Effect of the Inclusion of Different Levels of Dietary Cactus (Opuntia ficus-indica) on Gilts’ Biochemical Parameters and Feed Intake during Lactation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Diets and Housing
2.2. Blood Sampling
2.3. Blood Analyses
2.4. Statistical Analyses
3. Results
3.1. Gilt Performance
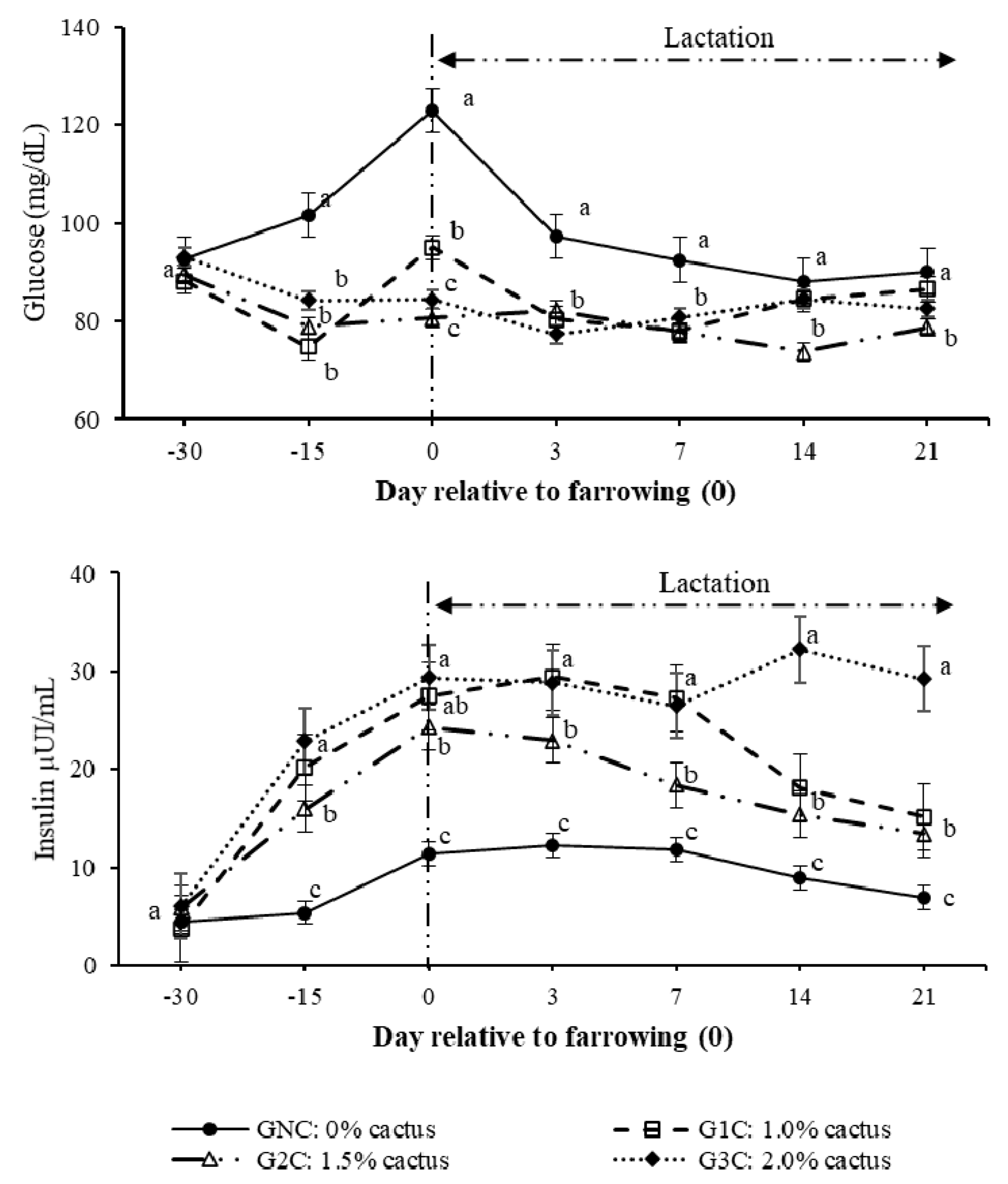
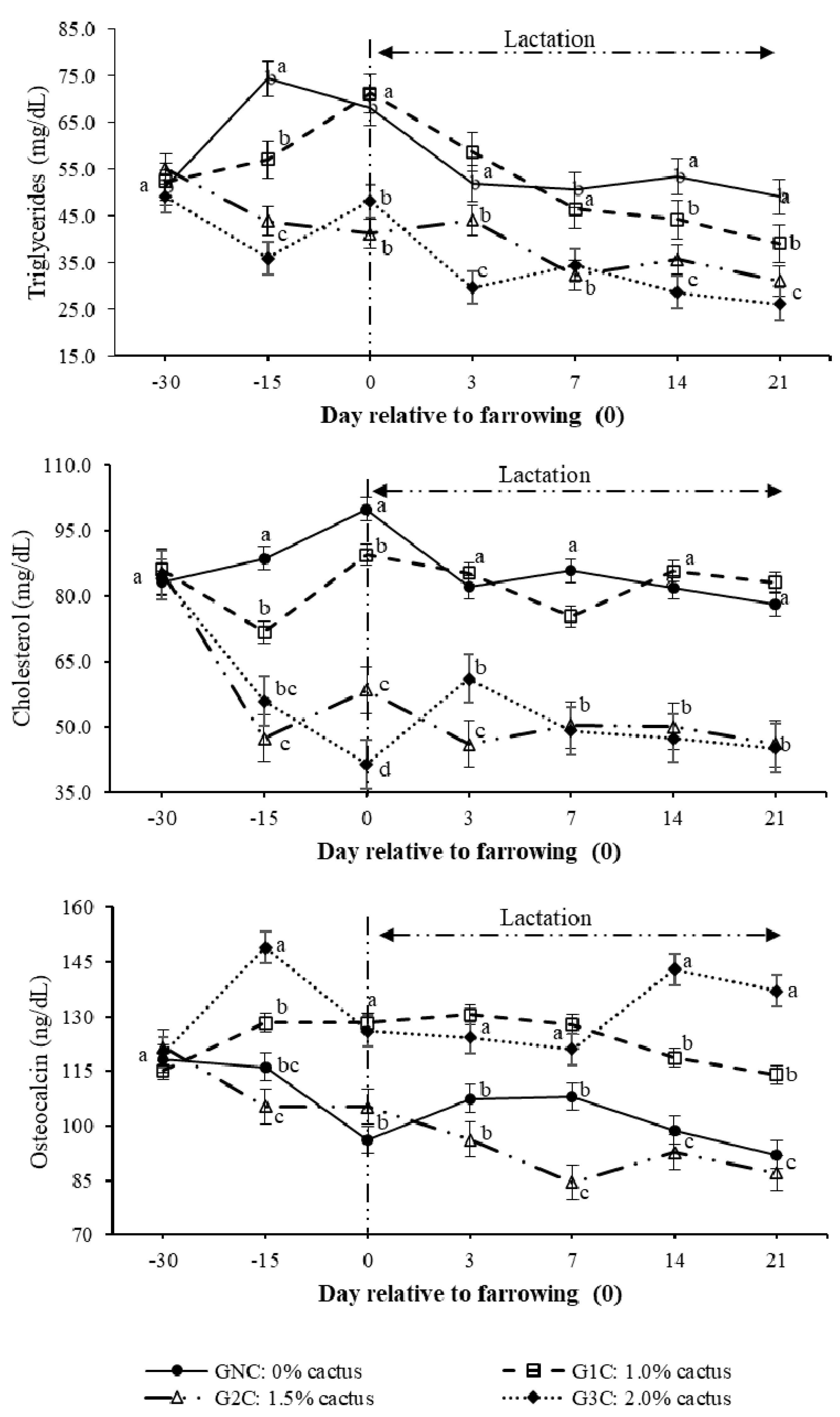
3.2. Hormones and Metabolites
3.3. Principal Component Analysis, Regression Models, and Optimal Cactus Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solà-Oriol, D.; Gasa, J. Feeding strategies in pig production: Sows and their piglets. Anim. Feed Sci. Technol. 2017, 233, 34–52. [Google Scholar] [CrossRef]
- Rempel, L.; Vallet, A.J.L.; Lents, C.A.; Nonneman, J. Measurements of body composition during late gestation and lactation in first and second parity sows and its relationship to piglet production and post-weaning reproductive performance. Livest. Sci. 2015, 178, 289–295. [Google Scholar] [CrossRef]
- Père, M.C.; Etienne, M.; Dourmad, J.Y. Adaptations of glucose metabolism in multiparous sows: Effects of pregnancy and feeding level. J. Anim. Sci. 2000, 78, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, E.; Etienne, M.; Ramaekers, P.; Pére, M.C. The metabolic status during the peri partum period affects the voluntary feed intake and the metabolism of the lactating multiparous sow. Livest. Sci. 2010, 127, 127–136. [Google Scholar] [CrossRef]
- Cools, A.; Maesb, D.A.Y.; Decaluwéa, R.; Buysec, J.; Kempend, T.; Liesegange, A.; Janssens, P.J. Ad libitum feeding during the peripartal period affects bodycondition, reproduction results and metabolism of sows. Anim. Feed Sci. Technol. 2014, 145, 130–140. [Google Scholar] [CrossRef]
- Barb, C.R.; Hausman, G.H.; Lents, C.A. Energy Metabolism and Leptin: Effects on Neuroendocrine Regulation of Reproduction in the Gilt and Sow. Reprod. Domest. Anim. 2008, 43, 324–330, doi;101111/j1439. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C. Altering prolactin concentrations in sows. Domest. Anim. Endocrinol. 2016, 56, 155–164. [Google Scholar] [CrossRef]
- Britt, J.H. Improving sow productivity through management during gestation, lactation and after weaning. J. Anim. Sci. 1986, 63, 1288–1296. [Google Scholar] [CrossRef]
