Diversity of Bacterial Soft Rot-Causing Pectobacterium Species Affecting Cabbage in Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Pathogen Isolation
2.2. Pathogenicity on Cabbage
2.3. Genotyping Methods
2.3.1. DNA Extraction
2.3.2. Preliminary Identification
2.3.3. Repetitive Element Palindromic PCR (Rep-PCR)
2.3.4. Multilocus Sequence Typing and Analysis (MLST/MLSA)
2.4. Virulence Assessment
3. Results
3.1. Isolation, Preliminary Identification, and Pathogenicity
3.2. Genetic Characterization
3.2.1. Rep-PCR
3.2.2. MLST and MLSA
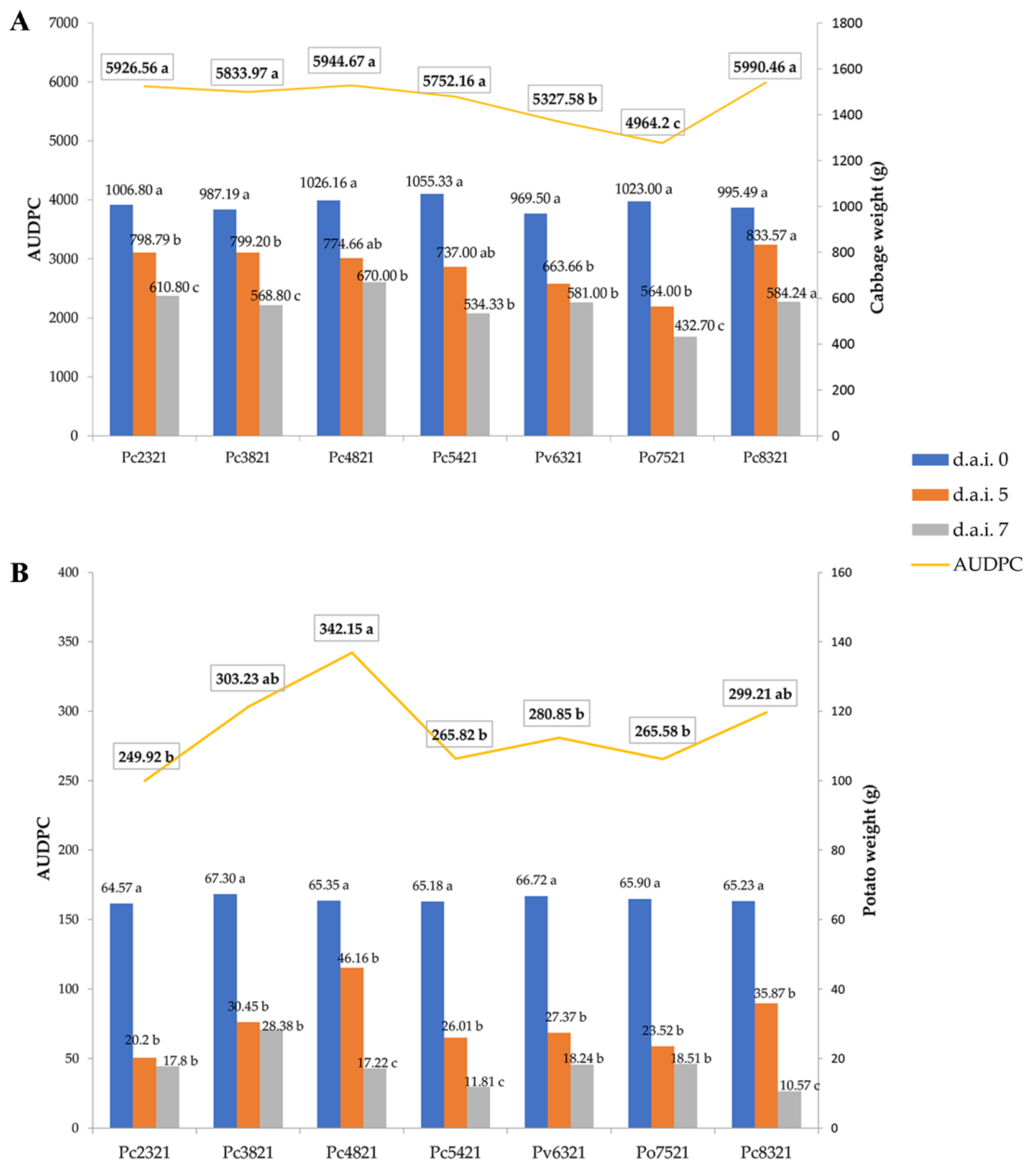
3.3. Virulence Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galal, T.M.; Khalafallah, A.A.; Elawa, O.E.; Hassan, L.M. Human health risks from consuming cabbage (Brassica oleracea L. var. capitata) grown on wastewater irrigated soil. Int. J. Phytoremediation 2018, 20, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Pavlović, I.; Salopek-Sondi, B. White cabbage (Brassica oleracea var. capitata f. alba): Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2017, 16, 117–135. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 21 November 2022).
