PCR-Based Versus Conventional Stool Testing in Hospitalized Patients with Diarrhea: Diagnostic Yield, Clinical Impact, and Stewardship Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Stool Analysis
2.2.1. Conventional Stool Culture
2.2.2. Microscopy (Ova and Parasite Tests)
2.2.3. Immunochromatographic Assays
2.2.4. PCR-Based Stool Testing
2.3. Data Collection Variables
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Presentation and Laboratory Findings
3.3. Diagnostic Yield
3.4. Predictors of Diagnostic Tests Positivity
3.5. Antibiotic Stewardship Analysis
3.5.1. Empiric Antibiotic Use Prior to Diagnostic Results
3.5.2. Inappropriate Antibiotic Use
3.6. Impact of Diagnostic Results on Antibiotic Stewardship
3.7. Clinical Outcomes and Predictors of Negative Oucomes
3.8. Predictors of ICU Admission
3.9. Predictors of Length of Hospital Stay (LOS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCR | polymerase chain reaction |
| AMR | antimicrobial resistance |
| ICU | intensive care unit |
| LOS | hospital length of stay |
| AST | antimicrobial susceptibility testing |
| C. difficile | Clostridioides difficile |
| EHEC | Enterohemorrhagic Escherichia coli |
| EPEC | Enteropathogenic Escherichia coli |
| EIEC | Enteroinvasive Escherichia coli |
| STEC | Shigatoxigenic Escherichia coli |
| CCI | Charlson Comorbidity Index |
| SD | standard deviation |
| IQR | interquartile range |
| WBC | white blood cells |
| Hgb | hemoglobin |
| CRP | C reactive protein |
| US | ultrasound |
| CT | computed tomography |
References
- Shane, A.L.; Mody, R.K.; Crump, J.A.; Tarr, P.I.; Steiner, T.S.; Kotloff, K.; Langley, J.M.; Wanke, C.; Warren, C.A.; Cheng, A.C.; et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin. Infect. Dis. 2017, 65, e45–e80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buss, S.N.; Leber, A.; Chapin, K.; Fey, P.D.; Bankowski, M.J.; Jones, M.K.; Rogatcheva, M.; Kanack, K.J.; Bourzac, K.M. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J. Clin. Microbiol. 2015, 53, 915–925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, L.J.; Hsiao, C.J.; Chen, B.; Liu, T.Y.; Ding, J.; Hsu, W.T.; Su-Ortiz, V.; Chen, S.T.; Su, K.Y.; Wu, H.P.; et al. Accuracy and comparison of two rapid multiplex PCR tests for gastroenteritis pathogens: A systematic review and meta-analysis. BMJ Open Gastroenterol. 2021, 8, e000553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kahlau, P.; Malecki, M.; Schildgen, V.; Schulz, C.; Winterfeld, I.; Messler, S.; Mattner, F.; Schildgen, O. Utility of two novel multiplexing assays for the detection of gastrointestinal pathogens—A first experience. Springerplus 2013, 2, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beal, S.G.; Tremblay, E.E.; Toffel, S.; Velez, L.; Rand, K.H. A Gastrointestinal PCR Panel Improves Clinical Management and Lowers Health Care Costs. J. Clin. Microbiol. 2017, 56, e01457-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yandag, M.; Tsend-Ayush, A.; Gunregjav, N.; Erdenebayar, O.; Byambadorj, B.; Juniichiro, N.; Jav, S. Detection and antibiotic resistance of diarrheagenic Escherichia coli from patients with diarrhea in Ulaanbaatar, Mongolia. J. Infect. Dev. Ctries. 