3.2. Microbial Responses to Variable Temperature
Mineral sulphides are known to oxidise exothermally, mainly due to the contents of pyrite (FeS
2) or pyrrhotite (Fe
1–xS, where
x = 0–0.17). Heat generation is an important parameter in managed sulphide heaps, because some sulphide minerals exhibit strongly increased dissolution kinetics with increased temperature, thus enhancing metal productivity. For example, the secondary copper sulphide chalcocite (Cu
2S) is oxidised by ferric ions in two stages (Equations (6) and (7)), the first being a rapid reaction that delivers approximately half of the copper to solution. The subsequent much slower oxidation of the “CuS” secondary product (
blaubleibender; Equation (7)) is strongly temperature dependent; for every ten-degree rise in temperature, the intrinsic rate of “CuS” oxidation increases three-fold [
25]. The oxidation of chalcopyrite (CuFeS
2) is similarly temperature dependent, exhibiting a five-fold increase in copper extraction at 65 °C compared with 35 °C.
Heat generation in sulphide heaps impacts the microbial communities that colonise the ores and catalyse the oxidation reactions. It is well known that different bacterial species have defined temperature ranges (“operating windows”) within which they grow well and are active. The ferric-ion generation “doubling times” for “
Ab. cupritolerans”,
S. thermosulfidooxidans and
At. ferrooxidans grown on ferrous ion and the sulphate-ion “doubling times” for
At. caldus grown on tetrathionate ion obtained for a range of temperatures were modelled using the Ratkowsky Equation (8),
where
b is the regression coefficient of the square root of growth rate constant versus degrees Kelvin for temperatures below the optimal temperature, and
c is an additional parameter to enable the model to fit the data for temperatures above the optimal temperature [
21] The Ratkowsky plots (
Figure 6) generate extrapolated values for the minimum (
TMIN), optimum (
TOPT) and maximum (
TMAX) temperatures for the activities of the four species (
Table 2)
The data shown for
S. thermosulfidooxidans are consistent with those reported by Golovacheva and Karavaiko [
11], where it was noted that at the temperature limits of approximately 20 °C or 60 °C, the species was practically inactive.
Figure 6.
Ratkowsky plots showing the relationships between temperature and ferric-ion generation (“Ab. cupritolerans”, S. thermosulfidooxidans and At. ferrooxidans) or sulphate generation (At. caldus) under the experimental conditions used in this study. SQRT = square root.
Figure 6.
Ratkowsky plots showing the relationships between temperature and ferric-ion generation (“Ab. cupritolerans”, S. thermosulfidooxidans and At. ferrooxidans) or sulphate generation (At. caldus) under the experimental conditions used in this study. SQRT = square root.
Table 2.
Extrapolated values of the cardinal temperatures derived from the Ratkowsky Equation [
21] for the activity of four bioleaching microorganisms used in this study.
Table 2.
Extrapolated values of the cardinal temperatures derived from the Ratkowsky Equation [21] for the activity of four bioleaching microorganisms used in this study.
| Microorganisms | Substrate | Extrapolated Values | Fitting Parameters |
|---|
| TMIN | TOPT | TMAX | “b” | “c” |
|---|
| “Ab. cupritolerans” | Fe2+ | 18.7 | 36.4 | 45.6 | 0.05924 | 0.0946 |
| S. thermosulfidooxidans | Fe2+ | 26.2 | 50.7 | 59.0 | 0.02024 | 0.2243 |
| At. caldus | S4O62− | 17.3 | 44.7 | 51.4 | 0.02127 | 0.3522 |
| At. ferrooxidans | Fe2+ | 13.0 | 30.0 | 45.5 | 0.01633 | 0.0110 |
In a heap colonised with these four species, metal extraction from sulphide minerals could be enhanced compared with chemical leaching in the temperature range 15–60 °C. Franzmann
et al. [
16] noted that, in a typical sulphide heap leach operation subject to increasing temperature, a succession of active mesophilic, moderately thermophilic and thermophilic microorganisms would be expected to contribute to the extraction of metals from sulphide ores in an environment.
An important aspect of heat generation in heaps is the speed with which heap temperatures increase. Readett
et al. [
3] operated an aerated, irrigated test heap equipped with temperature probes, constructed using siliceous shale ore containing copper oxide and chalcocite. For this heap, heat generation commenced during the curing period and rose rapidly from about 25–30 °C to 70 °C in the first two weeks when aeration, but not irrigation, was applied. In the absence of aeration (Days 19–25), heap temperatures dropped temporarily. Other reported examples of heat generation in commercially-operated heaps include:
A full scale heap of ROM ore, averaging 0.2%–0.3% Cu mainly as CuFeS
2, in which exhaust gases from monitored holes were typically 30 degrees Celsius above ambient temperatures, the maximum temperature (66 °C) occurring between a 6–12-m depth [
26].
An irrigated heap of pyrrhotite-rich copper-nickel sulphide ore, in which temperatures increased to 80 °C within a few days of the commencement of aeration [
27,
28].
Non-aerated chalcocite heaps in which internal temperatures up to 46 °C were measured [
5].
Aerated biooxidation heaps of pyritic gold ore in which temperatures ranged from 25 to 80 °C and into which iron-oxidising thermophiles (archaea) were introduced to facilitate biooxidation at temperatures above those suited to bacteria [
23].
Initially, ore parameters, such as ore grade, mineral sulphide reactivity and surface exposure, and bed permeability for both solution and air flows are at their maximum. Not surprisingly, therefore, heap temperatures may pass rapidly through the microbial “operating window” and exceed the temperature at which bacteria are active.
Based on the above examples, the effect of short-duration exposure to above-optimum temperature on bacterial activity was tested for Fe(II)-oxidising species. Initially, these “heat-stress” tests were conducted at 10 degrees above the
TOPT of the test species. Subsequently, Fe(II)-oxidising activity at
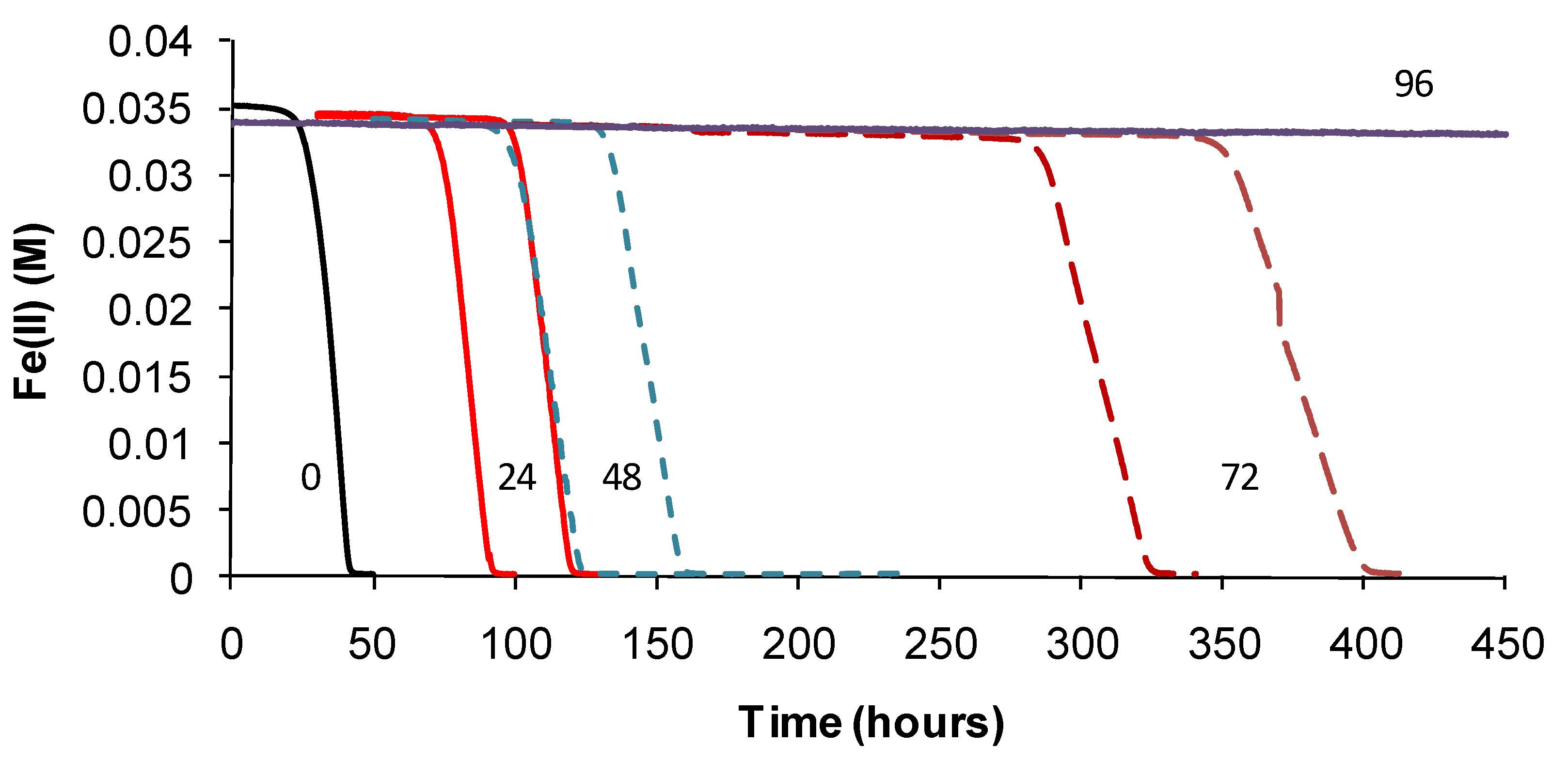
TOPT was monitored using the methods described. Periodic examinations of cells were conducted after heat treatment and during monitoring. Example Fe(II)-oxidation data are shown for “
Ab. cupritolerans” (
Figure 7).