- Živanović Miljković, J.; Popović, V.; Gajić, A. Land Take Processes and Challenges for Urban Agriculture: A Spatial Analysis for Novi Sad, Serbia. Land 2022, 11, 769. [Google Scholar] [CrossRef]
- Cui, W.; He, P.; Munir, S.; He, P.; He, Y.; Li, X.; Yang, L.; Wang, B.; Wu, Y.; He, P. Biocontrol of soft rot of Chinese cabbage using an endophytic bacterial strain. Front. Microbiol. 2019, 10, 1471. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.A.; Masood, S.D.; Bhat, N.A.; Bhat, M.A.; Razvi, S.M.; Mir, M.R.; Akhtar, S.; Wani, N.; Habib, M. Current status of post harvest soft rot in vegetables: A review. Asian J. Plant Sci. 2010, 9, 200–208. [Google Scholar] [CrossRef]
- Harter, L.L.; Jones, L.R. Cabbage Diseases; Farmers’ Bulletin No. 1351; US Government Printing Office: Washington, DC, USA, 1923; p. 20.
- Popović, T.; Jelušić, A.; Marković, S.; Iličić, R. Characterization of Pectobacterium carotovorum subsp. carotovorum isolates from a recent outbreak on cabbage in Bosnia and Herzegovina. Pestic. Phytomedicine 2019, 34, 211–222. [Google Scholar] [CrossRef]
- Van Gijsegem, F.; Toth, I.K.; van der Wolf, J.M. Outlook—Challenges and perspectives for management of diseases caused by Pectobacterium and Dickeya species. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Springer: Cham, Switzerland, 2021; pp. 283–289. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant diseases caused by prokaryotes: Bacteria and mollicutes. Bacterial Soft Rots. In Plant Patholog; Academic Press: Cambridge, MA, USA, 2005; pp. 615–703. [Google Scholar] [CrossRef]
- Czajkowski, R.; Perombelon, M.C.; van Veen, J.A.; van der Wolf, J.M. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Charkowski, A.O. The changing face of bacterial soft-rot diseases. Annu. Rev. Phytopathol. 2018, 56, 269–288. [Google Scholar] [CrossRef]
- Jonkheer, E.M.; Brankovics, B.; Houwers, I.M.; van der Wolf, J.M.; Bonants, P.J.; Vreeburg, R.A.; Bollema, R.; de Haan, J.R.; Berke, L.; Smit, S.; et al. The Pectobacterium pangenome, with a focus on Pectobacterium brasiliense, shows a robust core and extensive exchange of genes from a shared gene pool. BMC Genom. 2021, 22, 265. [Google Scholar] [CrossRef]
- Achtman, M. Evolution, population structure, and phylogeography of genetically monomorphic bacterial pathogens. Annu. Rev. Microbiol. 2008, 62, 53–70. [Google Scholar] [CrossRef]
- Nabhan, S.; De Boer, S.H.; Maiss, E.; Wydra, K. Taxonomic relatedness between Pectobacterium carotovorum subsp. carotovorum, Pectobacterium carotovorum subsp. odoriferum and Pectobacterium carotovorum subsp. brasiliense subsp. nov. J. Appl. Microbiol. 2012, 113, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hibbing, M.E.; Kim, H.S.; Reedy, R.M.; Yedidia, I.; Breuer, J.; Charkowski, A.O. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology 2007, 97, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Moleleki, L.N.; Onkendi, E.M.; Mongae, A.; Kubheka, G.C. Characterisation of Pectobacterium wasabiae causing blackleg and soft rot diseases in South Africa. Eur. J. Plant Pathol. 2013, 135, 279–288. [Google Scholar] [CrossRef]
