Chelerythrine-Mediated Growth Inhibition and Resistance Mechanism in Bacillus tropicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Minimum Inhibitory Concentrations (MICs) of Wild-Type B. tropicus (BT)
2.3. Domestication of Drug-Resistant B. tropicus (CheRBT)
2.4. Differential Growth and Metabolism of BT and CheRBT
2.4.1. Growth Curve Determination
2.4.2. Scanning Electron Microscopy (SEM)
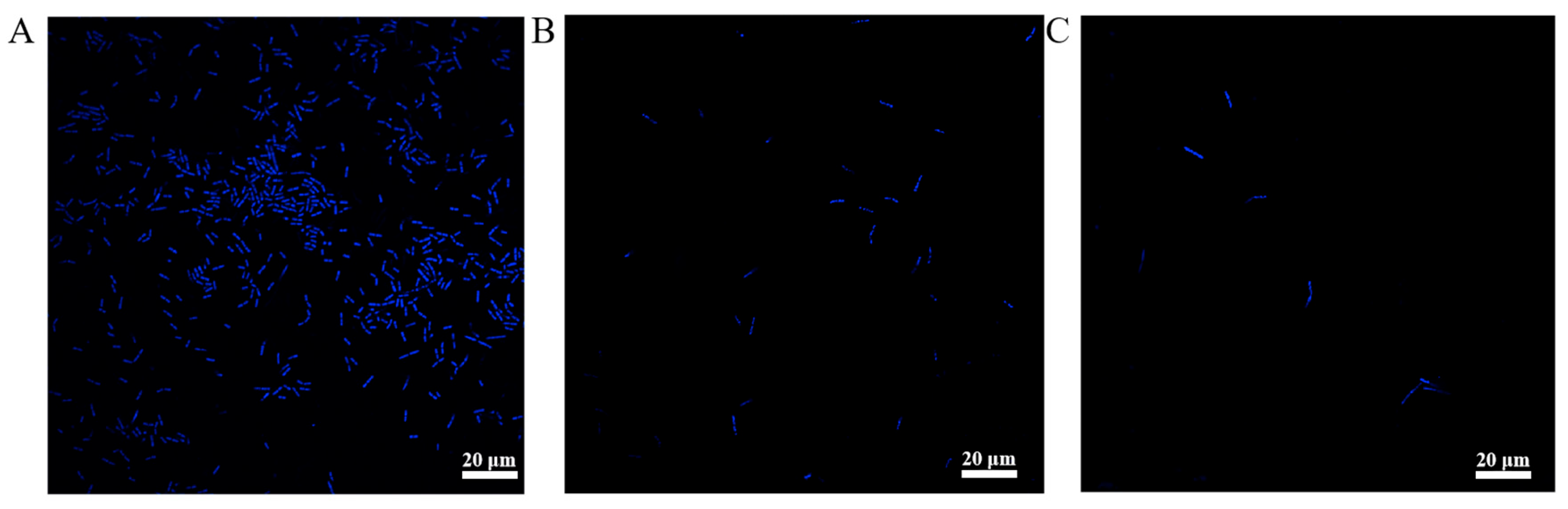
2.4.3. Biofilm Formation
2.4.4. Glucose Content in the BT and CheRBT Cultures
2.4.5. BT and CheRBT DNA Content
2.5. Transcriptome Analysis
2.5.1. RNA Preparation, Library Construction, and Sequencing
2.5.2. Read Mapping, Annotation, and Analysis
2.5.3. Defining and Analyzing the Differentially Expressed Genes
2.6. Statistical Analysis
3. Results and Discussion
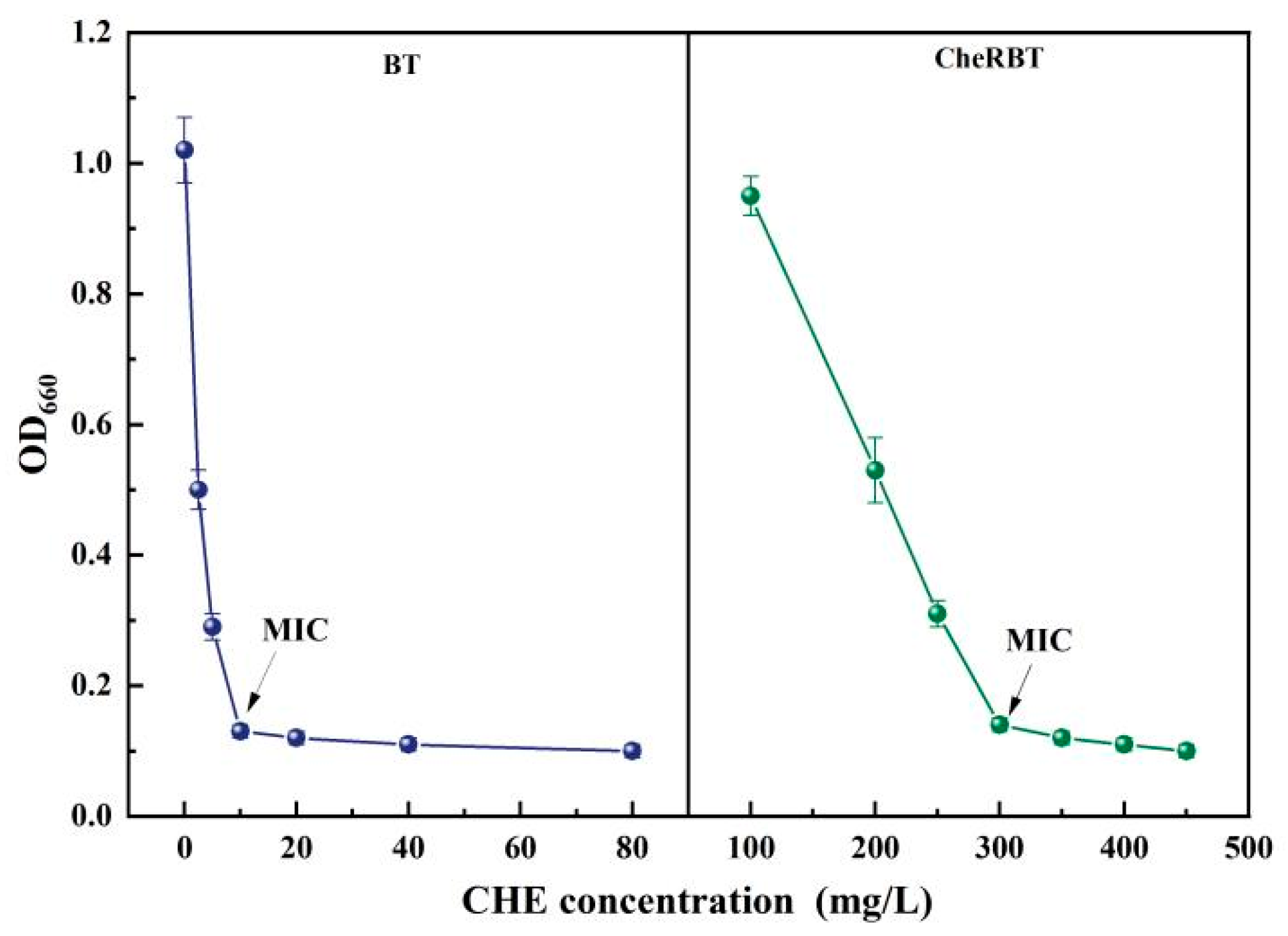
3.1. MICs of the Wild-Type and Chelerythrine-Resistant B. tropicus Strains
3.2. Growth and Metabolism Changes in BT and CheRBT
3.2.1. Growth Curve Changes in CheRBT with CHE Addition
3.2.2. Morphological Characteristics of the Two Strains
3.2.3. Glucose Content in the BT and CheRBT Culturing Systems
3.2.4. DNA Content of BT and CheRBT
3.3. Transcriptome Analysis
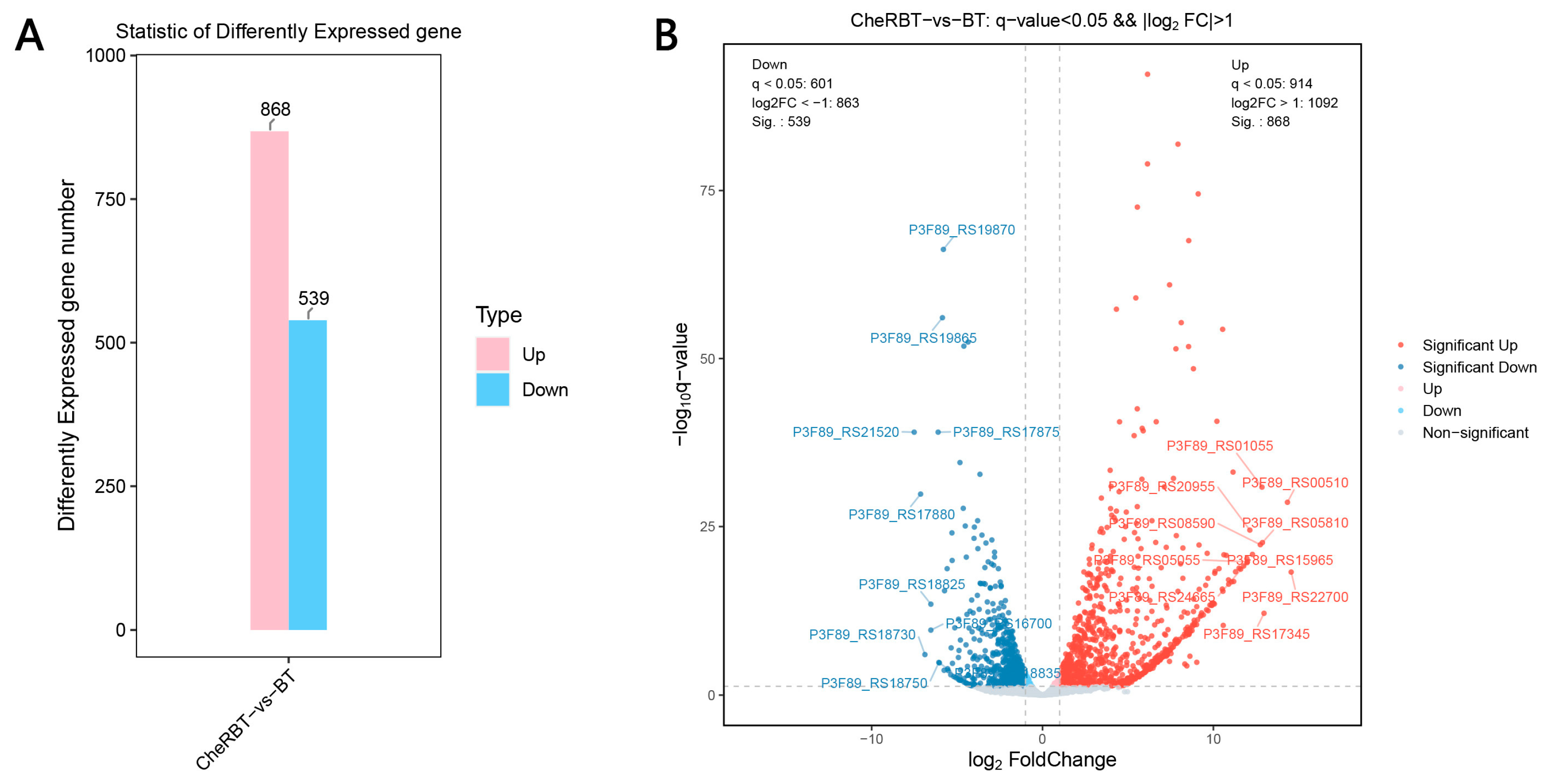
3.3.1. Identification of Differentially Expressed Genes (DEGs)
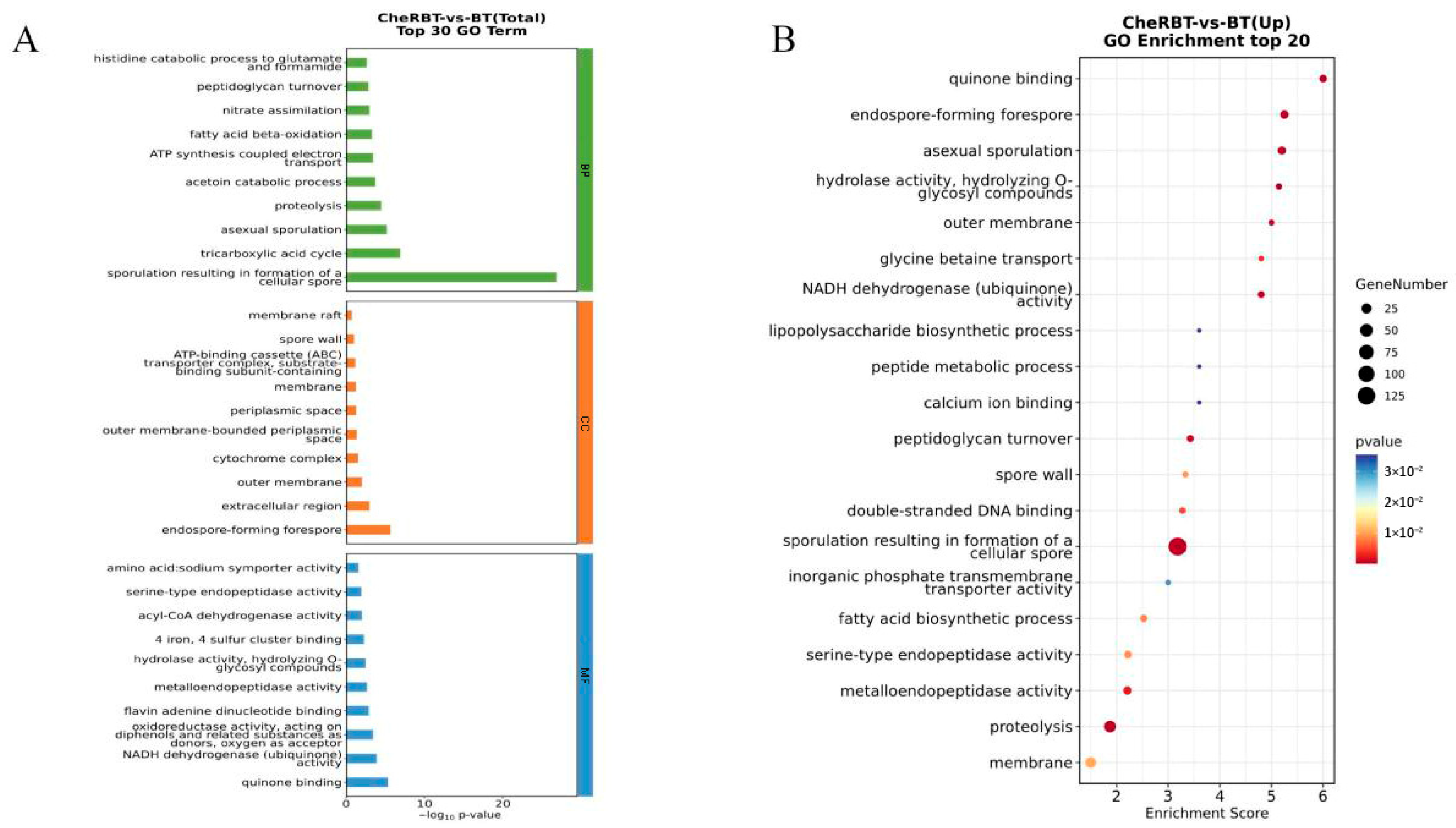
3.3.2. GO Enrichment Analysis
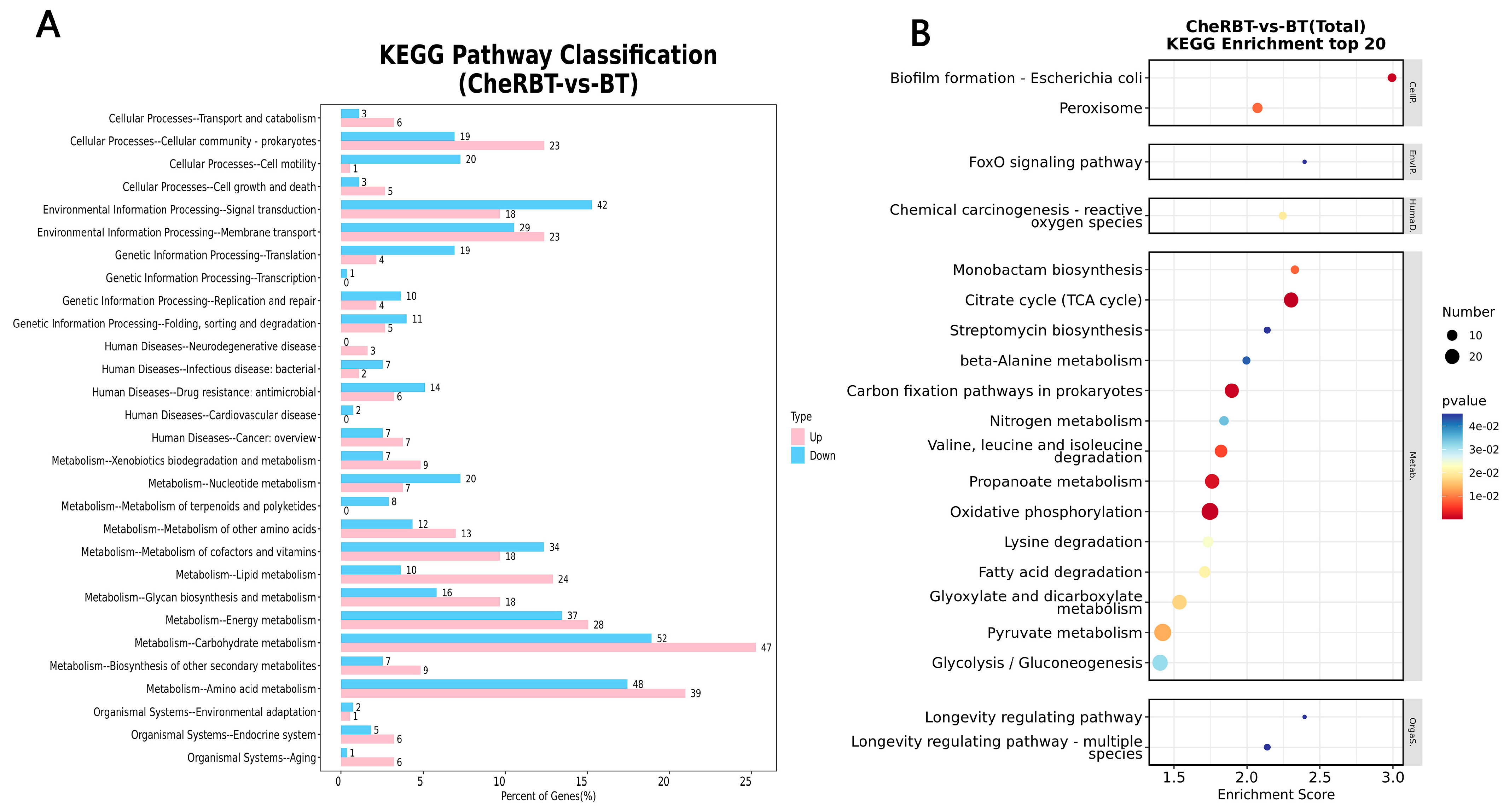
3.3.3. KEGG Pathway Analysis
3.4. Drug Resistance Mechanism Analysis
3.4.1. Glycolysis and Gluconeogenesis