2023, 17, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jiang, Y.; Yang, L.; Qin, J.; Guo, M.; Lu, Y.; Chen, H.; Zhuang, Y.; Zhang, J.; Zhang, H.; et al. Molecular and Conventional Analysis of Acute Diarrheal Isolates Identifies Epidemiological Trends, Antibiotic Resistance and Virulence Profiles of Common Enteropathogens in Shanghai. Front. Microbiol. 2018, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Dao, T.K.; Nguyen, H.D.; Phung, T.B.T.; Pham, T.T.N.; Nguyen, T.V.H.; Trinh, T.H.; Le, H.C.; Le, T.T.H.; Do, T.H. Application of PCR-Based Techniques for the Identification of Genetic Fingerprint Diversity of Dominant Bacteria in Fecal Samples of Children with Diarrhea in Vietnam. Infect. Dis. Rep. 2024, 16, 932–951. [Google Scholar] [CrossRef]
- Sharif, N.; Ahmed, S.N.; Khandaker, S.; Monifa, N.H.; Abusharha, A.; Vargas, D.L.R.; Díez, I.D.L.T.; Castilla, A.G.K.; Talukder, A.A.; Parvez, A.K.; et al. Multidrug resistance pattern and molecular epidemiology of pathogens among children with diarrhea in Bangladesh, 2019–2021. Sci. Rep. 2023, 13, 13975. [Google Scholar] [CrossRef]
- Freeman, K.; Mistry, H.; Tsertsvadze, A.; Royle, P.; McCarthy, N.; Taylor-Phillips, S.; Manuel, R.; Mason, J. Multiplex tests to identify gastrointestinal bacteria, viruses and parasites in people with suspected infectious gastroenteritis: A systematic review and economic analysis. Health Technol. Assess. 2017, 21, 1–188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilber, E.; Baker, J.M.; Rebolledo, P.A. Clinical Implications of Multiplex Pathogen Panels for the Diagnosis of Acute Viral Gastroenteritis. J. Clin. Microbiol. 2021, 59, e0151319. [Google Scholar] [CrossRef]
- Riddle, M.S.; DuPont, H.L.; Connor, B.A. ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults. Am. J. Gastroenterol. 2016, 111, 602–622. [Google Scholar] [CrossRef] [PubMed]
- Leli, C.; Di Matteo, L.; Gotta, F.; Vay, D.; Cavallo, V.; Mazzeo, R.; Busso, S.; Carrabba, L.; Rocchetti, A. Evaluation of a multiplex gastrointestinal PCR panel for the aetiological diagnosis of infectious diarrhoea. Infect. Dis. 2020, 52, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.R.; Beniprashad, M.; Cardona, M.; Masney, S.; Low, D.E.; Gubbay, J.B. Evaluation and verification of the Seeplex Diarrhea-V ACE assay for simultaneous detection of adenovirus, rotavirus, and norovirus genogroups I and II in clinical stool specimens. J. Clin. Microbiol. 2011, 49, 3154–3162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruijnesteijn van Coppenraet, L.E.S.; Dullaert-de Boer, M.; Ruijs, G.J.H.M.; van der Reijden, W.A.; van der Zanden, A.G.M.; Weel, J.F.L.; Schuurs, T.A. Case-Control Comparison of Bacterial and Protozoan Microorganisms Associated with Gastroenteritis: Application of Molecular Detection. Clin. Microbiol. Infect. 2015, 21, 592.e9–592.e19. [Google Scholar] [CrossRef]
- Khare, R.; Espy, M.J.; Cebelinski, E.; Boxrud, D.; Sloan, L.M.; Cunningham, S.A.; Pritt, B.S.; Patel, R.; Binnicker, M.J. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. J. Clin. Microbiol. 2014, 52, 3667–3673. [Google Scholar] [CrossRef]