- Pitman, A.R.; Harrow, S.A.; Visnovsky, S.B. Genetic characterisation of Pectobacterium wasabiae causing soft rot disease of potato in New Zealand. Eur. J. Plant Pathol. 2010, 126, 423–435. [Google Scholar] [CrossRef]
- Waleron, M.; Misztak, A.; Waleron, M.; Franczuk, M.; Jońca, J.; Wielgomas, B.; Mikiciński, A.; Popović, T.; Waleron, K. Pectobacterium zantedeschiae sp. nov. a new species of a soft rot pathogen isolated from Calla lily (Zantedeschia spp.). Syst. Appl. Microbiol. 2019, 42, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Marković, S.; Milić Komić, S.; Jelušić, A.; Iličić, R.; Bagi, F.; Stanković, S.; Popović, T. First report of Pectobacterium versatile causing blackleg of potato in Serbia. Plant Dis. 2022, 106, 312. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Schloop, A.; Swingle, B.; Perry, K.L. Pectobacterium and Dickeya responsible for potato blackleg disease in New York State in 2016. Plant Dis. 2018, 102, 1834–1840. [Google Scholar] [CrossRef]
- Sławiak, M.; van Beckhoven, J.R.; Speksnijder, A.G.; Czajkowski, R.; Grabe, G.; van der Wolf, J.M. Biochemical and genetical analysis reveal a new clade of biovar 3 Dickeya spp. strains isolated from potato in Europe. Eur. J. Plant Pathol. 2009, 125, 245–261. [Google Scholar] [CrossRef]
- Marković, S.; Stanković, S.; Jelušić, A.; Iličić, R.; Kosovac, A.; Poštić, D.; Popović, T. Occurrence and Identification of Pectobacterium carotovorum subsp. brasiliensis and Dickeya dianthicola Causing Blackleg in some Potato Fields in Serbia. Plant Dis. 2021, 105, 1080–1090. [Google Scholar] [CrossRef]
- Norman, D.J.; Yuen, J.M.F.; Resendiz, R.; Boswell, J. Characterization of Erwinia populations from nursery retention ponds and lakes infecting ornamental plants in Florida. Plant Dis. 2003, 87, 193–196. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Nerurkar, A.S. Characterization and differentiation of soft rot causing Pectobacterium carotovorum of Indian origin. Eur. J. Plant Pathol. 2013, 136, 87–102. [Google Scholar] [CrossRef]
- Zoledowska, S.; Motyka, A.; Zukowska, D.; Sledz, W.; Lojkowska, E. Population structure and biodiversity of Pectobacterium parmentieri isolated from potato fields in temperate climate. Plant Dis. 2018, 102, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, J.Y.; Lee, H.W.; Ha, J.H. Inactivation of bacteria causing soft rot disease in fresh cut cabbage using slightly acidic electrolyzed water. Food Control. 2021, 128, 108217. [Google Scholar] [CrossRef]
- Oskiera, M.; Kałużna, M.; Kowalska, B.; Smolińska, U. Pectobacterium carotovorum subsp. odoriferum on cabbage and Chinese cabbage: Identification, characterization and taxonomic relatedness of bacterial soft rot causal agents. J. Plant Pathol. 2017, 99, 149–160. [Google Scholar] [CrossRef]
- Xie, H.; Li, X.Y.; Ma, Y.L.; Tian, Y. First report of Pectobacterium aroidearum causing soft rot of Chinese cabbage in China. Plant Dis. 2018, 102, 674. [Google Scholar] [CrossRef]
- Fujimoto, T.; Nakayama, T.; Ohki, T.; Maoka, T. First Report of Soft Rot of Cabbage Caused by Pectobacterium wasabiae in Japan. Plant Dis. 2021, 105, 2236. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Bo, Z.; Du, W.; Fu, L.; Tian, Y.; Cui, S.; Shi, Y.; Xie, H. Occurrence, Characteristics, and PCR-Based Detection of Pectobacterium polaris Causing Soft Rot of Chinese Cabbage in China. Plant Dis. 2021, 105, 2880–2887. [Google Scholar] [CrossRef]
