | 0.547 (26.9) | 0.071 (18.4) | 45.2 |
| Qpop (mL·Kg−1·h−1) | 0.0412 (28.3) | 0.1 (94.7) | 0.182 (37.4) | 9.89 |
| V2pop (L·Kg−1) | 1.53 (20.1) | 0.526 (27.4) | 0.151 (17.7) | −0.743 |
| QMHpop (mL·Kg−1·h−1) | 0.0239 (31.8) | 0.498 (30.1) | 0.33 (18) | 15 |
| VMHpop (L·Kg−1) | 0.0436 (32) | 0.569 (27.2) | 0.0256 (243) | 24.7 |
| QMIpop (mL·Kg−1·h−1) | 0.0211 (19.8) | 0.494 (30.1) | 0.318 (17.5) | −6.02 |
| VMIpop (L·Kg−1) | 0.05 (17) | 0.437 (28.1) | 0.0215 (833) | 13.7 |
| Covariates estimates | ||||
| beta_ka_DAY_2 | 1.45 (12.1) | - | - | - |
| beta_ka_DAY_3 | −0.224 (69.4) | - | - | - |
| beta_ka_DAY_4 | −1.21 (13.1) | - | - | - |
| beta_ka_DAY_5 | −0.44 (34.5) | - | - | - |
| beta_Cl_DAY_2 | −0.117 (58.6) | - | - | - |
| beta_Cl_DAY_3 | 0.0702 (91.7) | - | - | - |
| beta_Cl_DAY_4 | 0.166 (37.6) | - | - | - |
| beta_Cl_DAY_5 | 0.29 (20.4) | - | - | - |
| beta_V1_VOL_HEALTHY | 0.543 (36.9) | - | - | - |
| beta_Q_DAY_2 | −0.976 (32) | - | - | - |
| beta_Q_DAY_3 | −1.01 (31.3) | - | - | - |
| beta_Q_DAY_4 | −0.561 (54.3) | - | - | - |
| beta_Q_DAY_5 | −0.467(63.8) | - | - | - |
| beta_QMH_DAY_2 | 0.306(104) | - | - | - |
| beta_QMH_DAY_3 | −0.409(70.8) | - | - | - |
| beta_QMH_DAY_4 | 0.781(39.1) | - | - | - |
| beta_QMH_DAY_5 | 0.864(33.7) | - | - | - |
| beta_VMH_DAY_2 | 0.102(283) | - | - | - |
| beta_VMH_DAY_3 | 0.311(79.1) | - | - | - |
| beta_VMH_DAY_4 | 0.84(29.8) | - | - | - |
| beta_VMH_DAY_5 | 1.15(21.3) | - | - | - |
| Error model parameters | ||||
| b2 | 0.253(0.00763) | - | - | - |
| b3 | 0.233(0.0745) | - | - | - |
| b4 | 0.201(0.043) | - | - | - |
| Variable | Median | Min | Max | P(05) | P(95) |
|---|---|---|---|---|---|
| Serum | |||||
| fAUCfirst/MIC (MIC = 0.4 µg/mL) | 86.32 | 37.51 | 201.22 | 57.08 | 131.24 |
| fAUCfirst/MIC (MIC = 0.8 µg/mL) | 43.16 | 18.76 | 100.61 | 28.54 | 65.62 |
| fAUCmean/MIC (MIC = 0.4 µg/mL) | 72.68 | 31.64 | 185.88 | 47.45 | 113.45 |
| fAUCmean/MIC (MIC = 0.8 µg/mL) | 45.42 | 19.78 | 116.18 | 29.66 | 70.9 |
| Milk (infected mammary gland) | |||||
| fAUCfirst/MIC (MIC = 0.4 µg/mL) | 83.74 | 14.84 | 200.57 | 54.10 | 127.99 |
| fAUCfirst/MIC (MIC = 0.8 µg/mL) | 41.87 | 7.42 | 100.29 | 27.05 | 64.00 |
| fAUCmean/MIC (MIC = 0.4 µg/mL) | 89.87 | 41.89 | 223.92 | 56.42 | 145.55 |
| fAUCmean/MIC (MIC = 0.8 µg/mL) | 44.93 | 20.95 | 111.96 | 28.21 | 72.78 |
| Estimates (RSE; %) | IIV (RSE; %) | Shrinkage (%) | |
|---|---|---|---|
| Parameter estimates | |||
| Nmaxpop (log10 CFU/mL) | 7.51 (0.897) | 0.0297 (38.3) | 11.3 |
| kgpop (h−1) | 0.351 (7.65) | 0.533 (14.7) | −1.72 |
| N0pop (log10 CFU/mL) | 6 (2.02) | 0.0201 (106) | 4.72 |
| gammapop | 3.78 (13.2) | 0.715 (14.8) | 3.38 |
| Kmaxpop (h−1) | 0.283 (6.74) | 0.515 (12.4) | −1.12 |
| EC50pop (µg/mL) | 0.165 (8.76) | 0.0842 (34.8) | −8.52 |
| Covariate estimates | |||
| beta_N0_MIC | −0.129 (28.7) | - | - |
| beta_EC50_MILK | 0.453 (11.1) | - | - |
| beta_EC50_MIC | 2.13 (8.12) | - | - |
| Correlations estimates | |||
| Corr_kg_Kmax | 0.988 (1.26) | - | |
| Error model parameters | |||
| a | 0.512 (3.37) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzutti, A.M.; Vico, J.P.; Serrano-Rodríguez, J.M.; Himelfarb, M.A.; Andrés-Larrea, M.I.S.; Lucas-Burneo, J.J.d.; Litterio, N.J. PK/PD Analysis by Nonlinear Mixed-Effects Modeling of a Marbofloxacin Dose Regimen for Treatment of Goat Mastitis Produced by Coagulase-Negative Staphylococci. Animals 2021, 11, 3098. https://doi.org/10.3390/ani11113098
Lorenzutti AM, Vico JP, Serrano-Rodríguez JM, Himelfarb MA, Andrés-Larrea MIS, Lucas-Burneo JJd, Litterio NJ. PK/PD Analysis by Nonlinear Mixed-Effects Modeling of a Marbofloxacin Dose Regimen for Treatment of Goat Mastitis Produced by Coagulase-Negative Staphylococci. Animals. 2021; 11(11):3098. https://doi.org/10.3390/ani11113098
Chicago/Turabian StyleLorenzutti, Augusto Matías, Juan Pablo Vico, Juan Manuel Serrano-Rodríguez, Martín Alejandro Himelfarb, Manuel Ignacio San Andrés-Larrea, José Julio de Lucas-Burneo, and Nicolás Javier Litterio. 2021. "PK/PD Analysis by Nonlinear Mixed-Effects Modeling of a Marbofloxacin Dose Regimen for Treatment of Goat Mastitis Produced by Coagulase-Negative Staphylococci" Animals 11, no. 11: 3098. https://doi.org/10.3390/ani11113098






