Epidemiology of Usutu Virus: The European Scenario
Abstract
:1. Introduction
2. Austria
3. Belgium
4. Croatia
5. Czech Republic
6. France
7. Germany
8. Greece
9. Hungary
10. Italy
11. Netherlands
12. Poland
13. Serbia
14. Slovakia
15. Spain
16. Switzerland
17. United Kingdom
18. Conclusions
Funding
Conflicts of Interest
References
- Cadar, D.; Lühken, R.; van der Jeugd, H.; Garigliany, M.; Ziegler, U.; Keller, M.; Lahoreau, J.; Lachmann, J.; Becker, N.; Kik, M.; et al. Widespread activity of multiple lineages of Usutu virus, Western Europe, 2016. Eurosurveillance 2016, 22, 30452. [Google Scholar] [CrossRef]
- Williams, M.C.; Simpson, D.I.; Haddow, A.J.; Knight, E.M. The isolation of West Nile Virus from man and of Usutu virus from the bird-biting mosquito Mansonia aurites (Theobald) in the Entebbe area of Uganda. Ann. Trop. Med. Parasitol. 1964, 58, 367–374. [Google Scholar] [CrossRef]
- Nikolay, B.; Diallo, M.; Boye, C.S.; Sall, A.A. Usutu virus in Africa. Vector Borne Zoonotic Dis. 2011, 11, 1417–1423. [Google Scholar] [CrossRef]
- Durand, B.; Haskouri, H.; Lowenski, S.; Vachiery, N.; Beck, C.; Lecollinet, S. Seroprevalence of West Nile and Usutu viruses in military working horses and dogs, Morocco, 2012: Dog as an alternative WNV sentinel species? Epidemiol. Infect. 2016, 144, 1857–1864. [Google Scholar] [CrossRef] [Green Version]
- Ben Hassine, T.; De Massis, F.; Calistri, P.; Savini, G.; Bel Haj Mohamed, B.; Ranen, A.; Di Gennaro, A.; Sghaier, S.; Hammami, S. First detection of co-circulation of West Nile and Usutu viruses in equids in the south-west of Tunisia. Transbound. Emerg. Dis. 2014, 61, 385–389. [Google Scholar] [CrossRef]
- Diagne, M.M.; Henriette, M.; Ndione, D.; Di Paola, N.; Fall, G.; Pouwedeou Bedekelabou, A.; Mbacké Sembène, P.; Faye, O.; Marinho de Andrade Zanotto, P.; Sall, A.A. Usutu virus isolated from rodents in Senegal. Viruses 2019, 11, 181. [Google Scholar] [CrossRef] [Green Version]
- Weissenböck, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus, Italy, 1996. Emerg. Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef]
- Weissenböck, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef]
- Becker, N.; Jöst, H.; Ziegler, U.; Eiden, M.; Höper, D.; Emmerich, P.; Fichet-Calvet, E.; Ehichioya, D.U.; Czajka, C.; Gabriel, M.; et al. Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS ONE 2012, 7, e32604. [Google Scholar] [CrossRef]
- Rouffaer, L.O.; Steensels, M.; Verlinden, M.; Vervaeke, M.; Boonyarittichaikij, R.; Martel, A.; Lambrecht, B. Usutu virus epizootic and Plasmodium coinfection in Eurasian blackbirds (Turdus merula) in Flanders, Belgium. J. Wildl. Dis. 2018, 54, 859–862. [Google Scholar] [CrossRef]
- Zannoli, S.; Sambri, V. West Nile virus and Usutu virus co-circulation in Europe: Epidemiology and implications. Microorganisms 2019, 7, e184. [Google Scholar] [CrossRef] [Green Version]
- Benzarti, E.; Linden, A.; Desmecht, D.; Garigliany, M. Mosquito-borne epornitic flaviviruses: An updateand review. J. Gen. Virol. 2019, 100, 119–132. [Google Scholar] [CrossRef]
- Jöst, H.; Bialonski, A.; Maus, D.; Sambri, V.; Eiden, M.; Groschup, M.H.; Günther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation of Usutu virus in Germany. Am. J. Trop. Med. Hyg. 2011, 85, 551–553. [Google Scholar] [CrossRef] [Green Version]