- Lee, S.; Vu, N.-T.; Oh, E.-J.; Rahimi-Midani, A.; Thi, T.-N.; Song, Y.-R.; Hwang, I.-S.; Choi, T.-J.; Oh, C.-S. Biocontrol of soft rot caused by Pectobacterium odoriferum with bacteriophage phiPccP-1 in Kimchi cabbage. Microorganisms 2021, 9, 779. [Google Scholar] [CrossRef]
- Smoktunowicz, M.; Jonca, J.; Stachowska, A.; May, M.; Waleron, M.M.; Waleron, M.; Waleron, K. The International Trade of Ware Vegetables and Orna-Mental Plants-An Underestimated Risk of Accelerated Spreading of Phytopathogenic Bacteria in the Era of Globalisation and Ongoing Climatic Changes. Pathogens 2022, 11, 728. [Google Scholar] [CrossRef]
- Gavrilović, V.; Obradović, A.; Arsenijević, M. Bacterial soft rot of carrot, parsley and celery. In Plant Pathogenic Bacteria; De Boer, S.H., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 269–271. [Google Scholar]
- Gašić, K.; Gavrilović, V.; Dolovac, N.; Trkulja, N.; Živković, S.; Ristić, D.; Obradović, A. Pectobacterium carotovorum subsp. carotovorum-the causal agent of broccoli soft rot in Serbia. Pestic. Phytomedicine 2014, 29, 249–255. [Google Scholar] [CrossRef]
- Popović, T.; Jelušić, A.; Milovanović, P.; Janjatović, S.; Budnar, M.; Dimkić, I.; Stanković, S. First report of Pectobacterium atrosepticum, causing bacterial soft rot on calla lily in Serbia. Plant Dis. 2017, 101, 2145. [Google Scholar] [CrossRef]
- Zlatković, N.; Prokić, A.; Gašić, K.; Kuzmanović, N.; Ivanović, M.; Obradović, A. First report of Pectobacterium carotovorum subsp. brasiliense causing soft rot on squash and watermelon in Serbia. Plant Dis. 2019, 103, 2667. [Google Scholar] [CrossRef]
- Loc, M.; Milošević, D.; Ivanović, Ž.; Ignjatov, M.; Budakov, D.; Grahovac, J.; Grahovac, M. Genetic Diversity of Pectobacterium spp. on Potato in Serbia. Microorganisms 2022, 10, 1840. [Google Scholar] [CrossRef] [PubMed]
- Loc, M.; Milošević, D.; Ignjatov, M.; Ivanović, Ž.; Budakov, D.; Grahovac, J.; Vlajkov, V.; Pajčin, I.; Grahovac, M. First report of Pectobacterium punjabense causing potato soft rot and blackleg in Serbia. Plant Dis. 2022, 106, 1513. [Google Scholar] [CrossRef]
- Hélias, V.; Hamon, P.; Huchet, E.; Wolf, J.V.D.; Andrivon, D. Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathol. 2012, 61, 339–345. [Google Scholar] [CrossRef]
- Fahy, P.C.; Persley, G.J. Plant Bacterial Diseases: A diagnostic Guide; Academic Press: Sydney, Australia, 1983; 393p. [Google Scholar]
- Bertani, G. Studies on lysogenesis I: The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef]
- Popović, T.; Jelušić, A.; Dimkić, I.; Stanković, S.; Poštić, D.; Aleksić, G.; Veljović Jovanović, S. Molecular characterization of Pseudomonas syringae pv. coriandricola and biochemical changes attributable to the pathological response on its hosts carrot, parsley, and parsnip. Plant Dis. 2019, 103, 3072–3082. [Google Scholar] [CrossRef]
- Kettani-Halabi, M.; Terta, M.; Amdan, M.; El Fahime, E.M.; Bouteau, F.; Ennaji, M.M. An easy, simple inexpensive test for the specific detection of Pectobacterium carotovorum subsp. carotovorum based on sequence analysis of the pmrA gene. BMC Microbiol. 2013, 13, 176. [Google Scholar] [CrossRef]