3.4.2. Biofilm Formation Analysis
3.4.3. TCA Cycle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelosi, C.; Bertrand, C.; Daniele, G.; Coeurdassier, M.; Benoit, P.; Nélieu, S.; Lafay, F.; Bretagnolle, V.; Gaba, S.; Vulliet, E.; et al. Residues of Currently Used Pesticides in Soils and Earthworms: A Silent Threat? Agric. Ecosyst. Environ. 2021, 305, 107167. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous Effects of Chemical Pesticides on Human Health–Cancer and Other Associated Disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Manfo, F.P.T.; Mboe, S.A.; Nantia, E.A.; Ngoula, F.; Telefo, P.B.; Moundipa, P.F.; Cho-Ngwa, F. Evaluation of the Effects of Agro Pesticides Use on Liver and Kidney Function in Farmers from Buea, Cameroon. J. Toxicol. 2020, 2020, 2305764. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.; Upadhyay, N. Effects of Organophosphate Pesticides on Siderophore Producing Soils Microorganisms. Biocatal. Agric. Biotechnol. 2019, 21, 101359. [Google Scholar] [CrossRef]
- Nurmansyah; Idris, H.; Suryani, E.; Gustia, H.; Ramadhan, A.I. The Effect of Various Essential Oil and Solvent Additives on the Botanical Pesticide of Piper Aduncum Essential Oil on Formulation Antifungal Activity. Results Eng. 2022, 16, 100644. [Google Scholar] [CrossRef]
- Saberi, F.; Marzban, R.; Ardjmand, M.; Pajoum Shariati, F.; Tavakoli, O. Optimization of Culture Media to Enhance the Ability of Local Bacillus thuringiensis var. tenebrionis. J. Saudi Soc. Agric. Sci. 2020, 19, 468–475. [Google Scholar] [CrossRef]