The data showed that the primary effect on bacterial activity was to prolong the lag time before Fe(II) oxidation commenced once optimal conditions were restored, from approximately 25 h with no heat treatment to 330 h after 72 h of heat treatment. The recovery time for a 96-h treatment 10 degrees Celsius higher than the optimum temperature for growth was more than three weeks. Estimated mean Fe(III)-generation doubling times increased from 4 h (no heat treatment) to 9 h for the heat-stress tests of a 72-h duration. While cell numbers were not estimated in these tests, periodic examination of cells showed that neither the heat treatment nor the subsequent “optimum” conditions promoted spore formation. Note that Fe(II) oxidation did not commence within three weeks for heat-stress tests of a duration of 96–168 hours, for this species.
Figure 7.
Example data for duplicate heat-stress tests using “Ab. cupritolerans” grown in Fe(II) growth medium (pH 1.8). The notations indicate the duration of heat-stress treatments (hours) conducted at 46 °C preceding transfer to a 35 °C incubator.
Figure 7.
Example data for duplicate heat-stress tests using “Ab. cupritolerans” grown in Fe(II) growth medium (pH 1.8). The notations indicate the duration of heat-stress treatments (hours) conducted at 46 °C preceding transfer to a 35 °C incubator.
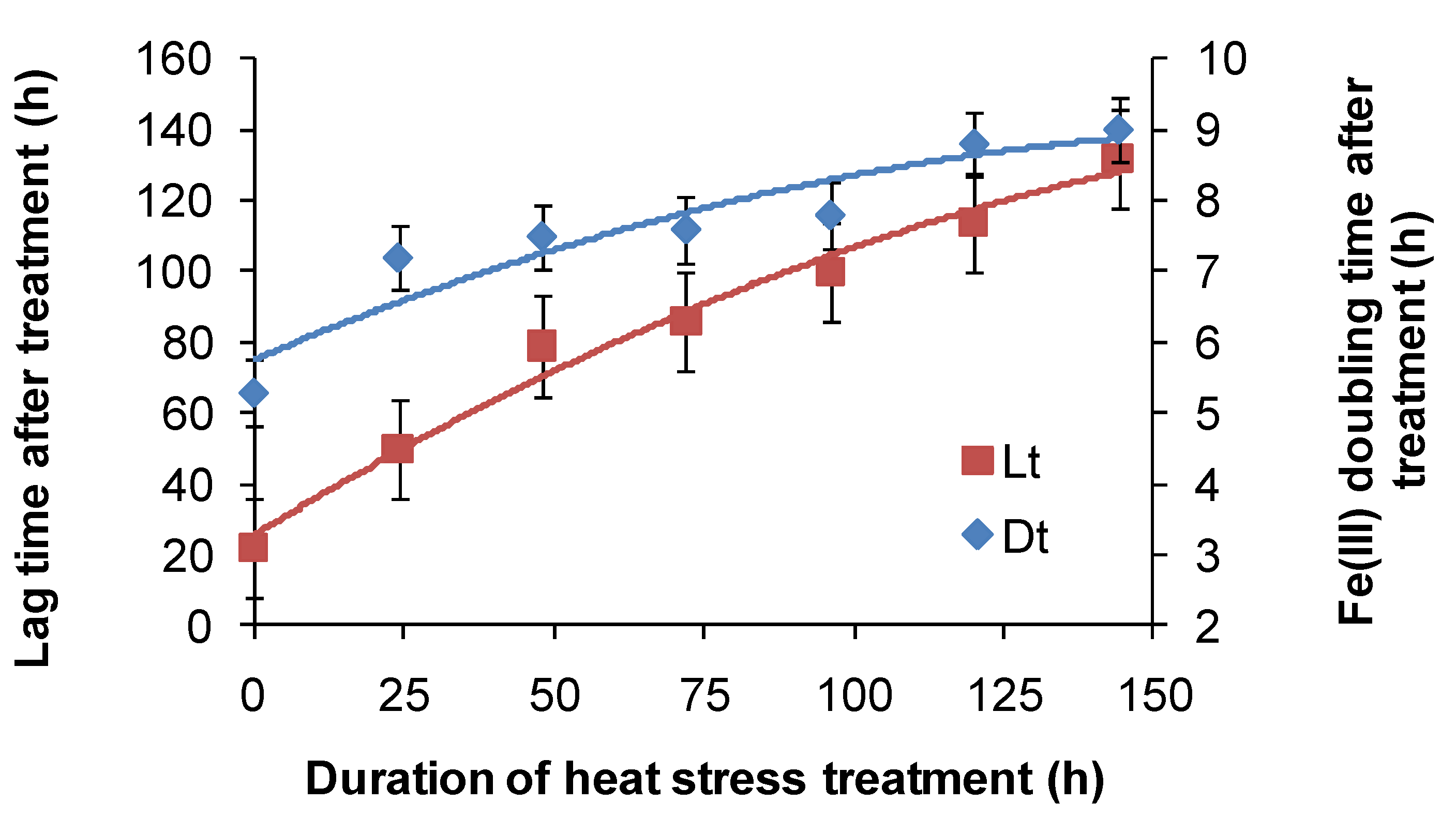
For
At. ferrooxidans, not a spore forming species, Fe(II)-oxidation heat-stress treatments at 10 degrees above
TOPT for up to 144 h generated similar data. Both lag times and Fe(III) doubling times increased with increased duration of heat-stress treatment, lag times from 20 to 130 h and Fe(III) doubling times from 5 to 9 h (
Figure 8). Estimates of final cell numbers for those tests where Fe(II) oxidation occurred were in the range 2 ± 2 × 10
7–7 ± 1 × 10
7, but estimate accuracy was compromised by the formation of insoluble Fe(III) hydroxy compounds (see
Figure 4). No Fe(II) oxidation occurred after 168 h of treatment within three weeks. Therefore, it is concluded that cell growth was the consequence of Fe(II) oxidation, and the prolonged lag times estimated from these tests were attributed to a large reduction in the number of active cells (see
Figure 2), in this case caused by the heat treatment.
Figure 8.
Variability in lag times (Lt) and Fe3+ generation doubling times (Dt) induced by short-duration heat stress (pH 1.8, 42 °C) for At. ferrooxidans after restoration of optimal conditions (pH 1.8, 32 °C).
Figure 8.
Variability in lag times (Lt) and Fe3+ generation doubling times (Dt) induced by short-duration heat stress (pH 1.8, 42 °C) for At. ferrooxidans after restoration of optimal conditions (pH 1.8, 32 °C).
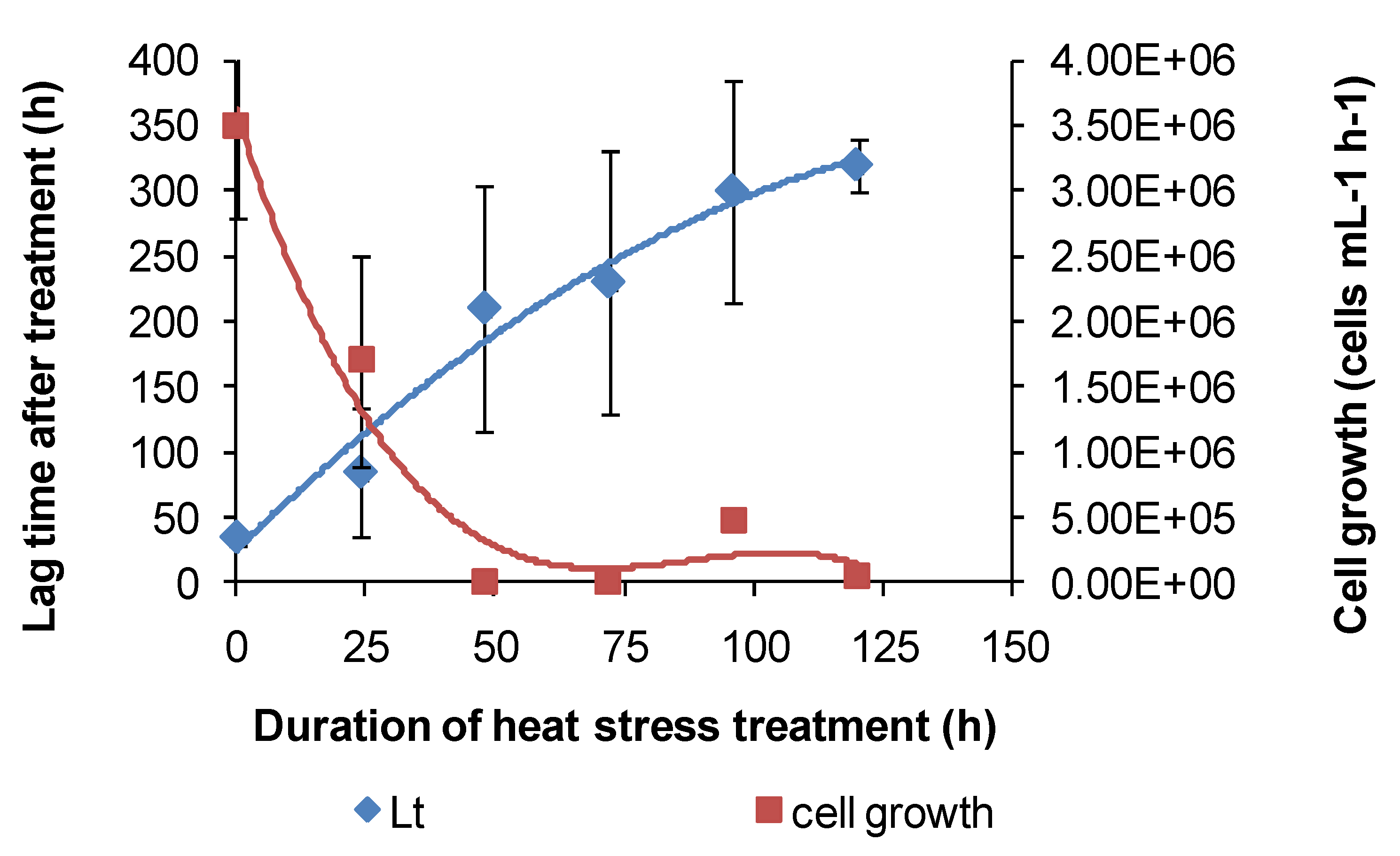
Similar heat-stress tests were conducted using
At. caldus grown on tetrathionate, using acid production as a guide to microbial activity. The results indicated that
At. caldus would be particularly sensitive to increased temperatures in heaps of sulphide ores. Individual lag times were extremely variable, but overall, the trend was towards increased lag times with increased periods of heat stress (
Figure 9). Likewise, cellular growth at 45 °C, following short-term heat treatments at 55 °C, was erratic between treatments and among replicates of the same treatment.