- Louws, F.J.; Fulbright, D.W.; Stephens, C.T.; De Bruijn, F.J. Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl. Environ. Microbiol. 1994, 60, 2286–2295. [Google Scholar] [CrossRef]
- Versalovic, J.; Schneider, M.; De Bruijn, F.J.; Lupski, J.R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 1994, 5, 25–40. [Google Scholar]
- Arsenijević, M.; Obradović, A. Occurrence of bacterial wilt and soft rot of seed cabbage plants (Brassica oleracea var. capitata L.) in Yugoslavia. J. Phytopathol. 1996, 144, 315–319. [Google Scholar] [CrossRef]
- Zhu, L.; Xie, H.; Chen, S.; Ma, R. Rapid isolation, identification and phylogenetic analysis of Pectobacterium carotovorum ssp. J. Plant Pathol. 2010, 92, 479–483. [Google Scholar]
- Alvarado, I.C.M.; Michereff, S.J.; Mariano, R.L.R.; Souza, E.B.; Quezado-Duval, A.M.; Resende, L.V.; Cardoso, E.; Mizubuti, E.S.G. Characterization and variability of soft rot-causing bacteria in Chinese cabbage in North Eastern Brazil. J. Plant Pathol. 2011, 93, 173–181. [Google Scholar]
- Nazerian, E.; Sijam, K.; Mior Ahmad, Z.A.; Vadamalai, G. First report of cabbage soft rot caused by Pectobacterium carotovorum subsp. carotovorum in Malaysia. Plant Dis. 2011, 95, 491. [Google Scholar] [CrossRef]
- Park, T.H.; Choi, B.S.; Choi, A.Y.; Choi, I.Y.; Heu, S.; Park, B.S. Genome sequence of Pectobacterium carotovorum subsp. carotovorum strain PCC21, a pathogen causing soft rot in Chinese cabbage. J. Bacteriol. 2012, 194, 6345–6346. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Taghavi, S.M. Phenotypic and genotypic characteristics of Iranian soft rot bacterial isolates from different hosts. Phytopathol. Mediterr. 2010, 49, 194–204. [Google Scholar]
- Portier, P.; Pédron, J.; Taghouti, G.; Fischer-Le Saux, M.; Caullireau, E.; Bertrand, C.; Laurent, A.; Chawki, K.; Oulgazi, S.; Moumni, M.; et al. Elevation of Pectobacterium carotovorum subsp. odoriferum to species level as Pectobacterium odoriferum sp. nov., proposal of Pectobacterium brasiliense sp. nov. and Pectobacterium actinidiae sp. nov., emended description of Pectobacterium carotovorum and description of Pectobacterium versatile sp. nov., isolated from streams and symptoms on diverse plants. Int. J. Syst. Evol. Microbiol. 2019, 69, 3207–3216. [Google Scholar] [CrossRef]
- Öztürk, M.; Umar, A.R. Occurrence, identification, and host range of Pectobacterium brasiliense causing soft rot on seed potato tubers in Turkey. J. Plant Dis. Prot. 2022, 1–12. [Google Scholar] [CrossRef]
- Zeigler, D.R. Gene sequences useful for predicting relatedness of whole genomes in bacteria. Int. J. Syst. Evol. Microbiol. 2003, 53, 1893–1900. [Google Scholar] [CrossRef]
- Cariddi, C.; Sanzani, S.M. A severe outbreak of bacterial lettuce soft rot caused by Pectobacterium carotovorum subsp. carotovorum in Apulia (Italy). J. Plant Pathol. 2013, 95, 441–446. [Google Scholar]
- Li, X.; Fu, L.; Chen, C.; Sun, W.; Tian, Y.; Xie, H. Characteristics and rapid diagnosis of Pectobacterium carotovorum ssp. associated with bacterial soft rot of vegetables in China. Plant Dis. 2020, 104, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Waleron, M.; Waleron, K.; Lojkowska, E. Characterization of Pectobacterium carotovorum subsp. odoriferum causing soft rot of stored vegetables. Eur. J. Plant Pathol. 2014, 139, 457–469. [Google Scholar] [CrossRef]