- Chen, N.; Qi, Y.; Ma, X.; Xiao, X.; Liu, Q.; Xia, T.; Xiang, J.; Zeng, J.; Tang, J. Rediscovery of traditional plant medicine: An underestimated anticancer drug of chelerythrine. Front. Pharmacol. 2022, 13, 906301. [Google Scholar] [CrossRef]
- Qian, W.; Yang, M.; Li, X.; Sun, Z.; Wang, T. Anti-microbial and anti-biofilm activities of combined chelerythrine-sanguinarine and mode of action against Candida albicans and Cryptococcus neoformans in vitro. Colloids Surf. B Biointerfaces 2020, 191, 111003. [Google Scholar] [CrossRef]
- Kang, S.M.; Kong, F.H.; Shi, X.Y.; Han, H.J.; Li, M.H.; Guan, B.Y.; Yang, M.; Cao, X.Y.; Tao, D.B.; Zheng, Y.; et al. Antibacterial activity and mechanism of lactobionic acid against Pseudomonas fluorescens and Methicillin-resistant Staphylococcus aureus and its application on whole milk. Food Control 2020, 108, 106876. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, J.; Zhao, N.; Cui, D.; Zhao, M. Antibacterial Effect and Mechanism of Chelerythrine on Xanthomonas oryzae pv. oryzae. Microorganisms 2025, 13, 953. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Rabby, S.M.F.; Gupta, D.R.; Rahman, M.; Paul, S.K.; Mahmud, N.U.; Rahat, A.A.M.; Jankuloski, L.; Islam, T. Natural Protein Kinase Inhibitors, Staurosporine, and Chelerythrine Suppress Wheat Blast Disease Caused by Magnaporthe oryzae Triticum. Microorganisms 2022, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Talontsi, F.M.; Matasyoh, J.C.; Ngoumfo, R.M.; Chepkorir, R. Mosquito Larvicidal Activity of Alkaloids from Zanthoxylum lemairei against the Malaria Vector Anopheles gambiae. Pestic. Biochem. Physiol. 