Figure 9.
Variability in lag times (Lt) and cell growth rates of At. caldus after heat-stress treatments; the reduction in pH was used as an indicator of tetrathionate utilisation.
Figure 9.
Variability in lag times (Lt) and cell growth rates of At. caldus after heat-stress treatments; the reduction in pH was used as an indicator of tetrathionate utilisation.
Some larger-scale studies in heaps or unsaturated columns (simulating heap conditions) have been undertaken to examine the effect of increased temperature and heat stress on microbial populations. Brierley [
29] inoculated a variable-temperature column charged with pyritic gold ore with a mixed consortium of mesophilic and moderately thermophilic bacteria and hyper-thermophilic archaea. Brierley [
29] reported that the hyper-thermophiles did not increase in numbers until the column was heated to > 50 °C, but that the moderate thermophiles, such as
S. thermosulfidooxidans, were detected in all columns up to 60 °C. In isothermal columns charged with low-grade CuFeS
2 ore and operated at different temperatures up to 60 °C, Mutch
et al. [
30] detected
At. caldus and a
Sulfobacillus strain at temperatures up to 50 °C, but surprisingly, the hyper-thermophiles included in the inoculum did not colonise even the 60 °C column. In both of those studies, the presence of mesophilic or moderately thermophilic bacteria in leachates of high-temperature columns was attributed to solution recycling through ambient-temperature reservoirs more amenable to their growth. Halinen
et al. [
31] inoculated isothermal columns containing polymetallic black schist ore with a mixed-microbial consortium enriched from mine water. In the 50 °C column, the bacteria detected in leachate or leach residue included
At. ferrooxidans and
At. caldus, both prevalent initially during leaching, when the sulphide content of the ore was at its greatest, and a
Sulfobacillus species that was detected after 200 days of leaching and prevailed thereafter.
A possible effect of self-heating in heaps is dehydration of microbial cells. Under dehydrating conditions, the ability to form spores should confer a significant advantage. In parallel with the heat-stress tests,
At. ferrooxidans and
At. caldus dehydrated and stored for up to six weeks were grown successfully when rehydrated in mixed Fe(II)-tetrathionate growth media or with chalcopyrite concentrate, demonstrating a natural resilience to the short-term effects of dehydration. Similarly, heat-dried cells of
S. thermosulfidooxidans attached to CuFeS
2 were revived successfully after four months, with high growth (>5 × 10
7 cells·mL
−1) a week after transfer to mixed Fe(II)-tetrathionate growth media. However, isolates obtained from dry samples from a “hot” heap after 2–3 years without irrigation were limited to strains closely related to
S. thermosulfidooxidans and
At. caldus, and the bacteria “revived” from heap samples after four years were all strains of
S. thermosulfidooxidans, indicative of their superior ability to survive for long periods in inhospitable hot and/or dry conditions [
17]. The results are consistent with the report that spore formation in
S. thermosulfidooxidans was strongest when cultures were grown on sulphide minerals and that cultures could survive heat treatments at 100–110 °C [
11].