 , P. odoriferum
, P. odoriferum  , and P. versatile
, and P. versatile  isolates examined in this study and 19 strains of P. carotovorum, P. odoriferum, and P. versatile isolated from various hosts and countries, which were retrieved from the GenBank. The tree was rooted with the D. solani strain RNS 05.1.2A.
isolates examined in this study and 19 strains of P. carotovorum, P. odoriferum, and P. versatile isolated from various hosts and countries, which were retrieved from the GenBank. The tree was rooted with the D. solani strain RNS 05.1.2A.
 , P. odoriferum
, P. odoriferum  , and P. versatile
, and P. versatile  isolates examined in this study and 19 strains of P. carotovorum, P. odoriferum, and P. versatile isolated from various hosts and countries, which were retrieved from the GenBank. The tree was rooted with the D. solani strain RNS 05.1.2A.
isolates examined in this study and 19 strains of P. carotovorum, P. odoriferum, and P. versatile isolated from various hosts and countries, which were retrieved from the GenBank. The tree was rooted with the D. solani strain RNS 05.1.2A.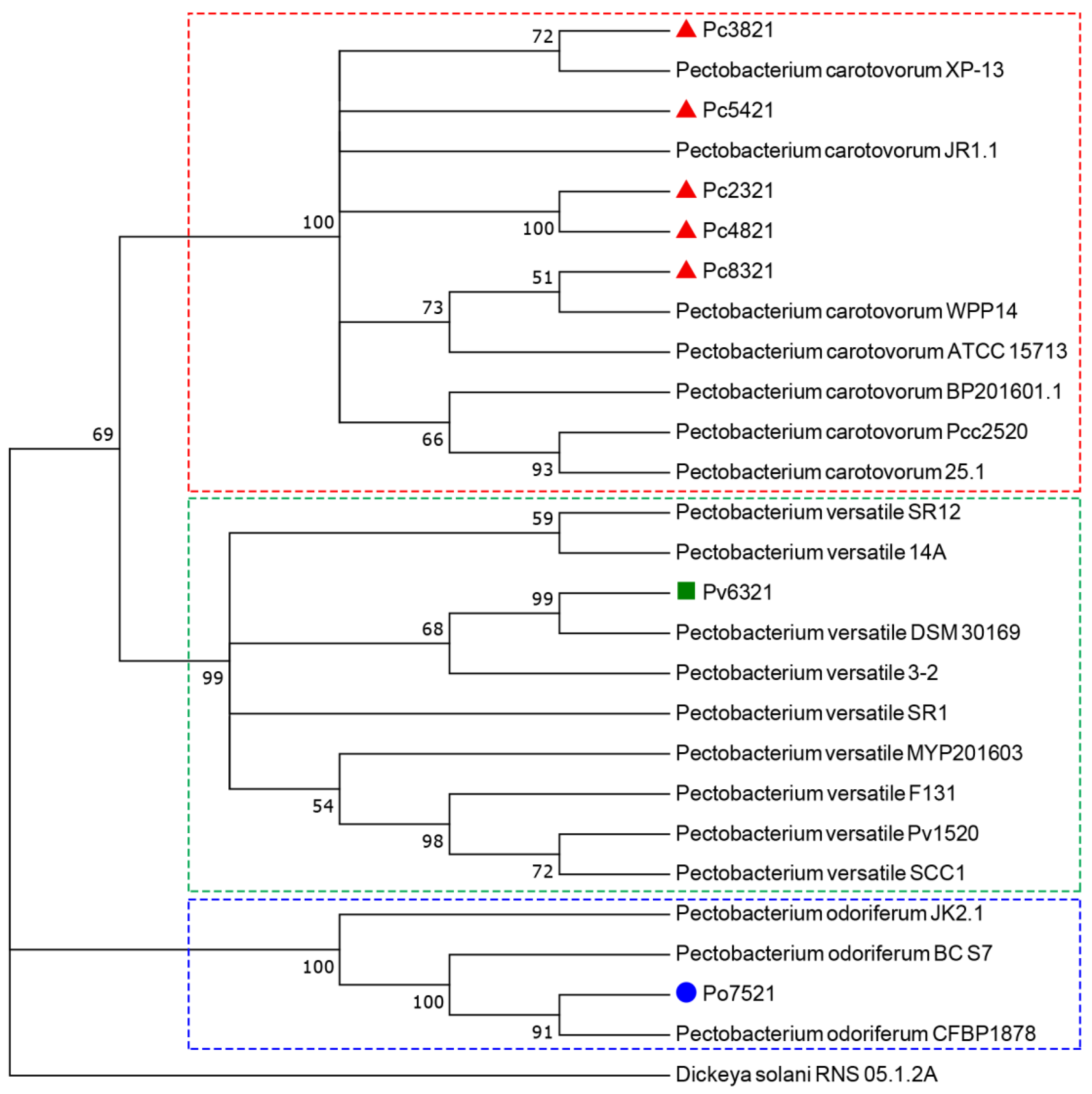

| Primer | Sequence (5′-3′) | Reference |
|---|---|---|
| Pectobacterium-specific primers | ||
| F0145 | TACCCTGCAGATGAAATTATTGATTGTTGAAGAC | [44] |
| E2477 | TACCAAGCTTTGGTTGTTCCCCTTTGGTCA | |
| Primers for rep-PCR | ||
| ERIC1R | ATGTAAGCTCCTGGGGATTCAC | [45] |
| ERIC2 | AAGTAAGTGACTGGGGTGAGCG | |
| REP1R-I | IIIICGICGICATCIGGC | |
| REP2-I | ICGICTTATCIGGCCTAC | |
| BOXA1R | CTACGGCAAGGCGACGCTGACG | |
| GTG5 | GTGGTGGTGGTGGTG | [46] |
| Primers for MLST | ||
| dnaX-F | TATCAGGTYCTTGCCCGTAAGTGG | [22] |
| dnaX-R | TCGACATCCARCGCYTTGAGATG | |
| icdA400F | GGTGGTATCCGTTCTCTGAACG | [16] |
| icdA977R | TAGTCGCCGTTCAGGTTCATACA | |
| proAF1 | CGGYAATGCGGTGATTCTGCG | |
| proAR1 | GGGTACTGACCGCCACTTC | |
| mdh2 | GCGCGTAAGCCGGGTATGGA | [17] |
| mdh4 | CGCGGCAGCCTGGCCCATAG | |
| Strain a | Isolation Source | Locality | Year | GenBank Accession Numbers | |||
|---|---|---|---|---|---|---|---|
| dnaX | icdA | mdh | proA | ||||
| Pectobacterium odoriferum | |||||||
| CFBP 1878 T | Chicory | France | 1978 | MK516907 | JF926783 | JF926793 | JF926823 |
| BC S7 | Chinese cabbage | Beijing (China) | 2007 | CP009678 | CP009678 | CP009678 | CP009678 |
| JK2.1 | Kimchi cabbage | South Korea | 2016 | CP034938 | CP034938 | CP034938 | CP034938 |
| Pectobacterium carotovorum | |||||||
| ATCC 15713 T | Potato | Denmark | 1952 | MW979047 | FJ895850 | FJ895851 | FJ895853 |
| 25.1 | Cucumber | Svietlahorsk (Belarus) | 2009 | CP088019 | CP088019 | CP088019 | CP088019 |
| WPP14 | Potato | Wisconsin (USA) | 2015 | CP051652 | CP051652 | CP051652 | CP051652 |
| BP201601.1 | Potato | Boseong (South Korea) | 2016 | CP034236 | CP034236 | CP034236 | CP034236 |
| JR1.1 | Radish | South Korea | 2016 | CP034237 | CP034237 | CP034237 | CP034237 |
| XP-13 | Potato | Zhouning (China) | 2018 | CP063242 | CP063242 | CP063242 | CP063242 |
| Pcc2520 | Potato | Serbia | 2020 | MW805307 | OP751390 | OP751392 | OP751393 |