- Calzolari, M.; Gaibani, P.; Bellini, R.; Defilippo, F.; Pierro, A.; Albieri, A.; Maioli, G.; Luppi, A.; Rossini, G.; Balzani, A.; et al. Mosquito, bird and human surveillance of West Nile and Usutu viruses in Emilia-Romagna Region (Italy) in 2010. PLoS ONE 2012, 7, e38058. [Google Scholar] [CrossRef] [Green Version]
- Klobucar, A.; Benic, N.; Krajcar, D.; Kosanovic-Licina, M.L.; Tesic, V.; Merdic, E.; Vrucina, I.; Savic, V.; Barbic, L.; Stevanovic, V.; et al. An overview of mosquitoes and emerging arboviral infections in the Zagreb area, Croatia. J. Infect. Dev. Ctries. 2016, 10, 1286–1293. [Google Scholar] [CrossRef] [Green Version]
- Calzolari, M.; Chiapponi, C.; Bonilauri, P.; Lelli, D.; Baioni, L.; Barbieri, I.; Lavazza, A.; Pongolini, S.; Dottori, M.; Moreno, A. Co-circulation of two Usutu virus strains in Northern Italy between 2009 and 2014. Infect. Genet. Evol. 2017, 51, 255–262. [Google Scholar] [CrossRef]
- Camp, J.V.; Kolodziejek, J.; Nowotny, N. Targeted surveillance reveals native and invasive mosquito species infected with Usutu virus. Parasit Vectors 2019, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- Hönig, V.; Palus, M.; Kaspar, T.; Zemanova, M.; Majerova, K.; Hofmannova, L.; Papezik, P.; Sikutova, S.; Rettich, F.; Hubalek, Z.; et al. Multiple lineages of Usutu virus (Flaviviridae, Flavivirus) in blackbirds (Turdus merula) and mosquitoes (Culex pipiens, Cx. modestus) in the Czech Republic (2016–2019). Microorganisms 2019, 7, 568. [Google Scholar] [CrossRef] [Green Version]
- Cadar, D.; Becker, N.; Campos Rde, M.; Börstler, J.; Jöst, H.; Schmidt-Chanasit, J. Usutu virus in bats, Germany, 2013. Emerg. Infect. Dis. 2014, 20, 1771–1773. [Google Scholar] [CrossRef]
- Benzarti, E.; Sarlet, M.; Franssen, M.; Cadar, D.; Schmidt-Chanasit, J.; Rivas, J.F.; Linden, A.; Desmecht, D.; Garigliany, M. Usutu virus epizootic in Belgium in 2017 and 2018: Evidence of virus endemization and ongoing introduction events. Vector Borne Zoonotic Dis. 2020, 20, 43–50. [Google Scholar] [CrossRef]
- Savini, G.; Monaco, F.; Terregino, C.; Di Gennaro, A.; Bano, L.; Pinoni, C.; De Nardi, R.; Bonilauri, P.; Pecorari, M.; Di Gialleonardo, L.; et al. Usutu virus in Italy: An emergence or a silent infection? Vet. Microbiol. 2011, 151, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Barbic, L.; Vilibic-Cavlek, T.; Listes, E.; Stevanovic, V.; Gjenero-Margan, I.; Ljubin-Sternak, S.; Pem-Novosel, I.; Listes, I.; Mlinaric-Galinovic, G.; Di Gennaro, A.; et al. Demonstration of Usutu virus antibodies in horses, Croatia. Vector Borne Zoonotic Dis. 2013, 13, 772–774. [Google Scholar] [CrossRef]
- Csank, T.; Drzewnioková, P.; Korytár, Ľ.; Major, P.; Gyuranecz, M.; Pistl, J.; Bakonyi, T. A Serosurvey of flavivirus infection in horses and birds in Slovakia. Vector Borne Zoonotic Dis. 2018, 18, 206–213. [Google Scholar] [CrossRef]
- Montagnaro, S.; Piantedosi, D.; Ciarcia, R.; Loponte, R.; Veneziano, V.; Fusco, G.; Amoroso, M.G.; Ferrara, G.; Damiano, S.; Iovane, G.; et al. Serological evidence of mosquito-borne flaviviruses circulation in hunting dogs in Campania Region, Italy. Vector Borne Zoonotic Dis. 2019, 19, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Romeo, C.; Lecollinet, S.; Caballero, J.; Isla, J.; Luzzago, C.; Ferrari, N.; García-Bocanegra, I. Are tree squirrels involved in the circulation of flaviviruses in Italy? Transbound. Emerg. Dis. 2018, 65, 1372–1376. [Google Scholar] [CrossRef]