On the basis of these studies, it is concluded that the self-heating of sulphide heaps poses a risk to successful microbial colonisation. Short-term heat-stress treatments at temperatures 10 degrees Celsius above the optimum temperatures for the growth of particular microbial strains caused prolonged periods of inactivity, and recovery from week-long heat treatments was extremely poor. The large masses of ores in bioleaching heaps mean that high temperatures arising from sulphide oxidation are hard to control initially, while the sulphide content of the ore is at its greatest. During that period, the numbers of mesophilic and moderately thermophilic bacteria will be markedly reduced in both numbers and activity. Recovery of a microbial population from prolonged heat stress will require re-inoculation via cells in the cooler process water being fed to the heap surface and, with prolonged detrimental hot, dry conditions, revival from microbial spores within the ore bed.
3.3. Microbial Responses to Variable Process Water Acidity (pH)
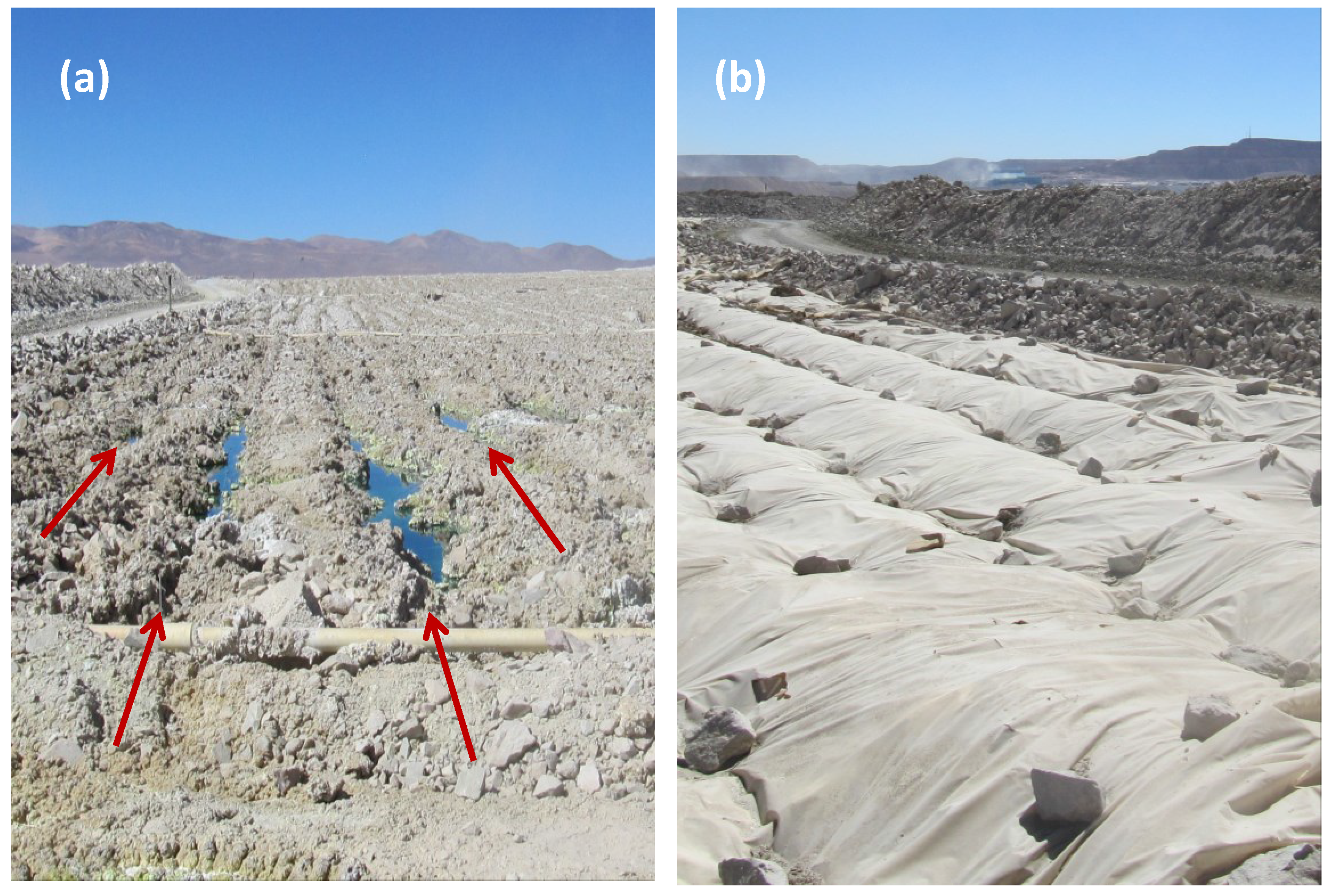
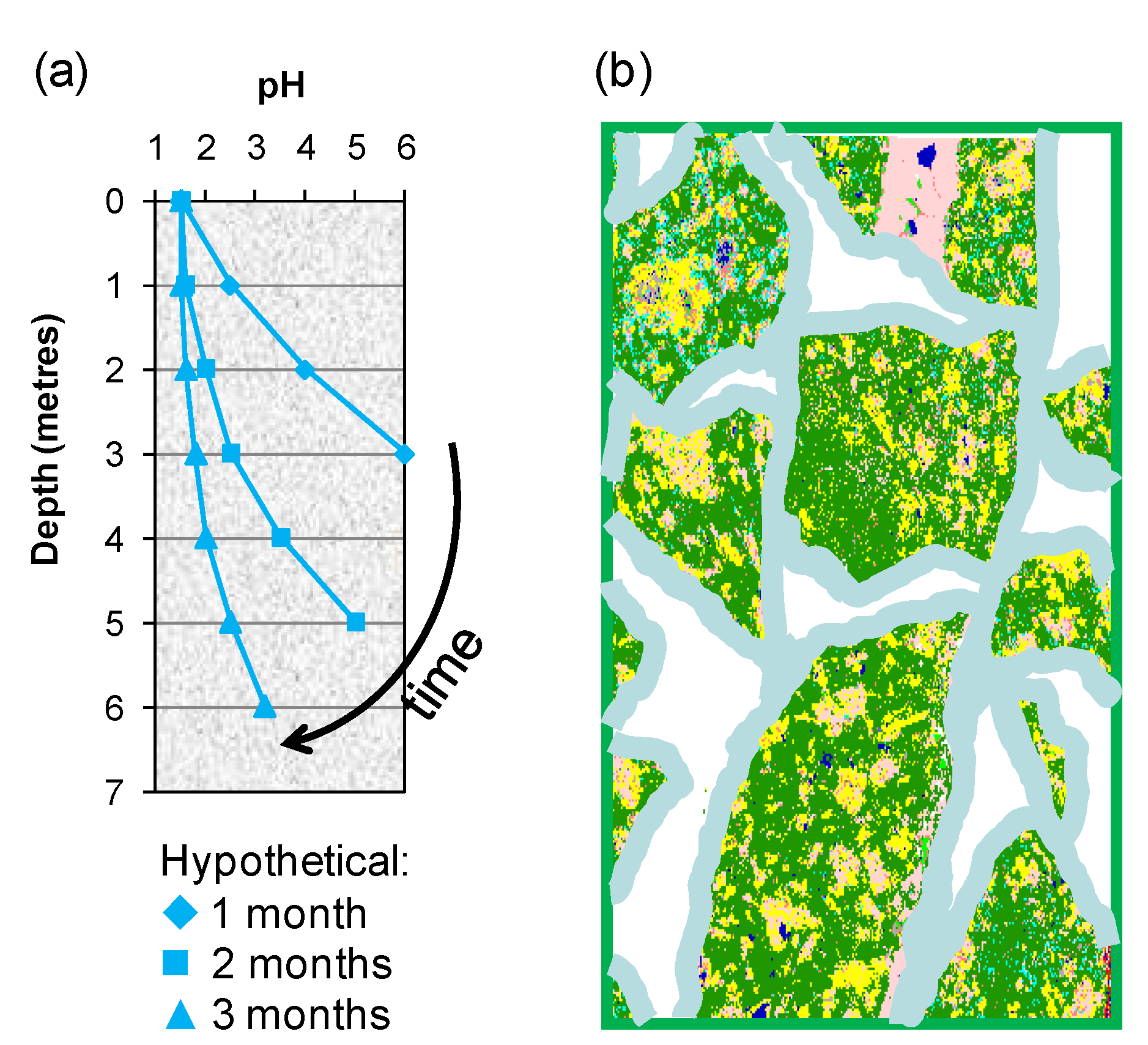
Two of the means by which variable process water acidity may arise in heaps are: (i) gangue-mineral acid consumption as the solution percolates through the bed from top to bottom, a bulk effect in which an “acid front” eventually reaches the base of the heap (
Figure 10a); and (ii) individual solution contact with particle surfaces that creates micro-environments differing in acidity from the bulk solution in the unsaturated bed (
Figure 10b).
It is thought that the population of acidophilic sulphur and Fe(II)-oxidisers will migrate through the ore bed with the acid front. Bacterial oxidation of Fe(II) requires a pH < 2, because in more alkaline solutions, the rate of chemical oxidation of Fe(II) to Fe(III) increases significantly and competes with biological Fe(II) oxidation. The Fe(III) generated forms insoluble compounds, thus removing the growth substrate from the solution. Halinen
et al. [
32] operated unsaturated pH-controlled columns of polymetallic black schist ore in the range pH 1.5–3.0 and concluded that pH 2 was optimum for good metal extraction and would minimise excess acid consumption due to gangue mineral dissolution. Tupikina
et al. [
33] reported that irrigation of unsaturated columns with a solution pH 1.7 or higher resulted in loss of the soluble iron, but with a solution feed of pH 1.4, there was a net increase in soluble iron.
Figure 10.
Schematic illustrating (a) the acid front moving through a porous ore bed and (b) polymineralic particles covered with a thin film of moisture within which chemical and microbial reactions take place.
Figure 10.
Schematic illustrating (a) the acid front moving through a porous ore bed and (b) polymineralic particles covered with a thin film of moisture within which chemical and microbial reactions take place.
It is not surprising, therefore, that Fe(II) oxidation by different bacteria occurs over a limited pH range (approximately 0.5–2.5) [
32], outside which those bacteria only able to oxidise Fe(II) will become inactive. The dual ability of
At. ferrooxidans to grow on Fe(II) and/or RISC is well established. The reported pH range for growth is pH 0.8–6 with an optimum of pH 1.8–2 and probably represents the range for RISC oxidation [
33]; the reported range for Fe(II) oxidation of pH 1.3–4.5 (optimum pH 2.5) [
34] would be expected to be somewhat narrower, but may have included a contribution from chemical Fe(II) oxidation at the high end of the range.
Under the conditions used in the present study, “
Ab. cupritolerans” oxidised Fe(II) in the range of pH 1–2.5 (
Figure 11) and
S. thermosulfidooxidans oxidised Fe(II) in the range of 1.2–2.2 (
Figure 12). Microbial Fe(II)-oxidation rates declined rapidly outside those limits. The data obtained for Fe(II) oxidation for
S. thermosulfidooxidans were consistent with those reported by Plumb
et al. [
34], with maximum growth at pH 1.5.
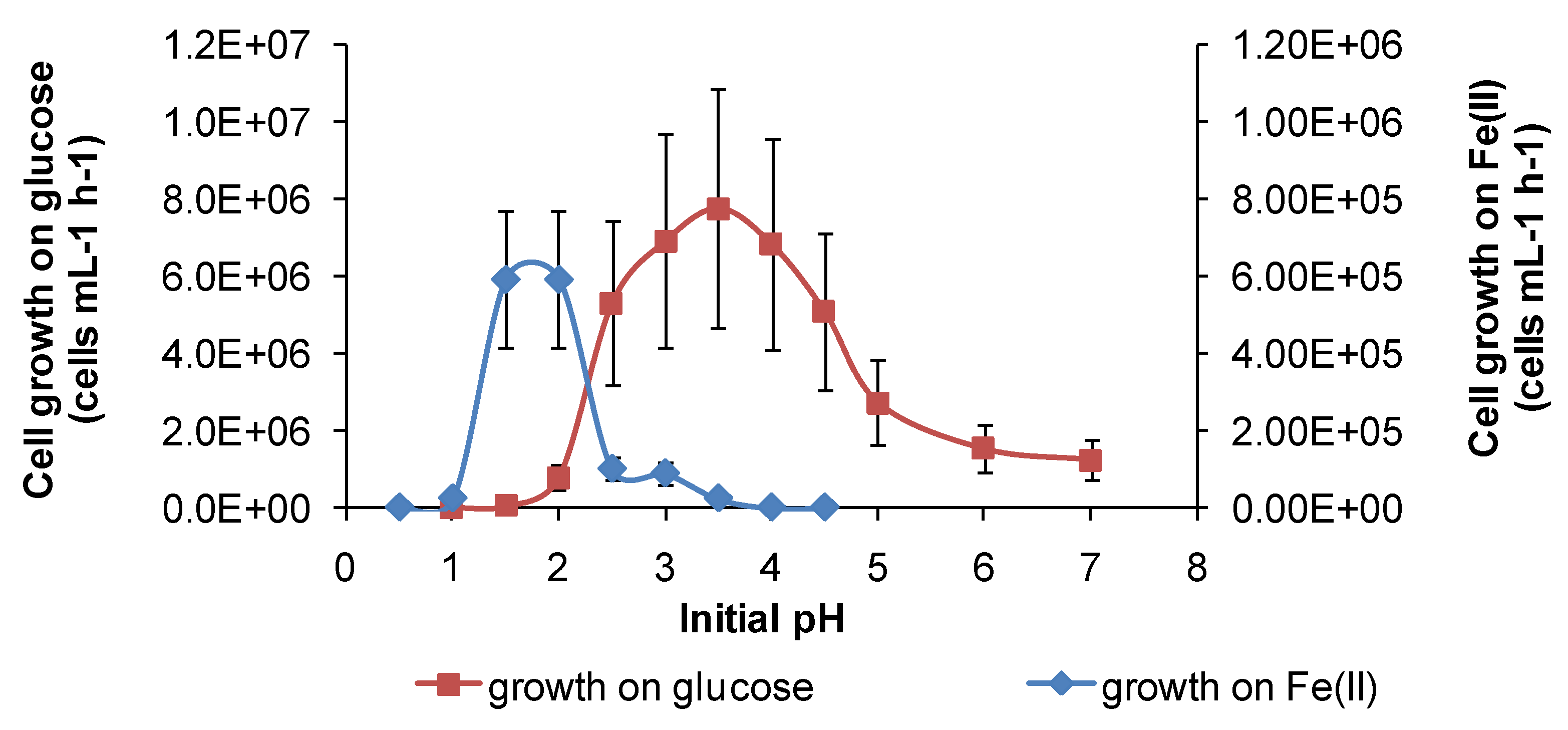
Figure 11.