- Spina, A.; Kerr, K.G.; Cormican, M.; Barbut, F.; Eigentler, A.; Zerva, L.; Tassios, P.; Popescu, G.A.; Rafila, A.; Eerola, E.; et al. Spectrum of enteropathogens detected by the FilmArray GI Panel in a multicentre study of community-acquired gastroenteritis. Clin. Microbiol. Infect. 2015, 21, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Sobczyk, J.; Jain, S.; Sun, X.; Karris, M.; Wooten, D.; Stagnaro, J.; Reed, S. Comparison of Multiplex Gastrointestinal Pathogen Panel and Conventional Stool Testing for Evaluation of Patients with HIV Infection. Open Forum Infect. Dis. 2020, 7, ofz547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ascierto, M.; Chianese, A.; Foglia, F.; Finamore, E.; Petrullo, L.; Zannella, C.; De Filippis, A.; Coppola, M.G.; Galdiero, M. Prevalence of Blastocystis spp. and Other Gastrointestinal Pathogens Among Patients Admitted to Research Hospitals in Campania Region, Italy. Pathogens 2025, 14, 425. [Google Scholar] [CrossRef]
- Mannstadt, I.; Choy, A.M.; Li, J.; Green, D.A.; Freedberg, D.E. Risk Factors and Clinical Outcomes Associated with Multiple as Opposed to Single Pathogens Detected on the Gastrointestinal Disease Polymerase Chain Reaction Assay. Gut Pathog. 2024, 16, 45. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, S.Y.; Hwang, H.S.; Ryu, J.Y.; Lee, J.; Song, I.D.; Kim, B.J.; Kim, J.W.; Chang, S.K.; Choi, C.H. Diagnostic yield of stool culture and predictive factors for positive culture in patients with diarrheal illness. Medicine 2017, 96, e7641. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Jia, S.; Qu, M.; Pei, Y.; Qiu, S.; Zhang, J.; Liu, Y.; Ma, S.; Lyu, N.; et al. A global atlas and drivers of antimicrobial resistance in Salmonella during 1900–2023. Nat. Commun. 2025, 16, 4611. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Freedberg, D.E.; Whittier, S.; Greendyke, W.; Lebwohl, B.; Green, D.A. Impact of Gastrointestinal Panel Implementation on Health Care Utilization and Outcomes. J. Clin. Microbiol. 2019, 57, e01775-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jansen, A.; Stark, K.; Kunkel, J.; Schreier, E.; Ignatius, R.; Liesenfeld, O.; Werber, D.; Göbel, U.B.; Zeitz, M.; Schneider, T. Aetiology of community-acquired, acute gastroenteritis in hospitalised adults: A prospective cohort study. BMC Infect. Dis. 2008, 8, 143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwack, W.G.; Lim, Y.J.; Kwon, K.H.; Chung, J.W.; Oh, J.Y. Outcomes and clinical relevance of stool multiplex bacterial polymerase chain reaction in patients with acute diarrhea: Single center experience. Korean J. Intern. Med. 2020, 35, 300–309. [Google Scholar] [CrossRef]
- Berdal, J.E.; Follin-Arbelet, B.; Bjørnholt, J.V. Experiences from multiplex PCR diagnostics of faeces in hospitalised patients: Clinical significance of Enteropathogenic Escherichia coli (EPEC) and culture negative campylobacter. BMC Infect. Dis. 2019, 19, 630. [Google Scholar] [CrossRef]