2011, 99, 82–85. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Saikkonen, K.; Damerau, A.; Yang, B.; Helander, M. Herbicide Residues in Soil Decrease Microbe-Mediated Plant Protection. Plant Biol. 2023, 25, 571–578. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural Products as Multidrug Resistance Modulators in Cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Liu, S.; Jan, S.; Shi, L.; Fernández-Grandon, G.M.; Gulzar, A.; Ali, B.; Rehman, M.; Wang, M. Knock-Down of Gossypol-Inducing Cytochrome P450 Genes Reduced Deltamethrin Sensitivity in Spodoptera exigua (Hübner). Int. J. Mol. Sci. 2019, 20, 2248. [Google Scholar] [CrossRef]
- Garcés Mejía, A.C.; Pino, N.J.; Peñuela, G.A. Effect of Secondary Metabolites Present in Brassica nigra Root Exudates on Anthracene and Phenanthrene Degradation by Rhizosphere Microorganism. Environ. Eng. Sci. 2018, 35, 203–209. [Google Scholar] [CrossRef]
- Singh, S.; Shyu, D.J.H.; Singh, S.; Shyu, D.J.H. Perspective on Utilization of Bacillus Species as Plant Probiotics for Different Crops in Adverse Conditions. AIMS Microbiol. 2024, 10, 220–238. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus Species in Soil as a Natural Resource for Plant Health and Nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragos, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis Stimulates