- Bournez, L.; Umhang, G.; Faure, E.; Boucher, J.M.; Boué, F.; Jourdain, E.; Sarasa, M.; Llorente, F.; Jiménez-Clavero, M.A.; Moutailler, S.; et al. Exposure of wild ungulates to the Usutu and tick-borne encephalitis viruses in France in 2009–2014: Evidence of undetected flavivirus circulation a decade ago. Viruses 2019, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Csank, T.; Pikalík, M.; Majláthová, V.; Majláth, I.; Pistl, J. Detection of neutralizing antibodies against Usutu virus in green lizards (Lacerta viridis). In Proceedings of the Joint Czechoslovak Virology Conference 2019 and 1st SK-AT Structural Virology Meeting, Bratislava, Slovakia, 13–15 February 2019; pp. 48–49. [Google Scholar]
- Cavrini, F.; Gaibani, P.; Longo, G.; Pierro, A.M.; Rossini, G.; Bonilauri, P.; Gerunda, G.E.; Di Benedetto, F.; Pasetto, A.; Girardis, M.; et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August-September 2009. Eurosurveillance 2009, 14, 19448. [Google Scholar]
- Pecorari, M.; Longo, G.; Gennari, W.; Grottola, A.; Sabbatini, A.; Tagliazucchi, S.; Savini, G.; Monaco, F.; Simone, M.; Lelli, R.; et al. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Eurosurveillance 2009, 14, 19446. [Google Scholar]
- Vilibic-Cavlek, T.; Kaic, B.; Barbic, L.; Pem-Novosel, I.; Slavic-Vrzic, V.; Lesnikar, V.; Kurecic-Filipovic, S.; Babic-Erceg, A.; Listes, E.; Stevanovic, V.; et al. First evidence of simultaneous occurrence of West Nile virus and Usutu virus neuroinvasive disease in humans in Croatia during the 2013 outbreak. Infection 2014, 42, 689–695. [Google Scholar] [CrossRef]
- Simonin, Y.; Sillam, O.; Carles, M.J.; Gutierrez, S.; Gil, P.; Constant, O.; Martin, M.F.; Girard, G.; Van de Perre, P.; Salinas, S.; et al. Human Usutu virus infection with atypical neurologic presentation, Montpellier, France, 2016. Emerg. Infect. Dis. 2018, 24, 875–878. [Google Scholar] [CrossRef] [Green Version]
- Vilibic-Cavlek, T.; Savic, V.; Sabadi, D.; Peric, L.; Barbic, L.; Klobucar, A.; Miklausic, B.; Tabain, I.; Santini, M.; Vucelja, M.; et al. Prevalence and molecular epidemiology of West Nile and Usutu virus infections in Croatia in the ‘One health’ context, 2018. Transbound. Emerg. Dis. 2019, 66, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Pacenti, M.; Sinigaglia, A.; Martello, T.; De Rui, M.E.; Franchin, E.; Pagni, S.; Peta, E.; Riccetti, S.; Milani, A.; Montarsi, F.; et al. Clinical and virological findings in patients with Usutu virus infection, northern Italy, 2018. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef]
- Bakonyi, T.; Erdélyi, K.; Brunthaler, R.; Dán, Á.; Weissenböck, H.; Nowotny, N. Usutu virus, Austria and Hungary, 2010-2016. Emerg. Microbes Infect. 2017, 6, e85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberle, S.W.; Kolodziejek, J.; Jungbauer, C.; Stiasny, K.; Aberle, J.H.; Zoufaly, A.; Hourfar, M.K.; Weidner, L.; Nowotny, N. Increase in human West Nile and Usutu virus infections, Austria, 2018. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef] [Green Version]
- Garigliany, M.; Linden, A.; Gilliau, G.; Levy, E.; Sarlet, M.; Franssen, M.; Benzarti, E.; Derouaux, A.; Francis, F.; Desmecht, D. Usutu virus, Belgium, 2016. Infect. Genet. Evol. 2017, 48, 116–119. [Google Scholar] [CrossRef]