- Chen, S.; Feuille, C. Enteropathogenic Escherichia coli (EPEC) Causes Chronic Diarrhea and Hyponatremia in an Adult. Clin. Case Rep. 2025, 13, e70288. [Google Scholar] [CrossRef]
- Eze, P.; Balsells, E.; Kyaw, M.H.; Nair, H. Risk factors for Clostridium difficileinfections—An overview of the evidence base and challenges in data synthesis. J. Glob. Health 2017, 7, 010417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, K.; Lawrence, J.; Berry, C.; Davis, G.; Yu, H.; Cai, B.; Gonzalez, E.; Prantner, I.; Kurcz, A.; Macovei, I.; et al. Risk Factors for Primary Clostridium Difficile Infection; Results from the Observational Study of Risk Factors for Clostridium Difficile Infection in Hospitalized Patients with Infective Diarrhea (ORCHID). Front. Public Health 2020, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crobach, M.J.; Planche, T.; Eckert, C.; Barbut, F.; Terveer, E.M.; Dekkers, O.M.; Wilcox, M.H.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the diagnostic guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 2016, 22 (Suppl. 4), S63–S81. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Morillas, J.A.; Brizendine, K.D.; Fraser, T.G. Predictors of Clostridioides difficile Infection-Related Complications and Treatment Patterns among Nucleic Acid Amplification Test-Positive/Toxin Enzyme Immunoassay-Negative Patients. J. Clin. Microbiol. 2020, 58, e01764-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Svenungsson, B.; Lagergren, A.; Ekwall, E.; Evengård, B.; Hedlund, K.O.; Kärnell, A.; Löfdahl, S.; Svensson, L.; Weintraub, A. Enteropathogens in adult patients with diarrhea and healthy control subjects: A 1-year prospective study in a Swedish clinic for infectious diseases. Clin. Infect. Dis. 2000, 30, 770–778. [Google Scholar] [CrossRef]
- Cadwgan, A.M.; Watson, W.A.; Laing, R.B.S.; MacKenzie, A.R.; Smith, C.C.; Douglas, J.G. Presenting clinical features and C-reactive protein in the prediction of a positive stool culture in patients with diarrhoea. J. Infect. 2000, 41, 159–161. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Zhang, Q.; Shen, J. Antimicrobial Resistance in Campylobacter spp. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Kelly, G.A.; Iordanov, R.; Franklin, A.; Ahmed, A.; Srinivasan, K.; Hayon, J.; Lasco, T.; Amini, R.; Shay, S.; Kulkarni, P.A.; et al. Impact of gastrointestinal polymerase chain reaction panels on antibiotic utilization in hospitalized adult patients. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, V.; Yune, P.; Rasul, R.; Schwartz, R.; Niknam, N.; Khameraj, A.; Malhotra, P.; Farber, B. 724. Gastrointestinal (GI) PCR vs Stool Cultures: Impact on Length of Hospital Stay (LOS) and Antibiotic Use. Open Forum Infect. Dis. 2020, 7 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed Central]