- Estienne, M.J.; Harper, A.F.; Barb, C.R.; Azain, M.J. Concentrations of leptin in serum and milk collected from lactating sows differing in body condition. Domest. Anim. Endocrinol. 2000, 19, 275–280. [Google Scholar] [CrossRef]
- Van den Brand, H.; Heetkamp, M.J.; Soede, N.M.; Schrama, J.W.; Kemp, B. Energy balance of lactating primiparous sows as affected by feeding level and dietary energy source. J. Anim. Sci. 2000, 78, 1520–1528. [Google Scholar] [CrossRef]
- Neill, C.; Williams, N. Milk production and nutritional requirements of modern sows—Casey. In Proceedings of the London Swine Conference 2010, London, ON, Canada, 31 March–1 April 2010; Available online: https://thepigsite.com/articles/milk-production-and-nutritional-requirements-of-modern-sows (accessed on 27 September 2020).
- Kim, S.W.; Hurley, W.L.; Wu, G.; Ji, F. Ideal amino acid balance for sows during gestation and lactation. J. Anim. Sci. 2009, 87, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesnel, H.; Meunier, S.M.C.; Hamard, A.; Guillemet, R.; Etienne, M.; Farmer, C.; Dourmad, J.Y.; Père, M.C. Dietary fiber for pregnant sows: Influence on sow physiology and performance during lactation. J. Anim. Sci. 2009, 87, 532–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillemet, R.; Guérin, C.; Richard, F.; Dourmad, J.Y.; Meunier-Salaün, M.C. Feed transition between gestation and lactation is exhibited earlier in sows fed a high-fiber diet during gestation. J. Anim. Sci. 2010, 88, 2637–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordaz, O.G.; Juárez, C.A.; Pérez, S.E.R.; Román, B.R.M.; Ortiz, R.R. Effect of spineless cactus intake (Opuntia ficus-indica) on blood glucose levels in lactating sows and its impact on feed intake, body weight loss, and weaning-estrus interval. Trop. Anim Health Prod. 2017, 49, 1025–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordaz, O.G.; Juárez, C.A.; Pérez, S.E.R.; Martínez, F.H.E.; Esquivel, C.J.; Ortiz, R.R. Effect of lactating sows’ diet supplemented with cactus (Opuntia ficus-indica) on feed intake and reproductive and productive post-weaning performances. Trop. Anim Health Prod. 2018, 80, 1671–1682. [Google Scholar] [CrossRef] [Green Version]