- Engel, D.; Jöst, H.; Wink, M.; Börstler, J.; Bosch, S.; Garigliany, M.M.; Jöst, A.; Czajka, C.; Lühken, R.; Ziegler, U.; et al. Reconstruction of the evolutionary history and dispersal of Usutu virus, a neglected emerging arbovirus in Europe and Africa. mBio 2016, 7, e01938-15. [Google Scholar] [CrossRef] [Green Version]
- Weissenböck, H.; Kolodziejek, J.; Fragner, K.; Kuhn, R.; Pfeffer, M.; Nowotny, N. Usutu virus activity in Austria, 2001–2002. Microbes Infect. 2003, 5, 1132–1136. [Google Scholar] [CrossRef]
- Bakonyi, T.; Jungbauer, C.; Aberle, S.W.; Kolodziejek, J.; Dimmel, K.; Stiasny, K.; Allerberger, F.; Nowotny, N. Usutu virus infections among blood donors, Austria, July and August 2017—Raising awareness for diagnostic challenges. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef]
- Garigliany, M.M.; Marlier, D.; Tenner-Racz, K.; Eiden, M.; Cassart, D.; Gandar, F.; Beer, M.; Schmidt-Chanasit, J.; Desmecht, D. Detection of Usutu virus in a bullfinch (Pyrrhula pyrrhula) and a great spotted woodpecker (Dendrocopos major) in north-west Europe. Vet. J. 2014, 199, 191–193. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J.; Juricová, Z.; Sikutová, S.; Rudolf, I.; Honza, M.; Janková, J.; Chytil, J.; Marec, F.; Sitko, J. Serologic survey of birds for West Nile flavivirus in southern Moravia (Czech Republic). Vector Borne Zoonotic Dis. 2008, 8, 659–666. [Google Scholar] [CrossRef]
- Rudolf, I.; Bakonyi, T.; Šebesta, O.; Mendel, J.; Peško, J.; Betášová, L.; Blažejová, H.; Venclíková, K.; Strakova, P.; Nowotny, N.; et al. Co-circulation of Usutu virus and West Nile virus in a reed bed ecosystem. Parasites Vectors 2015, 8, 520. [Google Scholar] [CrossRef] [Green Version]
- Lecollinet, S.; Blanchard, Y.; Manson, C.; Lowenski, S.; Aloy, S.L.E.; Quenault, H.; Touzain, F.; Lucas, P.; Eraud, C.; Bahuon, C.; et al. Dual Emergence of Usutu Virus in Common Blackbirds, Eastern France, 2015. Emerg. Infect. Dis. 2016, 22, 2225. [Google Scholar] [CrossRef] [Green Version]
- Eiden, M.; Gil, P.; Ziegler, U.; Rakotoarivony, I.; Marie, A.; Francés, B.; L’Ambert, G.; Simonin, Y.; Foulongne, V.; Groschup, M.H.; et al. Emergence of two Usutu virus lineages in Culex pipiens mosquitoes in the Camargue, France, 2015. Infect. Genet. Evol. 2018, 61, 151–154. [Google Scholar] [CrossRef]
- Allering, L.; Jöst, H.; Emmerich, P.; Günther, S.; Lattwein, E.; Schmidt, M.; Seifried, E.; Sambri, V.; Hourfar, K.; Schmidt-Chanasit, J. Detection of Usutu virus infection in a healthy blood donor from south-west Germany, 2012. Eurosurveillance 2012, 17, 17. [Google Scholar]
- Chaintoutis, S.C.; Dovas, C.I.; Papanastassopoulou, M.; Gewehr, S.; Danis, K.; Beck, C.; Lecollinet, S.; Antalis, V.; Kalaitzopoulou, S.; Panagiotopoulos, T.; et al. Evaluation of a West Nile virus surveillance and early warning system in Greece, based on domestic pigeons. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 131–141. [Google Scholar] [CrossRef]
- Nagy, A.; Mezei, E.; Nagy, O.; Bakonyi, T.; Csonka, N.; Kaposi, M.; Koroknai, A.; Szomor, K.; Rigó, Z.; Molnár, Z.; et al. Extraordinary increase in West Nile virus cases and first confirmed human Usutu virus infection in Hungary, 2018. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef] [Green Version]