Growth rates of “Ab. cupritolerans” in media of different initial pH, with Fe(II) (30 °C, 168 h, n = 5) or glucose (35 °C, 72 h, n = 3) as substrate. Initially, 106 cells·mL−1.
Figure 11.
Growth rates of “Ab. cupritolerans” in media of different initial pH, with Fe(II) (30 °C, 168 h, n = 5) or glucose (35 °C, 72 h, n = 3) as substrate. Initially, 106 cells·mL−1.
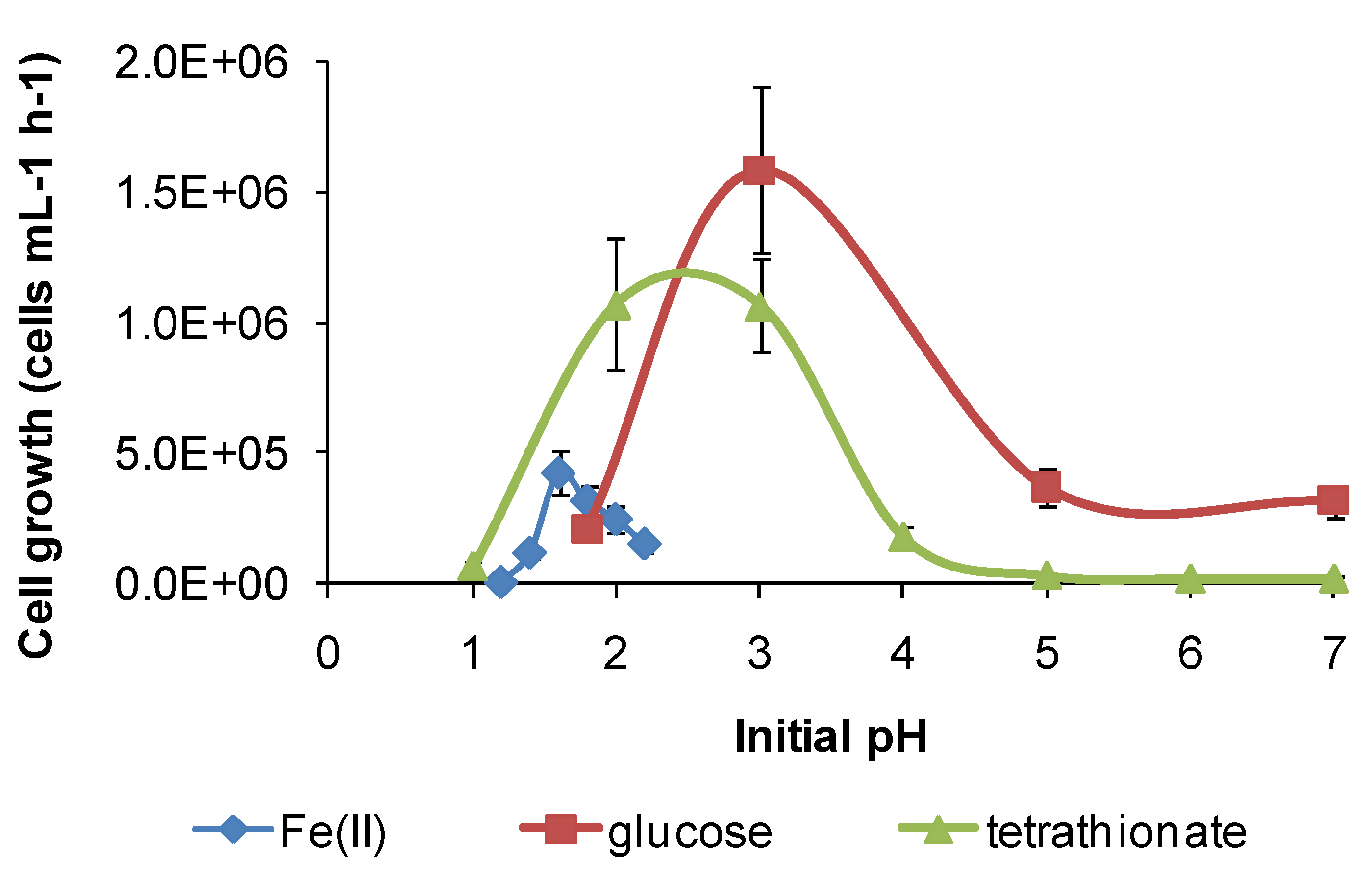
Figure 12.
Growth rates (cells·mL−1·h−1) of S. thermosulfidooxidans in media containing iron(II), glucose or tetrathionate as substrate at 50 °C with different initial pH. Initial cell concentration, 106 cells·mL−1. Duration of test, 168 h.
Figure 12.
Growth rates (cells·mL−1·h−1) of S. thermosulfidooxidans in media containing iron(II), glucose or tetrathionate as substrate at 50 °C with different initial pH. Initial cell concentration, 106 cells·mL−1. Duration of test, 168 h.
However, for both species, there was a broader pH window for growth when organic substrates were metabolised, for example glucose (
Figure 11 and
Figure 12), ensuring bacterial survival at solution pH where soluble Fe(II) is not available. The ability of
S. thermosulfidooxidans to grow on organic compounds after a short period of adjustment was reported, as was the enhanced leaching of sulphide ores in the presence of added glucose [
11], but the broadening of the pH window for growth (
Figure 12) was not previously noted. In addition, for
S. thermosulfidooxidans, RISC oxidation occurs in solutions of pH 1.2–3.6, providing a third metabolic strategy for survival in variable heap environments. The growth of
S. thermosulfidooxidans on sulphur in controlled pH experiments was reported to occur in the range of pH 2–4.5 [
11].
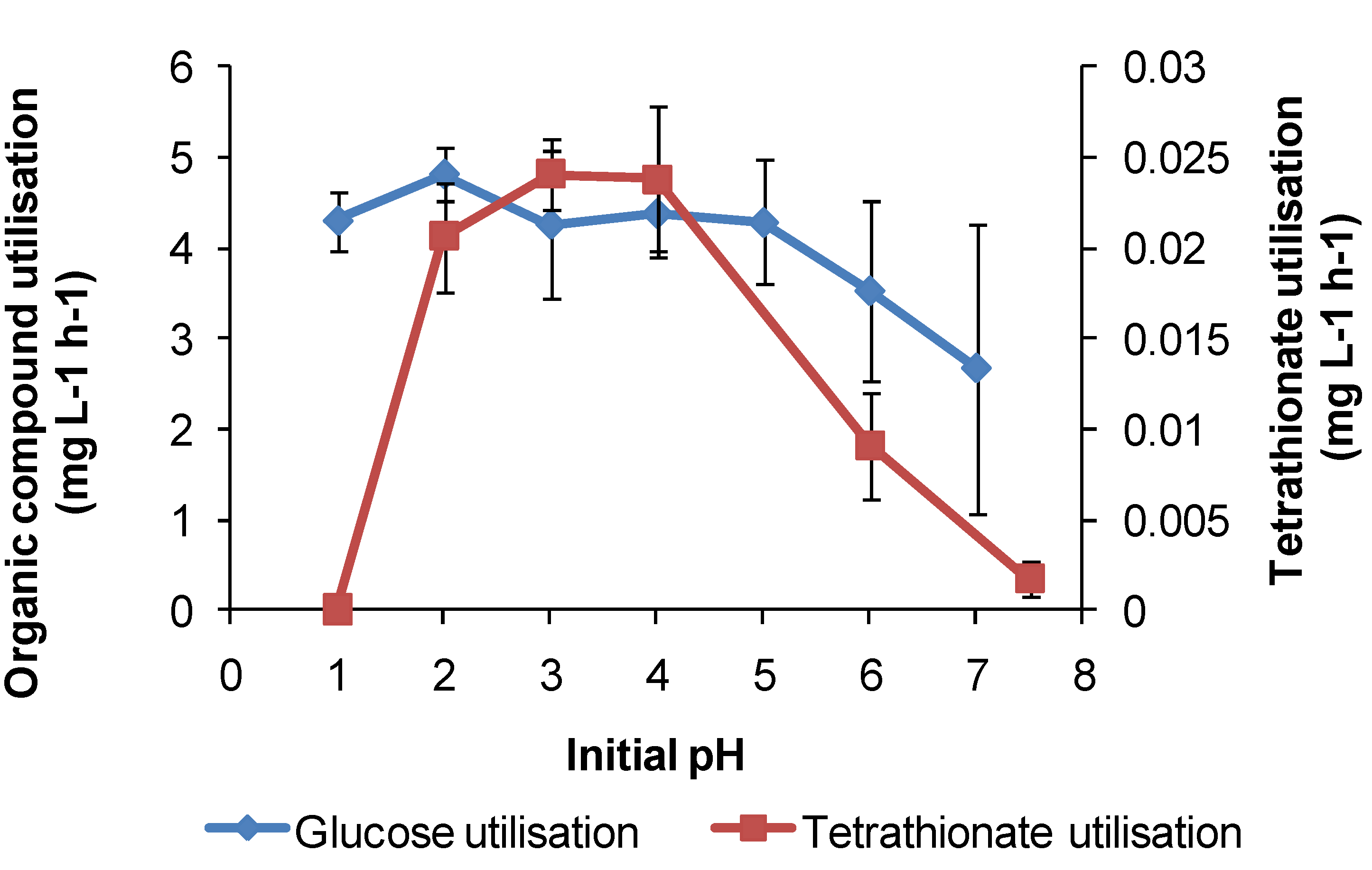
For
At. caldus in tetrathionate medium, cell growth occurred in the range of pH 1.5–5, with a maximum cell generation rate of 4 × 10
6 cells·mL
−1·h
−1 at pH 3. Tetrathionate utilisation occurred in the range of pH 1.5–6 with a maximum generation rate in the range of pH 3–4 (
Figure 13). The maximum growth and tetrathionate utilisation measured in this study at pH 3 was slightly higher than the optimum pH 2–2.5 reported previously [
12] or the optimum pH 2 for growth on elemental sulphur [
34].