- Mallmann, L.; da Silva, O.G.; Zacarias, R.J.; Basquera, B.F.; Pereira, F.D.; Ghiggi, F.J.E.; Andretta, I.; da Rosa, U.R.; Gonçalves, M.A.P.; Pandolfo, B.F. Proposal of equations for predicting post-farrowing sow weight. Acta Sci. Vet. 2018, 46, 1574. [Google Scholar] [CrossRef]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Ordaz, G.; Juárez, A.; Pérez, R.E.; Martínez, H.E.; Ortiz, R. Effects of Opuntia ficus-indica in the diet of primiparous sows on the metabolic profile during late gestation and lactation and feed intake during lactation. J. Anim. Physiol. Anim. Nutrit. 2020, 104, 1–12. [Google Scholar] [CrossRef]
- Nelson, R.W.; Ihle, S.L.; Lewis, L.D.; Salisbury, S.K.; Miller, T.; Bergdall, V.; Bottoms, G.D. Effects of dietary fiber supplementation on glycemic control in dogs with alloxan-induced diabetes mellitus. Am. J. Vet. Res. 1991, 52, 2060–2066. [Google Scholar]
- El-Razek, A.H.F.; Hassan, A.A. Nutritional value and hypoglycemic effect of prickly cactus pear (Opuntia ficus-indica) fruit extract in alloxan-induced diabetic rats. Aust. J. Basic Appl. Sci. 2011, 5, 356–377. [Google Scholar]
- Mokua, P.M.; Mayunzu, O.; Obonyo, M.; Thuita, J.; Mutuku, J.; Rutto, J.; Murilla, G. The prickly pear cactus (Opuntia fiscus-indica) cladode extracts modulate blood sugar in swiss white albino mice. Int. J. Diabetes Res. 2016, 5, 41–47. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, A.; Sharma, R. Pharmacological actions of Opuntia ficus indica: A Review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Faure, P.; Rossini, E.; Wiernsperger, N.; Richard, M.; Favier, A.; Halimi, S. An insulin sensitizer improves the free radical defense system potential and insulin sensitivity in high fructose-fed rats. Diabetes 1999, 48, 353–357. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutrit. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Chiteva, R.; Wairagu, N. Chemical and nutritional content of Opuntia ficus-indica (L.). Afr. J. Biotechnol. 2013, 112, 3309–3312. [Google Scholar] [CrossRef]
- Contreras, M.P.; Rivera, E.M.M.; Cortez, E.G.; López, A.R.; Rodríguez, M.E.G. Characterization of crystalline structures in Opuntia ficus-indica. J. Biol. 2015, 41, 99–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stelwagen, K. Mammary Gland, Milk Biosynthesis and Secretion Lactose; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Uebelhack, R.; Busch, R.; Alt, F.; Beah, Z.M.; Chong, P.W. Effects of cactus fiber on the excretion of dietary fat in healthy subjects: A double blind, randomized, placebo-controlled, crossover clinical investigation. Curr. Ther. Res. 2014, 76, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burley, V.J.; Paul, A.W.; Blundell, J.E. Sustained post-ingestive action of dietary fibre: Effects of a sugar-beet-fibre-supplemented breakfast on satiety. J. Hum. Nutr. Diet. 1993, 6, 253–260. [Google Scholar] [CrossRef]