- Meltzer, A.C.; Newton, S.; Lange, J.; Hall, N.C.; Vargas, N.M.; Huang, Y.; Moran, S.; Ma, Y. A randomized control trial of a multiplex gastrointestinal PCR panel versus usual testing to assess antibiotics use for patients with infectious diarrhea in the emergency department. J. Am. Coll. Emerg. Physicians Open 2022, 3, e12616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cybulski RJJr Bateman, A.C.; Bourassa, L.; Bryan, A.; Beail, B.; Matsumoto, J.; Cookson, B.T.; Fang, F.C. Clinical Impact of a Multiplex Gastrointestinal Polymerase Chain Reaction Panel in Patients with Acute Gastroenteritis. Clin. Infect. Dis. 2018, 67, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Keske, Ş.; Zabun, B.; Aksoy, K.; Can, F.; Palaoğlu, E.; Ergönül, Ö. Rapid Molecular Detection of Gastrointestinal Pathogens and Its Role in Antimicrobial Stewardship. J. Clin. Microbiol. 2018, 56, e00148-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Machiels, J.D.; Cremers, A.J.H.; van Bergen-Verkuyten, M.C.G.T.; Paardekoper-Strijbosch, S.J.M.; Frijns, K.C.J.; Wertheim, H.F.L.; Rahamat-Langendoen, J.; Melchers, W.J.G. Impact of the BioFire FilmArray gastrointestinal panel on patient care and infection control. PLoS ONE 2020, 15, e0228596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torres-Miranda, D.; Akselrod, H.; Karsner, R.; Secco, A.; Silva-Cantillo, D.; Siegel, M.O.; Roberts, A.D.; Simon, G.L. Use of Bio-Fire FilmArray Gastrointestinal PCR Panel Associated with Reductions in Antibiotic Use, Time to Optimal Antibiotics, and Length of Stay. BMC Gastroenterol. 2020, 20, 246. [Google Scholar] [CrossRef]
- Goldenberg, S.D.; Bacelar, M.; Brazier, P.; Bisnauthsing, K.; Edgeworth, J.D. A cost benefit analysis of the Luminex xTAG Gastrointestinal Pathogen Panel for detection of infectious gastroenteritis in hospitalised patients. J. Infect. 2015, 70, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, C.; Rogatcheva, M.; Harrel, B.; Vaughn, M.; Crisp, R.; Poritz, M.; Thatcher, S.; Korgenski, E.K.; Barney, T.; Daly, J.; et al. How well does physician selection of microbiologic tests identify Clostridium difficile and other pathogens in paediatric diarrhoea? Insights using multiplex PCR-based detection. Clin. Microbiol. Infect. 2015, 21, e9–e15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Z.; Zhang, X.; Chen, J.; Shi, Y.; Ji, S. Bacterial Infections in Acute-on-chronic Liver Failure: Epidemiology, Diagnosis, Pathogenesis, and Management. J. Clin. Transl. Hepatol. 2024, 12, 667–676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
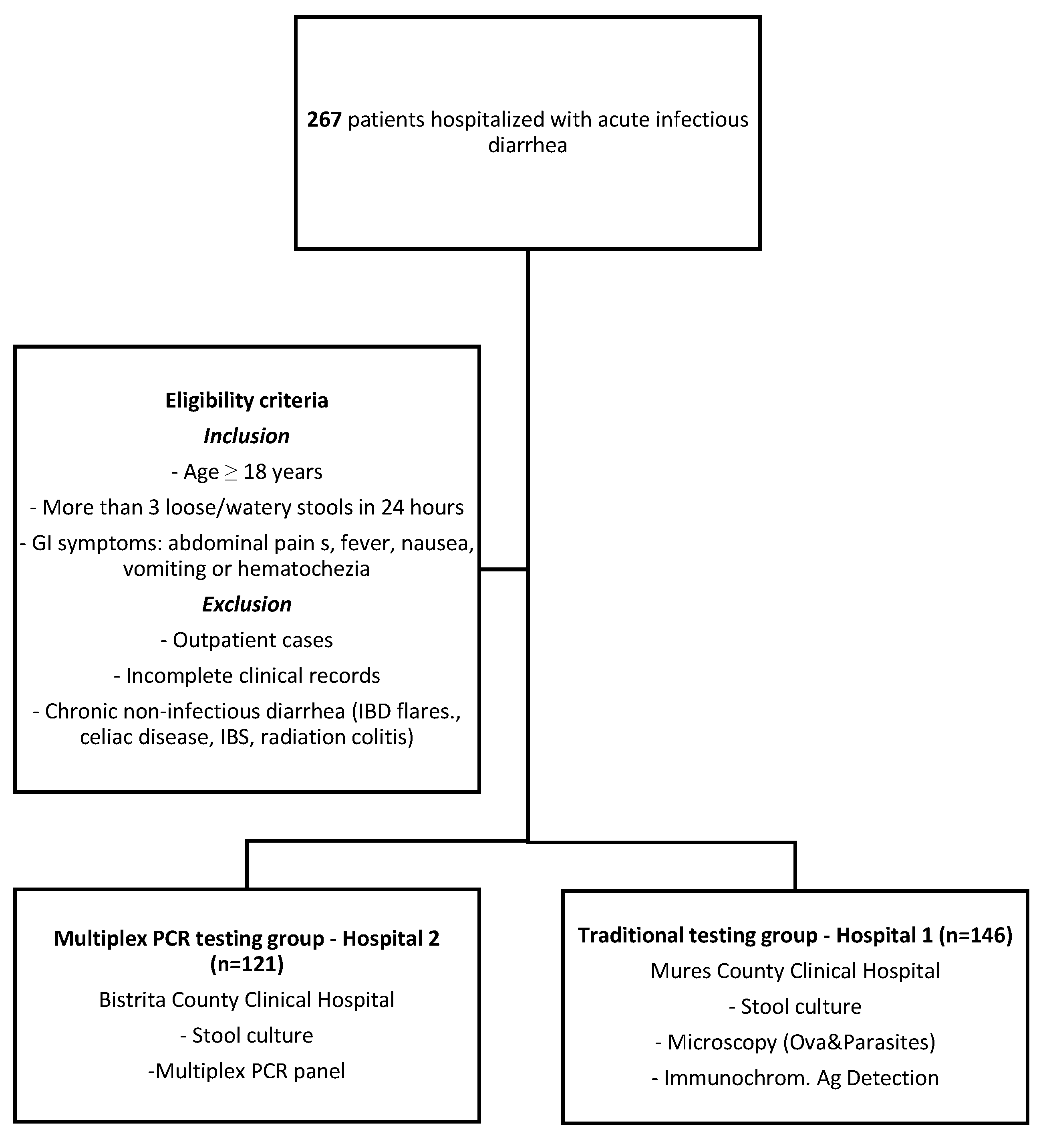
| Variable | Traditional Group (Hospital 1) (146) | Multiplex PCR Testing Group (Hospital 2) (121) | p Value |
|---|---|---|---|
| Age (median-IQR) | 60 (36–73) | 65 (53–75) | 0.02 |
| Min–max | 18–96 | 19–95 | |
| Sex, nr (%) | 0.537 | ||
| Male | 69 (48.3%) | 63 (52.1%) | |
| Female | 73 (51.7%) | 58 (47.9%) | |
| Length of hospital stay (median-IQR) | 6 (4–11) | 10 (6–15) | <0.0001 |
| Min–max | 1–45 | 3–39 | |
| Charlson Comorbidity Index (median-IQR) | 2 (0–5) | 5 (2–7) | <0.0001 |
| Min–max | 0–10 | 0–12 | |
| Comorbidities | |||
| Cardiovascular disease | 49 (35.8%) | 59 (48.8%) | 0.035 |
| Liver cirrhosis | 13 (9.4%) | 25 (20.7%) | 0.010 |
| Respiratory disease | 21 (15.2%) | 19 (15.7%) | 0.914 |
| Renal disease | 13 (9.4%) | 31 (25.6%) | 0.001 |
| Diabetes | 25 (18.0%) | 21 (17.4%) | 0.894 |