| Pectobacterium versatile | |||||||
| 14A | Potato | Minsk (Belarus) | 1978 | CP034276 | CP034276 | CP034276 | CP034276 |
| 3-2 | Potato | Minsk (Belarus) | 1979 | CP024842 | CP024842 | CP024842 | CP024842 |
| SCC1 | Potato | Finland | 1982 | CP021894 | CP021894 | CP021894 | CP021894 |
| F131 | Potato | Moscow (Russia) | 1993 | CP065030 | CP065030 | CP065030 | CP065030 |
| DSM 30169 | Cabbage | Germany | 2010 | CP065143 | CP065143 | CP065143 | CP065143 |
| MYP201603 | Potato | Miryang (South Korea) | 2013 | CP051628 | CP051628 | CP051628 | CP051628 |
| SR1 | Carrot | Iowa, Ames (USA) | 2019 | CP084656 | CP084656 | CP084656 | CP084656 |
| SR12 | Coleslaw | Iowa, Ames (USA) | 2019 | CP084654 | CP084654 | CP084654 | CP084654 |
| Pv1520 | Potato | Serbia | 2020 | MW805306 | OP751391 | MZ682621 | MZ682624 |
| Isolate Code | Hybrid | Pectolytic Activity | Genus Confirmation | DNA Fingerprinting Group | |||
|---|---|---|---|---|---|---|---|
| BOX | ERIC | GTG5 | REP | ||||
| Pc2021 | Cheers F1 | + | + | I | I | I | I |
| Pc2121 | Cheers F1 | + | + | I | I | I | I |
| Pc2221 | Cheers F1 | + | + | I | I | I | I |
| Pc2321 | Cheers F1 | + | + | I | I | I | I |
| Pc2421 | Cheers F1 | + | + | I | I | I | I |
| Pc2521 | Cheers F1 | + | + | I | I | I | I |
| Pc2621 | Cheers F1 | + | + | I | I | I | I |
| Pc2721 | Cheers F1 | + | + | I | I | I | I |
| Pc2821 | Cheers F1 | + | + | I | I | I | I |
| Pc3121 | Cheers F1 | + | + | II | II | II | II |
| Pc3221 | Cheers F1 | + | + | II | II | II | II |
| Pc3321 | Cheers F1 | + | + | II | II | II | II |
| Pc3421 | Cheers F1 | + | + | II | II | II | II |
| Pc3521 | Cheers F1 | + | + | II | II | II | II |
| Pc3621 | Cheers F1 | + | + | II | II | II | II |
| Pc3721 | Cheers F1 | + | + | II | II | II | II |
| Pc3821 | Cheers F1 | + | + | II | II | II | II |
| Pc3921 | Cheers F1 | + | + | II | II | II | II |
| Pc4221 | Cheers F1 | + | + | III | II | III | III |
| Pc4321 | Cheers F1 | + | + | III | II | III | III |
| Pc4421 | Cheers F1 | + | + | III | II | III | III |
| Pc4521 | Cheers F1 | + | + | III | II | III | III |
| Pc4621 | Cheers F1 | + | + | III | II | III | III |
| Pc4721 | Cheers F1 | + | + | III | II | III | III |
| Pc4821 | Cheers F1 | + | + | III | II | III | III |
| Pc4921 | Cheers F1 | + | + | III | II | III | III |
| Pc5021 | Cheers F1 | + | + | IV | III | IV | III |
| Pc5121 | Cheers F1 | + | + | IV | III | IV | III |
| Pc5221 | Cheers F1 | + | + | IV | III | IV | III |
| Pc5321 | Cheers F1 | + | + | IV | III | IV | III |
| Pc5421 | Cheers F1 | + | + | IV | III | IV | III |
| Pc5521 | Cheers F1 | + | + | IV | III | IV | III |
| Pc5621 | Cheers F1 | + | + | IV | III | IV | III |
| Pc5721 | Cheers F1 | + | + | IV | III | IV | III |
| Pv1021 | Hippo F1 | + | + | V | IV | V | IV |
| Pv1121 | Hippo F1 | + | + | V | IV | V | IV |
| Pv1221 | Hippo F1 | + | + | V | IV | V | IV |
| Pv1321 | Hippo F1 | + | + | V | IV | V | IV |
| Pv1421 | Hippo F1 | + | + | V | IV | V | IV |
| Pv1521 | Hippo F1 | + | + | V | IV | V | IV |
| Pv1621 | Hippo F1 | + | + | V | IV | V | IV |
| Pv6121 | Hippo F1 | + | + | V | IV | V | IV |
| Pv6221 | Hippo F1 | + | + | V | IV | V | IV |
| Pv6321 | Hippo F1 | + | + | V | IV | V | IV |