- Brockman, R. Effect of insulin on the utilization of propionate in gluconeogenesis in sheep. Br. J. Nutrit. 1990, 64, 95–101. [Google Scholar] [CrossRef]
- Lee, N.K.; Karsenty, G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol. Metab. 2009, 19, 161–166. [Google Scholar] [CrossRef]
- Rosen, C.J. Insulin-like growth factor I and bone mineral density: Experience from animal models and human observational studies. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.; Jung, Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, T.O.; Mackowiak, S.J.; Troiano, N.; Gundberg, C.M. Osteocalcin and its message: Relationship to bone histology in magnesium-deprived rats. Ame. J. Physiol. 1992, 263, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ordaz, O.G.; Juárez, C.A.; Portillo, M.L.; Pérez, S.R.E.; Ortiz, R.R. Productive evaluation and cost: Benefit analysis of lactating sows fed a diet containing nopal (Opuntia ficus-indica). Rev. Mex. Cienc. Pecu. 2019, 10, 1027–1041. [Google Scholar] [CrossRef]

| Item | Basal Diets | |
|---|---|---|
| Ingredients (g/kg) | Gestation | Lactation |
| Sorghum | 824.0 | 649.5 |
| Soy paste | 60.0 | 100.0 |
| Canola paste | 61.2 | 185.2 |
| Orthophosphate | 11.8 | 5.3 |
| Calcium carbonate | 14.0 | 12.4 |
| Soy oil | 22.0 | 38.5 |
| Lysine | 1.2 | 2.5 |
| Methionine + Cysteine | 0.9 | 1.5 |
| Salt | 3.0 | 3.0 |
| Vitamin and mineral premix a | 2.0 | 2.5 |
| Nutrient levels (%) | ||
| Metabolizable energy (MJ/kg) | 13.6 | 13.9 |
| Crude protein | 15.5 | 18.6 |
| Lysine | 0.79 | 0.95 |
| Methionine + cysteine | 0.43 | 0.59 |
| Calcium | 1.4 | 1.2 |
| Total phosphorus | 0.64 | 0.67 |
| Available phosphorus | 0.33 | 0.46 |
| Nutrient levels of Opuntia ficus-indica (%) | ||
| Crude protein | 5.6 | |
| Crude fat | 0.2 | |
| Fiber | 28.8 | |
| Humidity | 88.6 | |
| Ashes | 24.5 | |
| Nitrogen-free elements | 40.8 | |
| Mucilage (g/300 g dry base) | 2.6 | |
| Item | Feed Intake | SEM | p-Value & | ||||
|---|---|---|---|---|---|---|---|
| GNC: 0% Cactus | G1C: 1% Cactus | G2C: 1.5% Cactus | G3C: 2% Cactus | L | Q | ||
| Gestation | |||||||
| Day 85 to 115 | 2.24 | 2.22 | 2.23 | 2.24 | 0.05 | 0.8641 | 0.6518 |