| Inflammatory bowel disease | 6 (4.3%) | 12 (9.9%) | 0.076 |
| Cancer | 23 (16.5%) | 24 (19.8%) | 0.492 |
| Immunosuppressive medication | 16 (11.5%) | 42 (34.7%) | 0.000 |
| Recent surgery | 11 (7.7%) | 22 (18.2%) | 0.008 |
| Recent antibiotics | 23 (16.9%) | 14 (11.6%) | 0.223 |
| Symptoms | |||
| Loose stools | 71 (50%) | 65 (53.7%) | 0.547 |
| Watery diarrhea | 83 (60.6%) | 44 (36.4%) | 0.000 |
| Bloody diarrhea | 11 (8.0%) | 34 (28.1%) | 0.000 |
| Abdominal pain | 70 (51.1%) | 93 (76.9%) | 0.000 |
| Dehydration | 94 (68.6%) | 62 (51.2%) | 0.004 |
| Variables | Traditional Group—Hospital 1 (146) | Multiplex PCR Testing Group—Hospital 2 (121) | p Value |
|---|---|---|---|
| WBC (median-IQR) (/µL) | 8380 (6410–13,270) | 11,000 (6725–14,460) | <0.0001 |
| Hgb (g/dL) | 12.85 (11.08–14.23) | 11.2 (9.9–12) | <0.0001 |
| CRP (mg/L) | 4.35 (0.81–9.17) | 109 (42–194) | <0.0001 |
| Procalcitonin | 0.5 (0–10) | 0.5 (0.1–2.14) | 0.19 |
| Abdominal US | 30 (21.58%) | 30 (24.8%) | 0.539 |
| Abdominal CT scan | 32 (25.36%) | 41 (33.8%) | 0.159 |
| Endoscopy | 9 (6.52%) | 8 (6.61%) | 0.964 |
| Number of imagistic tests/patients (median-IQR) | 0 (0–1) | 0 (0–1) | 0.068 |
| Min–max | 0–3 | 0–3 | |
| Number of stool tests/patient (median-IQR) | 2 (2–3) | 1 (1–1) | <0.0001 |
| Min–max | 0–5 | 1–2 |
| Pathogen Detected | Multiplex PCR Testing Group—Hospital 2 (n = 121) | Traditional Group—Hospital 1 (n = 146) | p |
|---|---|---|---|
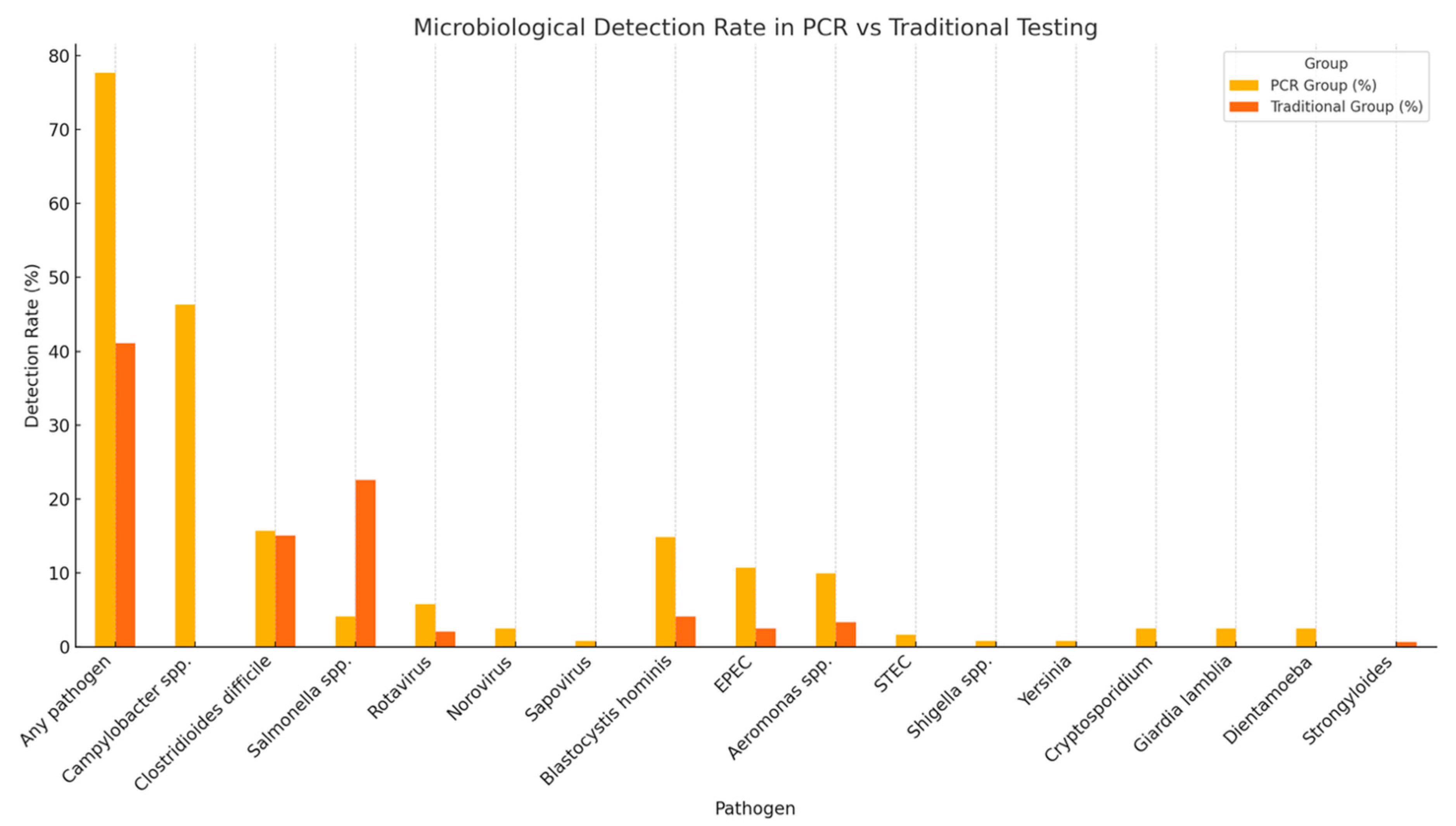
| Any pathogen detected | 94 (77.68%) | 60 (41.09%) | 0.0001 |
| Campylobacter | 56 (46.28%) | 0 | - |
| Single | 24 (19.83%) | ||
| Double | 22 (18.18%) | ||
| Triple | 10 (8.26%) | ||
| C. difficile | 19 (15.70%) | 22 (15.06%) | 0.531 |
| Salmonella | 5 (4.1%) | 33 (22.6%) | 0.00001 |
| Rotavirus | 7 (5.78%) | 3 (2.1%) | 0.110 |
| Single | 4 | ||
| Double | 1 | ||
| Triple | 2 | ||
| Adenovirus | 0 | 1 (0.68%) | - |
| Aeromonas spp. | 12 (9.91%) | 0 | |
| Single | 1 (0.82%) | ||
| Double | 7 (5.78%) | ||
| Triple | 4 (3.30%) | ||
| Blastocystis hominis | 18 (14.87%) | 0 | |
| Single | 4 (3.30%) | ||
| Double | 9 (7.43%) | ||
| Triple | 5 (4.13%) | ||
| EPEC | 13 (10.7%) | 0 | - |
| Single | 1 (0.82%) | ||
| Double | 9 (7.43%) | ||
| Triple | 3 (2.47%) | ||
| EHEC | 1 | 0 | - |
| STEC | 2 (1.65%) | 0 | - |
| Shigella | 1 (0.82%) | 0 | - |
| Norovirus | 3 (2.47%) | 0 | - |
| Sapovirus | 1 (0.82%) | 0 | |
| Yersinia | 1 (0.82%) | 0 | |
| Cryptosporidium | 3 (2.47%) | 0 | |
| Giardia lamblia | 3 (2.47%) | 0 | |
| Dientamoeba fragilis | 3 (2.47%) | 0 | |
| Strongyloides | 0 | 1 (0.68%) | |
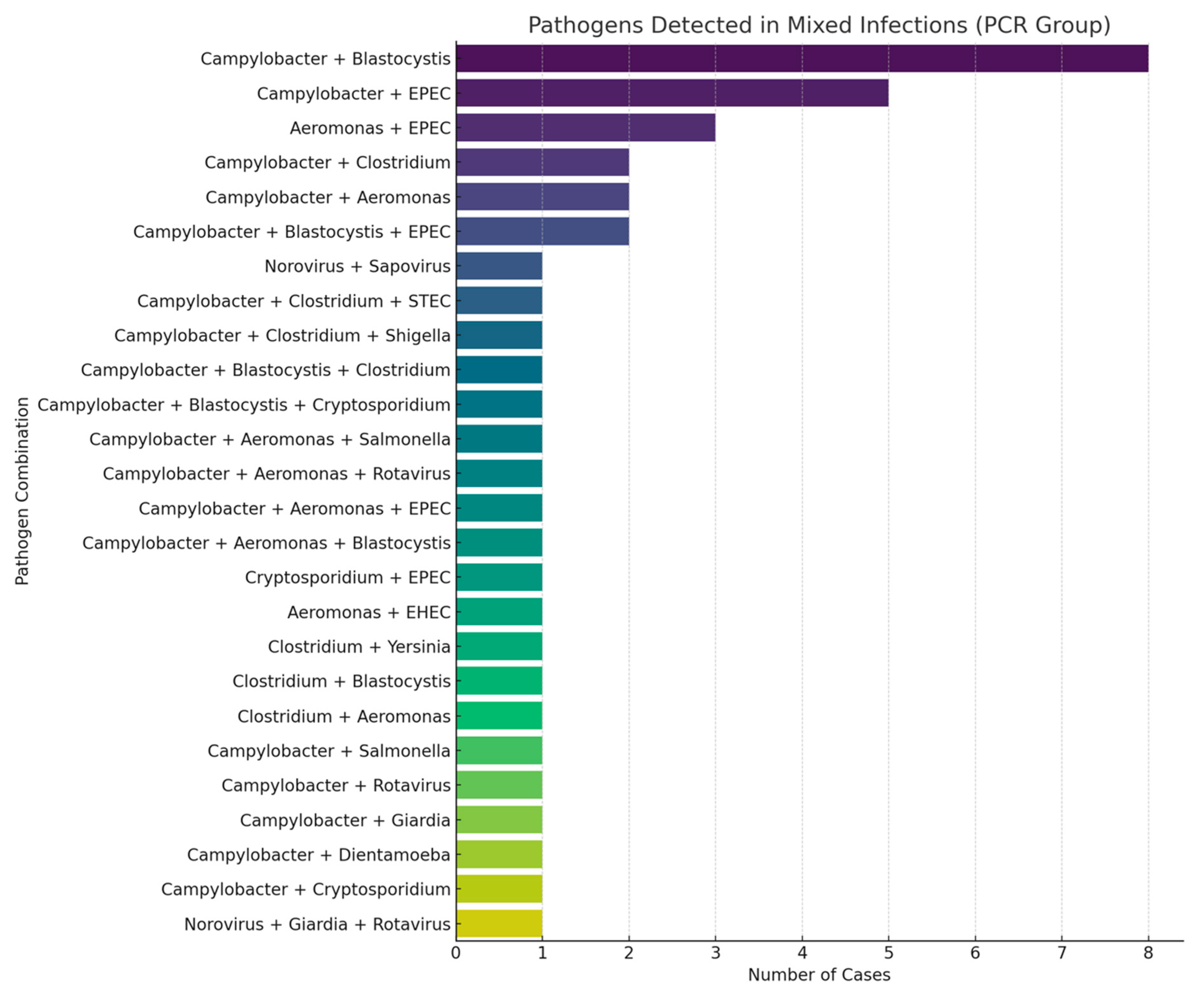
| Mixed infections (≥2 pathogens) | 42 (34.71%) | 0 | |
| Double pathogens | 31 (25.61%) | ||
| Triple pathogens | 11 (9.09%) |
| Multiplex PCR Testing Positivity (Hospital 2) | |||
|---|---|---|---|
| Predictor | OR | 95% CI | p Value |
| Symptoms | |||
| Watery diarrhea | 0.18 | 0.02–1.52 | 0.114 |
| Bloody diarrhea | 16.5 | 1.81–150.26 | 0.013 |
| Loose stool | 1.15 | 0.21–6.11 | 0.874 |
| Dehydration | 7.05 | 1.4–35.45 | 0.018 |
| CCI | 1.04 | 0.87–1.24 | 0.668 |