- Tamba, M.; Bonilauri, P.; Bellini, R.; Calzolari, M.; Albieri, A.; Sambri, V.; Dottori, M.; Angelini, P. Detection of Usutu Virus Within a West Nile Virus Surveillance Program in Northern Italy. Vector Borne Zoonotic Dis. 2011, 11, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Rijks, J.; Kik, M.; Slaterus, R.; Foppen, R.; Stroo, A.; Ijzer, J.; Stahl, J.; Gröne, A.; Koopmans, M.; Van Der Jeugd, H.P.; et al. Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Eurosurveillance 2016, 21, 30391. [Google Scholar] [CrossRef]
- Hubálek, Z.; Wegner, E.; Halouzka, J.; Tryjanowski, P.; Jerzak, L.; Šikutová, S.; Rudolf, I.; Kruszewicz, A.G.; Jaworski, Z.; Włodarczyk, R. Serologic Survey of Potential Vertebrate Hosts for West Nile Virus in Poland. Viral Immunol. 2008, 21, 247–254. [Google Scholar] [CrossRef]
- Bażanów, B.A.; Van Vuren, P.J.; Szymański, P.; Stygar, D.M.; Frącka, A.; Twardoń, J.; Kozdrowski, R.; Paweska, J.T. A Survey on West Nile and Usutu Viruses in Horses and Birds in Poland. Viruses 2018, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Lupulović, D.; Martín-Acebes, M.A.; Lazić, S.; Alonso-Padilla, J.; Blazquez, A.; Escribano-Romero, E.; Petrović, T.; Sáiz, J.-C. First Serological Evidence of West Nile Virus Activity in Horses in Serbia. Vector Borne Zoonotic Dis. 2011, 11, 1303–1305. [Google Scholar] [CrossRef] [Green Version]
- Escribano-Romero, E.; Lupulović, D.; Merino-Ramos, T.; Blazquez, A.; Lazic, G.; Lazić, S.; Sáiz, J.-C.; Petrović, T. West Nile virus serosurveillance in pigs, wild boars, and roe deer in Serbia. Vet. Microbiol. 2015, 176, 365–369. [Google Scholar] [CrossRef]
- Cvjetković, I.H.; Petrović, T.; Petrić, D.; Milošević, U.; Radovanov, J.; Kovačević, G.; Galović, A.J.; Patić, A.; Nikolić, N.; Cvjetković, D.; et al. Usutu Virus: An Emerging Flavivirus In Europe. Arch. Vet. Med. 2017, 10, 25–35. [Google Scholar] [CrossRef]
- Čabanová, V.; Sikutova, S.; Strakova, P.; Šebesta, O.; Víchová, B.; Zubriková, D.; Miterpakova, M.; Mendel, J.; Hurníková, Z.; Hubálek, Z.; et al. Co-Circulation of West Nile and Usutu Flaviviruses in Mosquitoes in Slovakia, 2018. Viruses 2019, 11, 639. [Google Scholar] [CrossRef] [Green Version]
- Busquets, N.; Alba, A.; Allepuz, A.; Aranda, C.; Núñez, J.I. Usutu Virus Sequences in Culex pipiens (Diptera:Culicidae), Spain. Emerg. Infect. Dis. 2008, 14, 861–863. [Google Scholar] [CrossRef]
- Jurado-Tarifa, E.; Napp, S.; Lecollinet, S.; Arenas, A.; Beck, C.; Cerdà-Cuéllar, M.; Fernández-Morente, M.; García-Bocanegra, I. Monitoring of West Nile virus, Usutu virus and Meaban virus in waterfowl used as decoys and wild raptors in southern Spain. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 58–64. [Google Scholar] [CrossRef]
- Llorente, F.; Perez-Ramírez, E.; Fernández-Pinero, J.; Soriguer, R.; Figuerola, J.; Jiménez-Clavero, M. Ángel Flaviviruses in Game Birds, Southern Spain, 2011–2012. Emerg. Infect. Dis. 2013, 19, 1023–1025. [Google Scholar] [CrossRef]
- Vanhomwegen, J.; Beck, C.; Desprès, P.; Figuerola, A.; García, R.; Lecollinet, S.; López-Roig, M.; Manuguerra, J.-C.; Serra-Cobo, J. Circulation of Zoonotic Arboviruses in Equine Populations of Mallorca Island (Spain). Vector Borne Zoonotic Dis. 2017, 17, 340–346. [Google Scholar] [CrossRef]
- Steinmetz, H.W.; Bakonyi, T.; Weissenböck, H.; Hatt, J.-M.; Eulenberger, U.; Robert, N.; Hoop, R.; Nowotny, N. Emergence and establishment of Usutu virus infection in wild and captive avian species in and around Zurich, Switzerland—Genomic and pathologic comparison to other central European outbreaks. Vet. Microbiol. 2011, 148, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Buckley, A.; Dawson, A.; Moss, S.R.; Hinsley, S.A.; E Bellamy, P.; Gould, E.A. Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J. Gen. Virol. 2003, 84, 2807–2817. [Google Scholar] [CrossRef]