At. caldus also grew on glucose (with 0.1 mM tetrathionate) across pH 1–7 at 45 °C, with the optimum at approximately pH 2, again demonstrating a possible alternative metabolism if RISC were absent in a heap. The requirement for RISC to be present for growth on glucose by
At. caldus was reported by Hallberg and Lindström [
12].
The results of some larger-scale studies on microbial stress induced by acidity have been published, but these tests, inoculated with mixed microbial cultures, were directed towards increased acidity in the region of pH 1 or lower (e.g., [
32,
35]), rather than under conditions of gangue mineral acid consumption and solution pH rising above pH 2. The results shown in
Figure 11,
Figure 12 and
Figure 13 are consistent with microbial activity being much reduced when the solution conditions approach pH 1 or less, as reported in the cited studies.
Figure 13.
Utilisation rates (mg·L−1·h−1) for At. caldus growth on tetrathionate or glucose as substrate at 45 °C in media with different initial pH. Initial cell concentration, 2 × 107 cells·mL−1; maximum duration of tests, 168 h (n = 3).
Figure 13.
Utilisation rates (mg·L−1·h−1) for At. caldus growth on tetrathionate or glucose as substrate at 45 °C in media with different initial pH. Initial cell concentration, 2 × 107 cells·mL−1; maximum duration of tests, 168 h (n = 3).
In summary, each of the study species has at least two metabolic pathways with different solution pH ranges for growth that could be activated in solutions of variable acidity. It appears that the restricted range of solution acidity for Fe(II) oxidation is largely determined by the solubility of Fe(III) and Fe(II) species. The two examples from this study (
Figure 11 and
Figure 12) both indicate optima for Fe(II) oxidation at pH < 2. At approximately pH 2 and higher, chemical oxidation of Fe(II) occurs in the presence of oxygen, and Fe(III) hydroxy compounds are precipitated and rates increase rapidly with increased pH. RISC oxidation occurs over a broader range of solution acidity, as does organic carbon utilisation (
Figure 11,
Figure 12 and
Figure 13). At pH < 1, microbial activity, whichever metabolism is employed, is curtailed by the need to restrict protons from entering the cells, facilitate acid tolerance and/or reduce the effects of acid on cells. A number of mechanisms employed by microorganisms to overcome the effects of acid have been reviewed [
36], but are outside the scope of this work.
3.4. Microbial Responses to Process Water Components
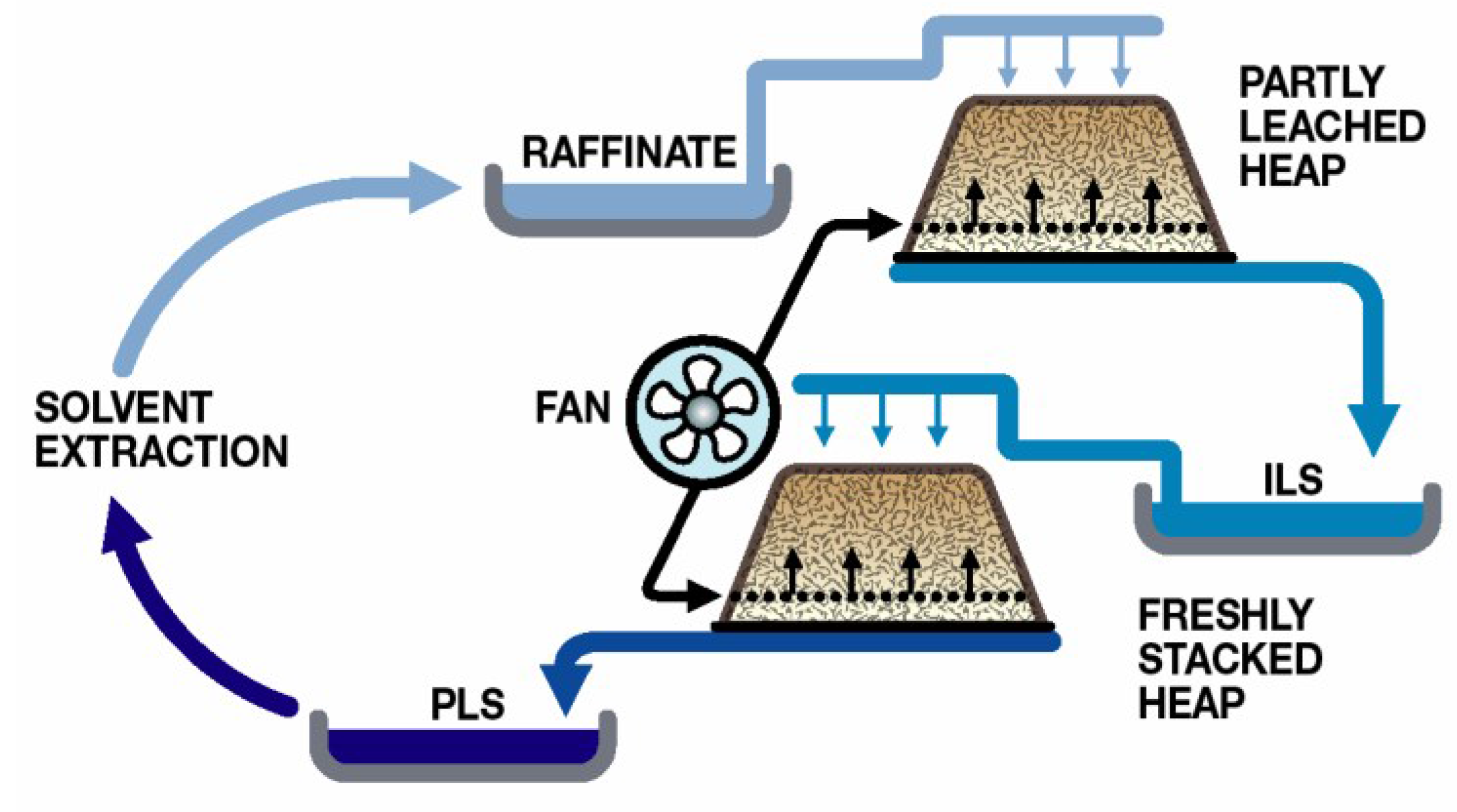
The majority of heap leaching operations use sulphuric acid as the lixiviant and rely upon microbial Fe(II) oxidation to generate the ferric ions necessary for sulphide oxidation. Process water management is directed towards minimal water use and involves recycling to the heaps after the removal of the target metal (e.g., Cu;
Figure 5). While some recent studies have examined the effects of low-pH process water on microbial colonisation of ores (e.g., [
35]), it is a reality that the application of low-pH water to heaps results in greater acid consumption, a major cost, and in increased gangue mineral dissolution, resulting in high total dissolved solids process water [
30]. Element concentrations in heap process waters can build to high concentrations, although, in the case of the data compiled from different case studies (
Table 3), only one or two of the elements were simultaneously at the upper concentration, depending on the ore being leached. For heaps irrigated with seawater, chloride in process waters strongly inhibits microbial activity, as do fluoride and/or nitrate leached from some ores. Acidophiles that colonise managed heaps develop resistance to heavy metals that provide them with a competitive selective advantage [
37].
Table 3.
Approximate upper limits (g·L−1) for individual cations/anions in heap process water.
Table 3.