Resident Rhizosphere Pseudomonas stutzeri for Plant Health through Metabolic Interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Wang, C.; Huang, J.; Feng, L.; Chen, X.; Wang, R. Developmental toxicity induced by chelerythrine in zebrafish embryos via activating oxidative stress and apoptosis pathways. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 273, 109719. [Google Scholar] [CrossRef]
- Yang, P.; Li, F.-J.; Huang, S.-W.; Luo, M.; Lin, W.; Yuan, G.-Q.; Li, Q.-Q. Physiological and Transcriptional Response of Xanthomonas oryzae pv. oryzae to Berberine, an Emerging Chemical Control. Phytopathology 2020, 110, 1027–1038. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for Linking Genomes to Life and the Environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for Integration and Interpretation of Large-Scale Molecular Data Sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Zhang, R.N.; Wang, P.F.; Zhang, W.L.; Li, Z.J.; Pang, X.Y.; Huang, F.F.; Wang, S.S.; Liu, X.N.; Zhang, H. Biofilm-mediated resistance to berberine in Escherichia coli. Front. Cell. Infect. Microbiol. 2025, 15, 1565714. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wen, J.; Zhao, X.; Ding, J.; Qi, G. Surfactin: A Quorum-Sensing Signal Molecule to Relieve CCR in Bacillus amyloliquefaciens. Front. Microbiol. 2020, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Omelon, S.; Georgiou, J.; Habraken, W. A Cautionary (Spectral) Tail: Red-Shifted Fluorescence by DAPI-DAPI Interactions. Biochem. Soc. Trans. 2016, 44, 46–49. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics Technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-Wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef]