- Weidinger, P.; Kolodziejek, J.; Bakonyi, T.; Brunthaler, R.; Erdélyi, K.; Weissenböck, H.; Nowotny, N. Different dynamics of Usutu virus infections in Austria and Hungary, 2017–2018. Transbound. Emerg. Dis. 2020, 67, 298–307. [Google Scholar] [CrossRef]
- Cadar, D.; Maier, P.; Muller, S.; Kress, J.; Chudy, M.; Bialonski, A.; Schlaphof, A.; Jansen, S.; Jöst, H.; Tannich, E.; et al. Blood donor screening for West Nile virus (WNV) revealed acute Usutu virus (USUV) infection, Germany, September 2016. Eurosurveillance 2017, 22, 30501. [Google Scholar] [CrossRef]
- Hubálek, Z.; Rudolf, I.; Čapek, M.; Bakonyi, T.; Betášová, L.; Nowotny, N. Usutu virus in blackbirds (Turdus merula), Czech Republic, 2011–2012. Transbound. Emerg. Dis. 2014, 61, 273–276. [Google Scholar] [CrossRef]
- Sieg, M.; Schmidt, V.; Ziegler, U.; Keller, M.; Höper, D.; Heenemann, K.; Rückner, A.; Nieper, H.; Muluneh, A.; Groschup, M.H.; et al. Outbreak and Cocirculation of Three Different Usutu Virus Strains in Eastern Germany. Vector Borne Zoonotic Dis. 2017, 17, 662–664. [Google Scholar] [CrossRef]
- Michel, F.; Sieg, M.; Fischer, D.; Keller, M.; Eiden, M.; Reuschel, M.; Schmidt, V.; Schwehn, R.; Rinder, M.; Urbaniak, S.; et al. Evidence for West Nile Virus and Usutu Virus Infections in Wild and Resident Birds in Germany, 2017 and 2018. Viruses 2019, 11, 674. [Google Scholar] [CrossRef] [Green Version]
- Bakonyi, T.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Csörgő, T.; Lussy, H.; Chvala, S.; Bukovsky, C.; Meister, T.; Weissenböck, H.; et al. Emergence of Usutu Virus in Hungary. J. Clin. Microbiol. 2007, 45, 3870–3874. [Google Scholar] [CrossRef] [Green Version]
- Caracciolo, I.; Cardenas, E.M.; Aloise, C.; Carletti, T.; Segat, L.; Burali, M.S.; Chiarvesio, A.; Totis, V.; Avšič–Županc, T.; Mastrangelo, E.; et al. Comprehensive response to Usutu virus following first isolation in blood donors in the Friuli Venezia Giulia region of Italy: Development of recombinant NS1-based serology and sensitivity to antiviral drugs. PLoS Negl. Trop. Dis. 2020, 14, e0008156. [Google Scholar] [CrossRef]
- Carletti, F.; Colavita, F.; Rovida, F.; Percivalle, E.; Baldanti, F.; Ricci, I.; De Liberato, C.; Rosone, F.; Messina, F.; Lalle, E.; et al. Expanding Usutu virus circulation in Italy: Detection in the Lazio region, central Italy, 2017 to 2018. Eurosurveillance 2019, 24, 1800649. [Google Scholar] [CrossRef]
- Munnink, B.B.O.; Münger, E.; Nieuwenhuijse, D.F.; Kohl, R.; Van Der Linden, A.; Schapendonk, C.M.E.; Van Der Jeugd, H.; Kik, M.; Rijks, J.M.; Reusken, C.B.E.M.; et al. Genomic monitoring to understand the emergence and spread of Usutu virus in the Netherlands, 2016–2018. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zaaijer, H.L.; Slot, E.; Molier, M.; Reusken, C.B.; Koppelman, M.H. Usutu virus infection in Dutch blood donors. Transfusion 2019, 59, 2931–2937. [Google Scholar] [CrossRef]
- Kemenesi, G.; Buzás, D.; Zana, B.; Kurucz, K.; Krtinić, B.; Kepner, A.; Földes, F.; Jakab, F. First genetic characterization of Usutu virus from Culex pipiens mosquitoes Serbia, 2014. Infect. Genet. Evol. 2018, 63, 58–61. [Google Scholar] [CrossRef]