| Lactation | |||||||
| Day 1 to 7 | 3.05 | 3.12 | 3.54 | 3.40 | 0.10 | 0.0015 | 0.2975 |
| Day 8 to 14 | 3.46 | 5.08 | 4.38 | 3.60 | 0.10 | 0.5622 | <0.0001 |
| Day 15 to 21 | 3.32 | 5.41 | 5.21 | 4.31 | 0.10 | <0.0001 | <0.0001 |
| Day 1 to 21 | 3.29 | 4.59 | 4.38 | 3.76 | 0.07 | <0.0001 | 0.0461 |
| Cactus Consumption | |||||||
| Gestation | |||||||
| Day 85 to 115 | -- | 1.12 | 1.21 | 1.41 | 0.04 | 0.0083 | 0.2318 |
| Lactation | |||||||
| Day 1 to 7 | -- | 1.60 | 1.55 | 2.38 | 0.07 | 0.0021 | 0.1195 |
| Day 8 to 14 | -- | 1.71 | 1.57 | 1.97 | 0.06 | 0.0715 | 0.0172 |
| Day 15 to 21 | -- | 1.74 | 1.65 | 2.23 | 0.06 | 0.0109 | 0.0037 |
| Day 1 to 21 | -- | 1.69 | 1.59 | 2.19 | 0.03 | <0.0001 | <0.0001 |
| Item | Day (D) | Groups (G) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| GNC: 0% Cactus | G1C: 1% Cactus | G2C: 1.5% Cactus | G3C: 2% Cactus | G | D | G * D | |||
| Gilts BW (kg) | |||||||||
| Day 85 of gestation | 179.71 | 185.53 | 181.42 | 180.91 | 0.34 | 0.0836 | -- | -- | |
| Day 110 of gestation | 191.32 | 196.38 | 190.33 | 190.72 | 0.34 | 0.0609 | -- | -- | |
| Post-farrowing & | 174.52 | 178.78 | 173.20 | 173.81 | 0.34 | 0.0813 | -- | -- | |
| Weaning | 164.22 a | 176.91 b | 171.11 c | 169.25 c | 0.34 | <0.0001 | -- | -- | |
| Gilt LBW (%) | 5.90 a | 1.05 b | 1.21 b | 2.62 c | 0.15 | 0.0415 | -- | -- | |
| Milk quality (%) | |||||||||
| 3 | 12.5 a | 11.6 a | 9.3 b | 9.0 b | 0.39 | ||||
| Fat | 11 | 6.6 a | 6.1 a | 6.2 a | 6.0 a | 0.39 | 0.0419 | <0.0001 | <0.0001 |
| 17 | 5.8 a | 5.0 b | 5.1 b | 4.7 c | 0.39 | ||||
| 3 | 7.1 a | 7.3 a | 6.1 b | 5.7 b | 0.39 | ||||
| Lactose | 11 | 6.4 a | 6.2 ab | 5.8 b | 5.6 b | 0.39 | 0.0218 | <0.0001 | <0.0001 |
| 17 | 6.3 a | 6.2 a | 5.9 b | 5.8 b | 0.39 | ||||
| 3 | 6.1 a | 6.1 a | 4.6 b | 4.5 b | 0.39 | ||||
| Protein | 11 | 5.3 a | 5.9 a | 5.5 a | 5.0 a | 0.39 | 0.0346 | <0.0001 | <0.0001 |
| 17 | 5.4 a | 5.7 a | 5.6 a | 4.6 b | 0.39 | ||||
| Piglet weight at birth (kg) | 1.6 | 1.5 | 1.5 | 1.5 | 0.12 | 0.8207 | -- | -- | |
| Piglet weight at weaning (kg) | 5.9 a | 5.7 a | 5.5 a | 4.8 b | 0.15 | 0.0432 | -- | -- | |
| Weaned piglets | 9.6 | 9.5 | 9.7 | 9.0 | 0.13 | 0.9741 | -- | -- | |
| Principal Component | ||
|---|---|---|
| 1 | 2 | |
| Variance explained | ||
| Eigenvalue | 1.842 | 1.767 |
| Variance (%) | 36.850 | 35.342 |
| Cumulative (%) | 36.850 | 72.192 |
| Factor loadings | ||