Approximate upper limits (g·L−1) for individual cations/anions in heap process water.
| Cu | Ni | Co | Zn | As * | Fe | Mg | Al | SO4 | Cl # | F | NO3 |
|---|
| 6 | 5 | <1 | 23 | 20 | 25 | 10 | 25 | 130 | 20 | 2 | 35 |
Methods used to investigate metal tolerance of microorganisms vary. Some rely only on the increase in cell numbers for a given time period, and others measure microbial activity during growth on a substrate. Initially, metal tolerance data for the four study species were obtained using the batch method (
Section 2.7) and estimating cell numbers after 168 h. The results (
Table 4) indicated that there was great variability between replicate estimates using the same strain and also between strains from different heaps. A cursory review of the literature showed that metal toxicity data for
At. ferrooxidans (e.g., [
38,
39,
40]) similarly exhibits a high degree of variability within and between studies.
Subsequently, in experiments to explore the variability further, metal tolerances were examined using the ORP- and pH-monitoring methods described for Fe(II) and RISC oxidation, supplemented by estimates of cell growth. Example data are given for the effect of copper on Fe(II) oxidation by
At. ferrooxidans. The main effect of copper was to slow Fe(II)-oxidation rates progressively with increased concentration (
Figure 14a), rather than to prolong the lag time markedly (
Figure 14b). The data are interpreted as indicating that cells did not become completely inactive, but performed their functions more slowly in the presence of Cu.
Table 4.
Ranges of metal tolerances estimated from batch tests after 168 h for “Ab. cupritolerans”, At. ferrooxidans, At. caldus and S. thermosulfidooxidans and some closely-related isolates obtained from copper heaps.
Table 4.
Ranges of metal tolerances estimated from batch tests after 168 h for “Ab. cupritolerans”, At. ferrooxidans, At. caldus and S. thermosulfidooxidans and some closely-related isolates obtained from copper heaps.
| Species | Element (g·L−1) |
|---|
| Cu | Ni | Co | Zn | As |
|---|
| “Ab. cupritolerans”, from heap, adapted | 175 | 15 | 15 | 60 | 15 |
| At. ferrooxidans, unadapted | 65 | 225 | 140 | 285 | 45 |
| and related isolates from heap, adapted | 75 | 265 | 170 | 285 | 60 |
| At. caldus, unadapted | 1–5 | 2–15 | 5–25 | 65–285 | 30 |
| and related isolates from heap, adapted | 5–25 | 5–50 | 5–50 | 50–185 | 60 |
| S. thermosulfidooxidans, unadapted | 20–75 | 9–40 | 1–2 | 15 | 15 |
| and related isolates from heap, adapted | 15–50 | 5–165 | 1–10 | 10–50 | 15 |
Figure 14.
Fe(II) oxidation by At. ferrooxidans in the presence of copper: (a) effect of copper (duplicate tests); the initial Cu concentrations are noted on the graph; (b) estimated Fe(III)-doubling times (Dt) and lag times (Lt) (n = 2).
Figure 14.
Fe(II) oxidation by At. ferrooxidans in the presence of copper: (a) effect of copper (duplicate tests); the initial Cu concentrations are noted on the graph; (b) estimated Fe(III)-doubling times (Dt) and lag times (Lt) (n = 2).
The same ORP-monitoring method was used to compare the effects of several contaminant cations and the anions chloride and sulphate (
Figure 15). In these tests, the control contained 35 mM of Fe(II) in BSM (Fe-BSM ionic strength 0.22 M) and amounts of contaminant elements were added to yield media with final ionic strength 1 M. While it may be considered that an ionic strength of 1 M is “high”, values from 1.4 to 7.6 M have been estimated from heap process water compositions ([
7] and the references therein), largely influenced by Fe(III), Al(III) and SO
4 concentrations. In the presence of cobalt sulphate (Co 10.7 g·L
−1), the Fe(II) oxidation rate was very slow, preventing the estimation of Dt; the lag time was estimated to be 108 hours. There was no Fe(II) oxidation in the presence of sodium chloride (Na 17.9 g·L
−1) in the time frame of the experiment (168 h). The particular sensitivity of acidophiles to chloride involves complex, pH-dependent mechanisms that result in the acidification of cell cytoplasm when the growth environment has low pH [
41]. This sensitivity restricts the use of seawater (0.5 M chloride) or more concentrated saline bore water for leaching operations [
7]. A comparative study using a batch-culture technique with Fe(II) or tetrathionate as the substrate showed that
At. ferrooxidans grew in medium with up to 7 g·L
−1 NaCl,
At. caldus with up to 15 g·L
−1 NaCl and
S. thermosulfidooxidans with up to 7 g·L
−1 NaCl [
42]. Thus, the expectation was low that growth at up to 1 M NaCl (58 g·L
−1) would occur in this comparative study (
Figure 15). When sodium nitrate was added as the contaminant, the Fe(II) was oxidised chemically before inoculation, nullifying the test. In the tests illustrated in
Figure 16, estimated Fe(III) doubling times and lag times were greater than those estimated for the control (lower ionic strength). On the basis of the estimated lag times, it was concluded that the most inhibitory cations tested were cobalt > copper and that chloride was more inhibitory than sulphate. With the exceptions of cobalt and chloride, Fe(II) oxidation by
At. ferrooxidans occurred in 1 M ionic strength synthetic “process water” containing a single contaminant.
Figure 15.
Comparative effects of some cations on Fe(II) oxidation by At. ferrooxidans in media of 1 M ionic strength. Cations (g·L−1) are indicated on the X axis. Dt, ferric ion generation doubling time; Lt, lag time; initial cell concentration, 106 cells·mL−1; maximum duration of tests, 168 h, or less if the substrate was fully utilised (n = 3).
Figure 15.
Comparative effects of some cations on Fe(II) oxidation by At. ferrooxidans in media of 1 M ionic strength. Cations (g·L−1) are indicated on the X axis. Dt, ferric ion generation doubling time; Lt, lag time; initial cell concentration, 106 cells·mL−1; maximum duration of tests, 168 h, or less if the substrate was fully utilised (n = 3).
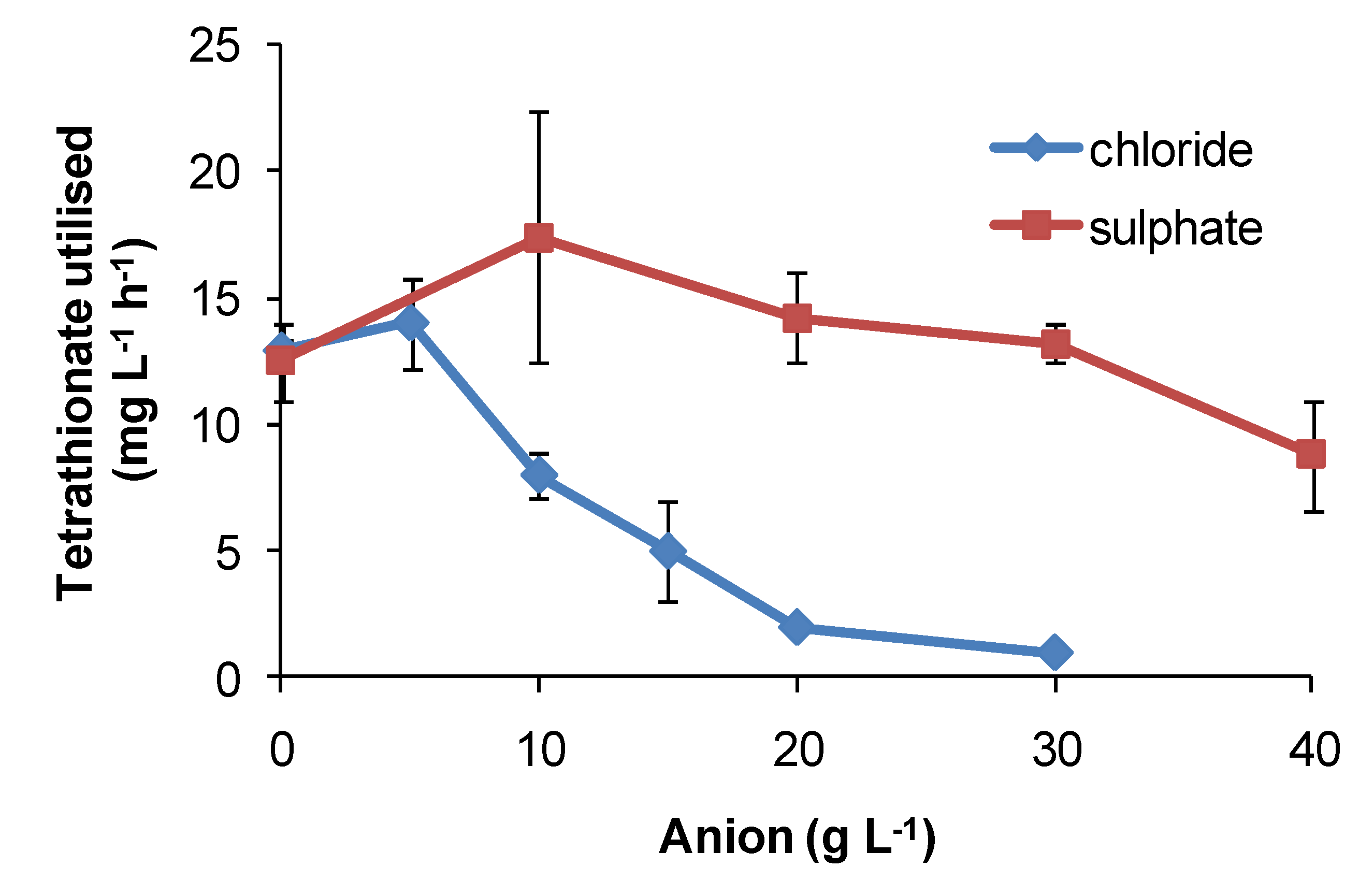
Regarding the effects of anions, in comparative one-week tests using
At. ferrooxidans or
S. thermosulfidooxidans, the presence of nitrate caused slower Fe(II)- and tetrathionate-oxidation with increased lag times and reduced cell numbers (
Figure 16) relative to nitrate-free tests. Chloride was more inhibitory than sulphate for
At. caldus grown in tetrathionate medium (
Figure 17). The rank order was nitrate > chloride > sulphate.