| Immunosuppression | 0.54 | 0.17–1.76 | 0.31 |
| CRP > 10 (mg/L) | 4.52 | 0.34–60.83 | 0.256 |
| WBC > 10,000/µL | 0.48 | 0.16–1.44 | 0.19 |
| Traditional stool test positivity (Hospital 1) | |||
| Predictor | OR | 95% CI | p value |
| Symptoms | |||
| Watery diarrhea | 0.27 | 0.09–0.85 | 0.025 |
| Bloody diarrhea | 1.46 | 0.29–7.35 | 0.649 |
| Loose stool | 2.57 | 1.01–6.55 | 0.048 |
| Dehydration | 2.86 | 0.94–8.73 | 0.065 |
| CCI | 0.88 | 0.76–1.02 | 0.088 |
| WBC > 10,000/µL | 0.67 | 0.29–1.59 | 0.367 |
| Detection of mixed infections | |||
| Predictor | OR | 95% CI | p value |
| Symptoms | |||
| Bloody diarrhea | 0.89 | 0.34–2.31 | 0.806 |
| Dehydration | 0.79 | 0.31–2 | 0.623 |
| ICU admission | 2.01 | 0.68–5.95 | 0.205 |
| WBC > 10,000/µL | 1.5 | 0.62–3.64 | 0.371 |
| CRP > 10 (mg/L) | 1 | 0.99–1 | 0.063 |
| Procalcitonin | 1 | 1–1 | 0.465 |
| CCI | 1.05 | 0.91–1.21 | 0.542 |
| Immunosuppression | 1.28 | 0.52–3.14 | 0.596 |
| Empiric Antibiotics Initiated Prior to Result | |||
|---|---|---|---|
| Predictor | OR | 95% CI | p Value |
| Symptoms | |||
| Watery diarrhea | 1.15 | 0.56–2.39 | 0.75 |
| Bloody diarrhea | 1.75 | 0.78–3.93 | 0.172 |
| Abdominal pain | 0.47 | 0.25–0.86 | 0.015 |
| Dehydration | 0.9 | 0.42–1.93 | 0.792 |
| CCI | 1.19 | 1.08–1.31 | 0.001 |
| Comorbidities | |||
| Cardiovascular | 0.89 | 0.46–1.74 | 0.739 |
| Liver cirrhosis | 0.89 | 0.37–2.17 | 0.798 |
| Respiratory | 0.9 | 0.4–2 | 0.794 |
| Renal | 0.31 | 0.13–0.75 | 0.01 |
| Diabetes | 1.05 | 0.46–2.38 | 0.907 |
| Cancer | 1.9 | 0.73–4.99 | 0.191 |
| Immunosuppressive therapy | 1.12 | 0.45–2.75 | 0.808 |
| Immunosuppressive status | 1.09 | 0.64–1.84 | 0.756 |
| WBC > 10,000/µL | 2.18 | 1.33–3.6 | 0.002 |
| Inappropriate Antibiotic Use | |||
|---|---|---|---|
| Predictor | OR | 95% CI | p Value |
| Symptoms | |||
| Watery diarrhea | 0.82 | 0.4–1.69 | 0.588 |
| Bloody diarrhea | 1.23 | 0.58–2.6 | 0.594 |
| Abdominal pain | 0.68 | 0.37–1.25 | 0.213 |
| Dehydration | 1.43 | 0.67–3.06 | 0.351 |
| CCI | 1.12 | 1.02–1.22 | 0.019 |
| Type of stool test (PCR vs. traditional) | 0.296 | 0.16–0.53 | <0.001 |
| Comorbidities | |||
| Cardiovascular | 1.47 | 0.75–2.91 | 0.263 |
| Liver cirrhosis | 1.1 | 0.46–2.65 | 0.831 |
| Respiratory | 1.4 | 0.63–3.13 | 0.409 |
| Renal | 0.36 | 0.15–0.84 | 0.018 |
| Diabetes | 1.31 | 0.58–2.96 | 0.52 |
| Cancer | 0.71 | 0.29–1.72 | 0.443 |
| Immunosuppressive therapy | 0.69 | 0.29–1.61 | 0.389 |
| ICU admission | 2.48 | 0.99–6.17 | 0.051 |
| WBC > 10,000/µL | 1.46 | 0.85–2.51 | 0.17 |
| Variable | Traditional Stool Tests (Hospital 1) (146) | Multiplex PCR Testing Group (Hospital 2) (121) | p Value |
|---|---|---|---|
| Empiric ATB initiated prior to result | 53 (36.3%) | 84 (70.0%) | 0.000 |
| Empiric ATB continued after result | 10 (6.84%) | 0 | |
| Change in ATB therapy after result | 40 (27.39%) | 51 (42.14%) | 0.011 |
| ATB discontinued after result | 3 (3.70%) | 15 (12.39%) | 0.033 |
| ATB added after result | 31 (21.23%) | 19 (15.70%) | 0.248 |
| Duration of ATB after result-median (IQR) | 7 (5–10) | 5 (0–7) | <0.0001 |
| Min–max | 0–12 | 0–10 |