- Weber, G.; Convery, H.J.; Lea, M.A.; Stamm, N.B. Feedback Inhibition of Key Glycolytic Enzymes in Liver: Action of Free Fatty Acids. Science 1966, 154, 1357–1360. [Google Scholar] [CrossRef]
- Zhang, C.-S.; Hawley, S.A.; Zong, Y.; Li, M.; Wang, Z.; Gray, A.; Ma, T.; Cui, J.; Feng, J.-W.; Zhu, M.; et al. Fructose-1,6-Bisphosphate and Aldolase Mediate Glucose Sensing by AMPK. Nature 2017, 548, 112–116. [Google Scholar] [CrossRef]
- Hardie, K.R.; Heurlier, K. Establishing bacterial communities by ‘word of mouth’: LuxS and autoinducer 2 in biofilm development. Nat. Rev. Microbiol. 2008, 6, 635–643. [Google Scholar] [CrossRef]
- Jackson, D.W.; Suzuki, K.; Oakford, L.; Simecka, J.W.; Hart, M.E.; Romeo, T. Biofilm Formation and Dispersal under the Influence of the Global Regulator CsrA of Escherichia coli. J. Bacteriol. 2002, 184, 290–301. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.; Wang, Y.; Wen, J.; Zhao, X.; Qi, G. Comparative Study of the Role of Surfactin-Triggered Signalling in Biofilm Formation among Different Bacillus Species. Microbiol. Res. 2022, 254, 126920. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, D.; Haagensen, J.A.J.; Rich, C.; Forestier, C. Characterization of Type 2 Quorum Sensing in Klebsiella pneumoniae and Relationship with Biofilm Formation. J. Bacteriol. 