- Petrović, T.; Šekler, M.; Petrić, D.; Vidanović, D.; Potkonjak, A.; Cvjetković, I.H.; Savić, S.; Debeljak, Z.; Lazić, G.; Ćupina, A.I.; et al. Flaviviruses at the territory of Serbia—Present situation and challenges. Arch. Vet. Med. 2018, 11, 53–70. [Google Scholar] [CrossRef]
- Vázquez, A.; Ruiz, S.; Herrero, L.; Moreno, J.; Molero, F.; Magallanes, A.; Sánchez-Seco, M.P.; Figuerola, J.; Tenorio, A. West Nile and Usutu Viruses in Mosquitoes in Spain, 2008–2009. Am. J. Trop. Med. Hyg. 2011, 85, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Chvala, S.; Bakonyi, T.; Bukovsky, C.; Meister, T.; Brugger, K.; Rubel, F.; Nowotny, N.; Weissenböck, H. Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003–2005. Vet. Microbiol. 2007, 122, 237–245. [Google Scholar] [CrossRef]
- Meister, T.; Lussy, H.; Bakonyi, T.; Sikutová, S.; Rudolf, I.; Vogl, W.; Winkler, H.; Frey, H.; Hubálek, Z.; Nowotny, N.; et al. Serological evidence of continuing high Usutu virus (Flaviviridae) activity and establishment of herd immunity in wild birds in Austria. Vet. Microbiol. 2008, 127, 237–248. [Google Scholar] [CrossRef]
- Buchebner, N.; Zenker, W.; Wenker, C.; Steinmetz, H.W.; Sós, E.; Lussy, H.; Nowotny, N. Low Usutu virus seroprevalence in four zoological gardens in central Europe. BMC Vet. Res. 2013, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Benzarti, E.; Garigliany, M.; Hauman, D.; Paternostre, J.; Linden, A.; Franssen, M.; Sarlet, M.; Cassart, D.; Desmecht, D. First evidence of fatal Usutu virus natural infections in an Anatidae, the Common Scoter (Melanitta nigra). Vector Borne Zoonotic Dis. 2019, 19, 777–780. [Google Scholar] [CrossRef]
- Vilibić-Čavlek, T.; Barbić, L.; Stevanović, V.; Mlinarić-Galinović, G. Usutu virus: A novel flavivirus in Croatia. Lijec. Vjesn. 2015, 137, 46–51. (In Croatian) [Google Scholar]
- Santini, M.; Vilibic-Cavlek, T.; Barsic, B.; Barbic, L.; Savic, V.; Stevanovic, V.; Listes, E.; Di Gennaro, A.; Savini, G. First cases of human Usutu virus neuroinvasive infection in Croatia, August-September 2013: Clinical and laboratory features. J. Neurovirol. 2015, 21, 92–97. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Savic, V.; Petrovic, T.; Toplak, I.; Barbic, L.; Petric, D.; Tabain, I.; Hrnjakovic-Cvjetkovic, I.; Bogdanic, M.; Klobucar, A.; et al. Emerging trends in the epidemiology of West Nile and Usutu virus infections in Southern Europe. Front. Vet. Sci. 2019, 6, 437. [Google Scholar] [CrossRef] [Green Version]
- Vittecoq, M.; Lecollinet, S.; Jourdain, E.; Thomas, F.; Blanchon, T.; Arnal, A.; Lowenski, S.; Gauthier-Clerc, M. Recent Circulation of West Nile Virus and Potentially Other Closely Related Flaviviruses in Southern France. Vector Borne Zoonotic Dis. 2013, 13, 610–613. [Google Scholar] [CrossRef]
- Roesch, F.; Fajardo, A.; Moratorio, G.; Vignuzzi, M. Usutu Virus: An Arbovirus on the Rise. Viruses 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, U.; Jöst, H.; Müller, K.; Fischer, D.; Rinder, M.; Tietze, D.T.; Danner, K.-J.; Becker, N.; Skuballa, J.; Hamann, H.