Figure 16.
Effect of nitrate (0–4 g·L−1) in mixed Fe(II)-tetrathionate medium, on the growth of At. ferrooxidans and S. thermosulfidooxidans. Tests conducted at 30 and 50 °C, respectively.
Figure 16.
Effect of nitrate (0–4 g·L−1) in mixed Fe(II)-tetrathionate medium, on the growth of At. ferrooxidans and S. thermosulfidooxidans. Tests conducted at 30 and 50 °C, respectively.
Figure 17.
Effect of sulphate and chloride anions added as sodium salts on rates of tetrathionate utilisation (mg·L−1·h−1) by At. caldus at 45 °C. Initial cell concentration, 106 cells·mL−1; rate data estimated over 100 h, or less if substrate fully utilised (n = 2).
Figure 17.
Effect of sulphate and chloride anions added as sodium salts on rates of tetrathionate utilisation (mg·L−1·h−1) by At. caldus at 45 °C. Initial cell concentration, 106 cells·mL−1; rate data estimated over 100 h, or less if substrate fully utilised (n = 2).
In general, the data presented in
Table 4 provided evidence of adaptation to the metals by some isolates obtained from the heaps, compared with the Deutsche Sammlung von Mikroorganismen und Zellkulturen (unadapted) strains of the same species. The data also showed that the four strains exhibit tolerances to Cu, Ni, Co, Zn and As that would allow them to oxidise Fe(II) in heap process waters close to or above the upper cation concentrations in
Table 3. While the benefits of adaptation have not been investigated in this study, it is assumed that adapted strains would exhibit reduced lag times and/or faster Fe(II) or RISC oxidation rates compared with “controls”. This assumption is based on the results of prolonged exposure to 4-nonylphenol, a minor, but strongly-inhibitory component of a solvent extraction reagent commonly used in the separation and purification of copper from heap process water. Using the electrochemical Fe(II)-oxidation monitoring method, Collinson
et al. [
43] demonstrated for
S. thermosulfidooxidans that “near normal” Fe(II)-oxidation rates and lag times were restored after a year of adaptation to low concentrations of 4-nonylphenol. Similarly, while
At. ferrooxidans and
S. thermosulfidooxidans were shown to be inhibited by nitrate in Fe(II) growth medium, in bioleaching tests (30 °C or 45 °C) with CuFeS
2, the mixed culture of bacteria and archaea, including
At. ferrooxidans and
S. thermosulfidooxidans, adapted to the presence of nitrate within nine weeks [
44]. In a related study using
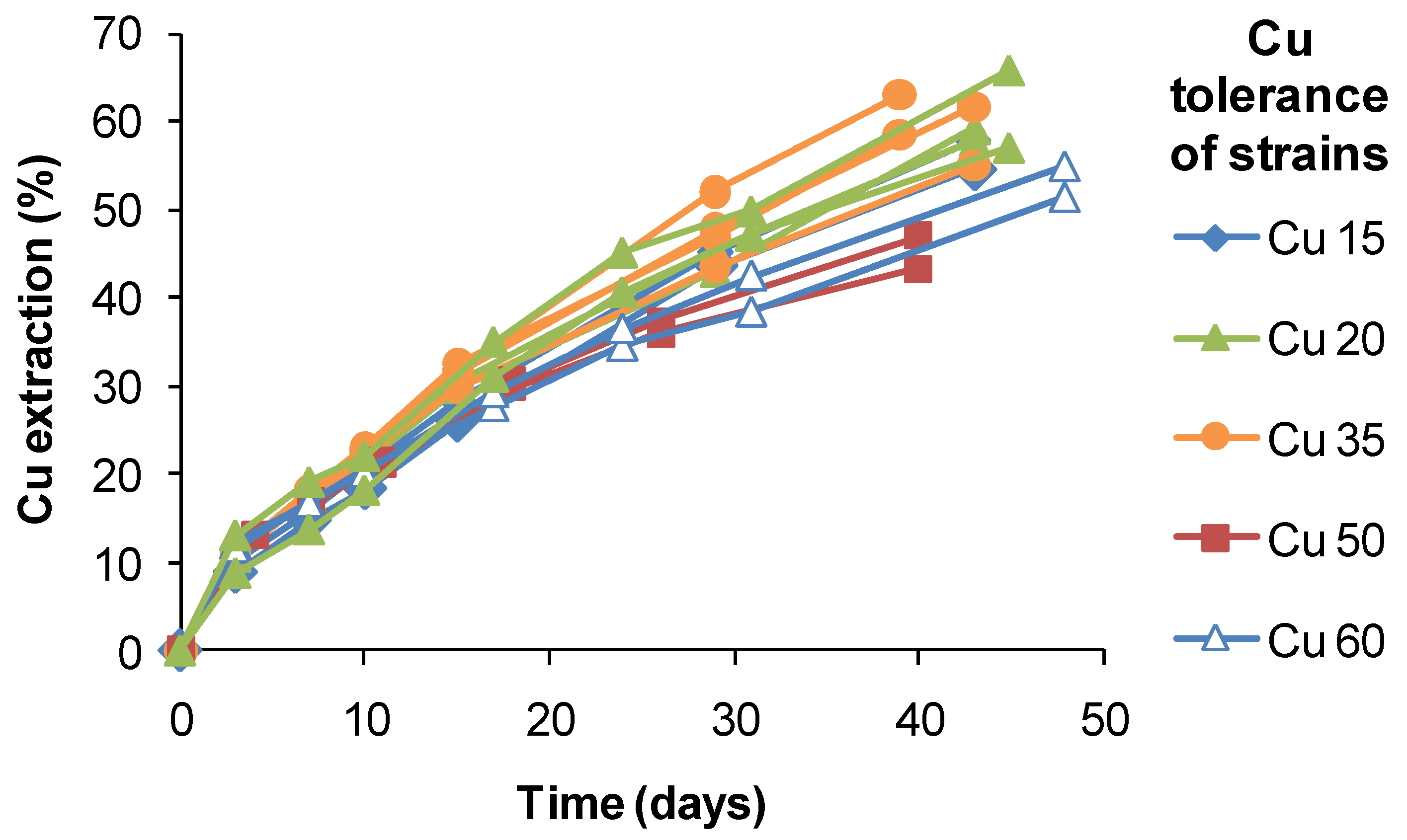
S. thermosulfidooxidans-like isolates from a copper heap, the range of extractions obtained after 40 days of leaching varied by approximately 20%, but did not correlate with estimated Cu tolerances (
Figure 18). It is possible that the slightly lower extractions obtained using strains tolerant to 50–60 g Cu L
−1 were a result of slower Fe(II) oxidation and limited Fe(III) production. Therefore, while attributing the relatively high Cu tolerances to adaptation to the ore, adaptation itself did not directly confer an enhanced ability to extract Cu from CuFeS
2 in bioleaching tests.
Figure 18.
Copper extraction by native strains of S. thermosulfidooxidans from copper sulphide heaps. Different strains had tolerances of up to 60 g Cu L−1. Test conditions were: initial pH 1.8, 1% w/v CuFeS2 concentrate, 45 °C, leach duration 30–48 days; test leach solutions did not exceed 5 g Cu L−1.
Figure 18.
Copper extraction by native strains of S. thermosulfidooxidans from copper sulphide heaps. Different strains had tolerances of up to 60 g Cu L−1. Test conditions were: initial pH 1.8, 1% w/v CuFeS2 concentrate, 45 °C, leach duration 30–48 days; test leach solutions did not exceed 5 g Cu L−1.
Nevertheless, adaptation has played a role in commercial bioleaching. In a recent review [
45], examples of microbial adaptation were identified in a number of pilot-, demonstration- and commercial-scale, stirred-tank plants and from the substantial test programmes. Such descriptions were scarce, but included microorganisms that adapted to 17–20 g·L
−1 Cu, 6–7 g·L
−1 Zn, 13–15 g·L
−1 Fe and 65–70 g·L
−1 sulphate when grown on a polymetallic concentrate [
46]. In a stirred-tank pilot plant for the oxidation of nickel sulphide concentrate, the microorganisms grew in the presence of 23 g·L
−1 Ni and 38 g·L
−1 Fe [
47]. During the development of a commercial-scale, stirred-tank process for the extraction of cobalt from pyritic tailings, microorganisms grew in the presence of >5 g·L
−1 Co and > 35 g·L
−1 Fe [
48], and mineral oxidation was at least 30% faster in continuous reactors than in batch reactors [
49]. Continuous culture of
At. ferrooxidans on arsenopyrite for one year resulted in cultures able to grow in the presence of 5 g·L
−1 arsenite and 30 g·L
−1 arsenate [
50], and the presence of arsenic-resistance genes in
S. thermosulfidooxidans [
51] could explain its prevalence (with other
Sulfobacillus spp.) in several continuous bioreactors processing refractory gold concentrates [
52].