2005, 187, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kim, J.-W. Function of the mdxR Gene Encoding a Novel Regulator for Carbohydrate Metabolism and Sporulation in Bacillus subtilis 168. Arch. Microbiol. 2023, 205, 78. [Google Scholar] [CrossRef] [PubMed]
- Meylan, S.; Porter, C.B.M.; Yang, J.H.; Belenky, P.; Gutierrez, A.; Lobritz, M.A.; Park, J.; Kim, S.H.; Moskowitz, S.M.; Collins, J.J. Carbon Sources Tune Antibiotic Susceptibility in Pseudomonas aeruginosa via Tricarboxylic Acid Cycle Control. Cell Chem. Biol. 2017, 24, 195–206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wan, H.; Chai, W.; Cui, D.; Zhao, M. Chelerythrine-Mediated Growth Inhibition and Resistance Mechanism in Bacillus tropicus. Microorganisms 2025, 13, 2731. https://doi.org/10.3390/microorganisms13122731
Wang J, Wan H, Chai W, Cui D, Zhao M. Chelerythrine-Mediated Growth Inhibition and Resistance Mechanism in Bacillus tropicus. Microorganisms. 2025; 13(12):2731. https://doi.org/10.3390/microorganisms13122731
Chicago/Turabian StyleWang, Jueyu, Hongxia Wan, Wenqi Chai, Daizong Cui, and Min Zhao. 2025. "Chelerythrine-Mediated Growth Inhibition and Resistance Mechanism in Bacillus tropicus" Microorganisms 13, no. 12: 2731. https://doi.org/10.3390/microorganisms13122731
APA StyleWang, J., Wan, H., Chai, W., Cui, D., & Zhao, M. (2025). Chelerythrine-Mediated Growth Inhibition and Resistance Mechanism in Bacillus tropicus. Microorganisms, 13(12), 2731. https://doi.org/10.3390/microorganisms13122731