| ICU Admission | |||
|---|---|---|---|
| Predictor | OR | 95% CI | p Value |
| Hospitalization days | 1.08 | 1.02–1.14 | 0.006 |
| Symptoms | |||
| Watery diarrhea | 0.83 | 0.27–2.58 | 0.75 |
| Bloody diarrhea | 2.04 | 0.72–5.79 | 0.18 |
| Abdominal pain | 0.79 | 0.3–2.1 | 0.641 |
| Dehydration | 1.42 | 0.45–4.49 | 0.553 |
| CCI | 1.26 | 1.1–1.43 | 0.001 |
| Comorbidities | |||
| Cardiovascular | 0.42 | 0.16–1.12 | 0.83 |
| Liver cirrhosis | 6.69 | 2.52–17.77 | <0.001 |
| Respiratory | 3.07 | 1.09–8.67 | 0.034 |
| Renal | 0.466 | 0.21–2.03 | 0.466 |
| Diabetes | 0.31 | 0.08–1.12 | 0.073 |
| Cancer | 0.92 | 0.24–3.47 | 0.904 |
| Immunosuppressive therapy | 2.34 | 0.75–7.32 | 0.143 |
| Recent ATB | 1.72 | 0.54–5.42 | 0.356 |
| WBC > 10,000/µL | 1.66 | 0.81–3.4 | 0.162 |
| Length of hospital stay | |||
| Predictor | B | 95% CI | p value |
| Positive stool test results | 2.78 | 1.16–4.39 | 0.001 |
| ICU admission | 5.23 | 2.73–7.74 | <0.001 |
| Inappropriate ATB use | −1.01 | −3.54–1.51 | 0.427 |
| Symptoms | |||
| Watery diarrhea | −0.22 | −2.62–2.18 | 0.856 |
| Bloody diarrhea | 1.20 | −1.37–3.78 | 0.355 |
| Dehydration | 1.59 | −0.93–4.10 | 0.213 |
| CCI | 0.56 | 0.10–1.02 | 0.017 |
| Comorbidities | |||
| Cardiovascular | 0.21 | −1.92–2.34 | 0.846 |
| Liver disease | 2.44 | −0.08–4.96 | 0.057 |
| Respiratory | 2.38 | 0.01–4.74 | 0.048 |
| Renal | −4.15 | −6.68–1.62 | 0.001 |
| Diabetes | −2.92 | −5.22–0.62 | 0.013 |
| Cancer | −0.24 | −3–2.53 | 0.866 |
| WBC > 10,000/µL | 2.67 | 0.84–4.50 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boeriu, A.; Andone, A.; Dobru, D.; Ciurea, C.N.; Nyulas, V.A.; Onișor, D.; Tilea, B.; Matei, L.A.; Imreh-Ferenci, R.-B.; Fofiu, C. PCR-Based Versus Conventional Stool Testing in Hospitalized Patients with Diarrhea: Diagnostic Yield, Clinical Impact, and Stewardship Implications. Microorganisms 2025, 13, 2785. https://doi.org/10.3390/microorganisms13122785
Boeriu A, Andone A, Dobru D, Ciurea CN, Nyulas VA, Onișor D, Tilea B, Matei LA, Imreh-Ferenci R-B, Fofiu C. PCR-Based Versus Conventional Stool Testing in Hospitalized Patients with Diarrhea: Diagnostic Yield, Clinical Impact, and Stewardship Implications. Microorganisms. 2025; 13(12):2785. https://doi.org/10.3390/microorganisms13122785
Chicago/Turabian StyleBoeriu, Alina, Adina Andone, Daniela Dobru, Cristina Nicoleta Ciurea, Victoria Ancuta Nyulas, Danusia Onișor, Brindusa Tilea, Lavinia Andrada Matei, Reka-Bernadett Imreh-Ferenci, and Crina Fofiu. 2025. "PCR-Based Versus Conventional Stool Testing in Hospitalized Patients with Diarrhea: Diagnostic Yield, Clinical Impact, and Stewardship Implications" Microorganisms 13, no. 12: 2785. https://doi.org/10.3390/microorganisms13122785
APA StyleBoeriu, A., Andone, A., Dobru, D., Ciurea, C. N., Nyulas, V. A., Onișor, D., Tilea, B., Matei, L. A., Imreh-Ferenci, R.-B., & Fofiu, C. (2025). PCR-Based Versus Conventional Stool Testing in Hospitalized Patients with Diarrhea: Diagnostic Yield, Clinical Impact, and Stewardship Implications. Microorganisms, 13(12), 2785. https://doi.org/10.3390/microorganisms13122785