| Glucose | 0.614 | 0.050 |
| Total cholesterol | 0.925 | −0.217 |
| Triglycerides | 0.819 | −0.222 |
| Insulin | −0.197 | 0.755 |
| Osteocalcin | −0.011 | 0.997 |
| Model | Non-Standardized Coefficients | Standardized Coefficients | t | p-Value | Confidence Interval for β (95.0%) | ||
|---|---|---|---|---|---|---|---|
| Β | SD | β | LL | UL | |||
| Constant | 2.711 | 0.223 | 12.164 | <0.0001 | 2.267 | 3.155 | |
| Day | 0.099 | 0.019 | 0.495 | 5.203 | <0.0001 | 0.061 | 0.137 |
| PC 1 | −0.332 | 0.141 | −0.222 | −2.343 | 0.022 | −0.613 | −0.050 |
| PC 2 | −0.151 | 0.139 | −0.102 | −1.092 | 0.278 | −0.427 | 0.125 |
| Item & | Regression Estimators * | Critical Point | R2 | |||||
|---|---|---|---|---|---|---|---|---|
| CL (%) | IV | |||||||
| Intercept | β1 | β2 | β3 | |||||
| Feed intake (kg) | 3.13 (<0.0001) | 2.36 (<0.0001) | −1.28 (<0.0001) | -- -- | 0.92 | 4.20 | 0.26 | |
| PC 1 | Glucose (mg/dL) | 93.97 (<0.0001) | −22.32 (<0.0001) | 8.62 (0.0040) | -- -- | 1.23 | 79.32 | 0.23 |
| Triglycerides (mg/dL) | 53.13 (<0.0001) | −47.58 (0.0010) | 70.46 (0.0018) | −31.40 (0.0011) | 0.98 | 44.61 | 0.31 | |
| Cholesterol (mg/dL) | 81.92 (<0.0001) | −85.87 (<0.0001) | 122.40 (<0.0001) | −52.43 (<0.0001) | 1.02 | 66.03 | 0.28 | |
| PC 2 | Insulin (µUI/mL) | 13.87 (<0.0001) | 20.78 (<0.0001) | −9.69 (<0.0001) | -- -- | 1.07 | 25.05 | 0.39 |
| Osteocalcin (ng/mL) | 105.39 (<0.0001) | 33.88 (0.0079) | −16.43 (0.0554) | -- -- | 1.03 | 122.85 | 0.21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, R.; López, M.; Pérez, R.E.; Ramírez, P.d.l.P.; Ordaz, G. Effect of the Inclusion of Different Levels of Dietary Cactus (Opuntia ficus-indica) on Gilts’ Biochemical Parameters and Feed Intake during Lactation. Animals 2020, 10, 1881. https://doi.org/10.3390/ani10101881
Ortiz R, López M, Pérez RE, Ramírez PdlP, Ordaz G. Effect of the Inclusion of Different Levels of Dietary Cactus (Opuntia ficus-indica) on Gilts’ Biochemical Parameters and Feed Intake during Lactation. Animals. 2020; 10(10):1881. https://doi.org/10.3390/ani10101881
Chicago/Turabian StyleOrtiz, Ruy, Manuel López, Rosa E. Pérez, Paola de la Paz Ramírez, and Gerardo Ordaz. 2020. "Effect of the Inclusion of Different Levels of Dietary Cactus (Opuntia ficus-indica) on Gilts’ Biochemical Parameters and Feed Intake during Lactation" Animals 10, no. 10: 1881. https://doi.org/10.3390/ani10101881