| Pv6421 | Hippo F1 | + | + | V | IV | V | IV |
| Pv6521 | Hippo F1 | + | + | V | IV | V | IV |
| Pv6621 | Hippo F1 | + | + | V | IV | V | IV |
| Po7021 | Hippo F1 | + | + | VI | V | VI | V |
| Po7121 | Hippo F1 | + | + | VI | V | VI | V |
| Po7221 | Hippo F1 | + | + | VI | V | VI | V |
| Po7321 | Hippo F1 | + | + | VI | V | VI | V |
| Po7421 | Hippo F1 | + | + | VI | V | VI | V |
| Po7521 | Hippo F1 | + | + | VI | V | VI | V |
| Po9121 | Hippo F1 | + | + | VI | V | VI | V |
| Po9221 | Hippo F1 | + | + | VI | V | VI | V |
| Po9321 | Hippo F1 | + | + | VI | V | VI | V |
| Po9421 | Hippo F1 | + | + | VI | V | VI | V |
| Po9521 | Hippo F1 | + | + | VI | V | VI | V |
| Po9621 | Hippo F1 | + | + | VI | V | VI | V |
| Pc8021 | Hippo F1 | + | + | VII | VI | VII | VI |
| Pc8121 | Hippo F1 | + | + | VII | VI | VII | VI |
| Pc8221 | Hippo F1 | + | + | VII | VI | VII | VI |
| Pc8321 | Hippo F1 | + | + | VII | VI | VII | VI |
| Pc8421 | Hippo F1 | + | + | VII | VI | VII | VI |
| Pc8521 | Hippo F1 | + | + | VII | VI | VII | VI |
| Pc8621 | Hippo F1 | + | + | VII | VI | VII | VI |
| Pc8721 | Hippo F1 | + | + | VII | VI | VII | VI |
| Isolate Code | Hybrid | Identification According to the NCBI BLASTn (Per. Ident) | |||||
|---|---|---|---|---|---|---|---|
| Species | dnaX | icdA | mdh | proA | |||
| Pc2321 (group Pc2021–Pc2821) | Cheers F1 | P. carotovorum | 99.79% | 99.81% | 99.75% | 100% | |
| Pc3821 (group Pc3121–Pc3921) | Cheers F1 | P. carotovorum | 99.79% | 100% | 100% | 99.25% | |
| Pc4821 (group Pc4221–Pc4921) | Cheers F1 | P. carotovorum | 100% | 100% | 99.75% | 99.85% | |
| Pc5421 (group Pc5021–Pc5721) | Cheers F1 | P. carotovorum | 99.36% | 99.44% | 99.02% | 97.76% | |
| Pv6321 | (group Pv1021–Pv1621, Pv6121–Pv6621) | Hippo F1 | P. versatile | 99.78% | 100% | 100% | 99.85% |
| Po7521 | (group Po7021–Po7521, Po9121–Po9621) | Hippo F1 | P. odoriferum | 99.79% | 100% | 100% | 99.57% |
| Pc8321 (group Pc8021–Pc8721) | Hippo F1 | P. carotovorum | 100% | 100% | 100% | 97.91% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelušić, A.; Mitrović, P.; Marković, S.; Iličić, R.; Milovanović, P.; Stanković, S.; Popović Milovanović, T. Diversity of Bacterial Soft Rot-Causing Pectobacterium Species Affecting Cabbage in Serbia. Microorganisms 2023, 11, 335. https://doi.org/10.3390/microorganisms11020335
Jelušić A, Mitrović P, Marković S, Iličić R, Milovanović P, Stanković S, Popović Milovanović T. Diversity of Bacterial Soft Rot-Causing Pectobacterium Species Affecting Cabbage in Serbia. Microorganisms. 2023; 11(2):335. https://doi.org/10.3390/microorganisms11020335
Chicago/Turabian StyleJelušić, Aleksandra, Petar Mitrović, Sanja Marković, Renata Iličić, Predrag Milovanović, Slaviša Stanković, and Tatjana Popović Milovanović. 2023. "Diversity of Bacterial Soft Rot-Causing Pectobacterium Species Affecting Cabbage in Serbia" Microorganisms 11, no. 2: 335. https://doi.org/10.3390/microorganisms11020335
APA StyleJelušić, A., Mitrović, P., Marković, S., Iličić, R., Milovanović, P., Stanković, S., & Popović Milovanović, T. (2023). Diversity of Bacterial Soft Rot-Causing Pectobacterium Species Affecting Cabbage in Serbia. Microorganisms, 11(2), 335. https://doi.org/10.3390/microorganisms11020335