-P.; et al. Epidemic Spread of Usutu Virus in Southwest Germany in 2011 to 2013 and Monitoring of Wild Birds for Usutu and West Nile Viruses. Vector Borne Zoonotic Dis. 2015, 15, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Lühken, R.; Jöst, H.; Cadar, D.; Thomas, S.M.; Bosch, S.; Tannich, E.; Becker, N.; Ziegler, U.; Lachmann, L.; Schmidt-Chanasit, J. Distribution of Usutu Virus in Germany and Its Effect on Breeding Bird Populations. Emerg. Infect. Dis. 2017, 23, 1994–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheuch, D.E.; Schäfer, M.; Eiden, M.; Heym, E.C.; Ziegler, U.; Walther, D.; Schmidt-Chanasit, J.; Keller, M.; Groschup, M.H.; Kampen, H. Detection of Usutu, Sindbis, and Batai Viruses in Mosquitoes (Diptera: Culicidae) Collected in Germany, 2011–2016. Viruses 2018, 10, 389. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, U.; Fast, C.; Eiden, M.; Bock, S.; Schulze, C.; Hoeper, D.; Ochs, A.; Schlieben, P.; Keller, M.; Zielke, D.E.; et al. Evidence for an independent third Usutu virus introduction into Germany. Vet. Microbiol. 2016, 192, 60–66. [Google Scholar] [CrossRef]
- Manarolla, G.; Bakonyi, T.; Gallazzi, D.; Crosta, L.; Weissenböck, H.; Dorrestein, G.; Nowotny, N. Usutu virus in wild birds in northern Italy. Vet. Microbiol. 2010, 141, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Lelli, R.; Savini, G.; Teodori, L.; Filipponi, G.; Di Gennaro, A.; Leone, A.; Di Gialleonardo, L.; Venturi, L.; Caporale, V. Serological Evidence of USUTU Virus Occurrence in North-Eastern Italy. Zoonoses Public Health 2008, 55, 361–367. [Google Scholar] [CrossRef]
- Llopis, I.V.; Rossi, L.; Di Gennaro, A.; Mosca, A.; Teodori, L.; Tomassone, L.; Grego, E.; Monaco, F.; Lorusso, A.; Savini, G. Further circulation of West Nile and Usutu viruses in wild birds in Italy. Infect. Genet. Evol. 2015, 32, 292–297. [Google Scholar] [CrossRef]
- Puggioli, A.; Bonilauri, P.; Calzolari, M.; Lelli, D.; Carrieri, M.; Urbanelli, S.; Pudar, D.; Bellini, R. Does Aedes albopictus (Diptera: Culicidae) play any role in Usutu virus transmission in Northern Italy? Experimental oral infection and field evidences. Acta Trop. 2017, 172, 192–196. [Google Scholar] [CrossRef]
- Pautasso, A.; Radaelli, M.C.; Ballardini, M.; Francese, D.R.; Verna, F.; Modesto, P.; Grattarola, C.; Desiato, R.; Bertolini, S.; Vitale, N.; et al. Detection of West Nile and Usutu Viruses in Italian Free Areas: Entomological Surveillance in Piemonte and Liguria Regions, 2014. Vector Borne Zoonotic Dis. 2016, 16, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Llopis, I.V.; Tomassone, L.; Grego, E.; Silvano, F.; Rossi, L. Investigation into Usutu and West Nile viruses in ticks from wild birds in Northwestern Italy, 2012–2014. New Microbiol. 2016, 40, 56–57. [Google Scholar]
- Grottola, A.; Marcacci, M.; Tagliazucchi, S.; Gennari, W.; Di Gennaro, A.; Orsini, M.; Monaco, F.; Marchegiano, P.; Marini, V.; Meacci, M.; et al. Usutu virus infections in humans: A retrospective analysis in the municipality of Modena, Italy. Clin. Microbiol. Infect. 2017, 23, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaibani, P.; Pierro, A.; Alicino, R.; Rossini, G.; Cavrini, F.; Landini, M.P.; Sambri, V. Detection of Usutu-Virus-Specific IgG in Blood Donors from Northern Italy. Vector Borne Zoonotic Dis. 2012, 12, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Percivalle, E.; Sassera, D.; Rovida, F.; Isernia, P.; Fabbi, M.; Baldanti, F.; Marone, P. Usutu Virus Antibodies in Blood Donors and Healthy Forestry Workers in the Lombardy Region, Northern Italy. Vector Borne Zoonotic Dis. 2017, 17, 658–661. [Google Scholar] [CrossRef]
- Percivalle, E.; Cassaniti, I.; Sarasini, A.; Rovida, F.; Adzasehoun, K.M.G.; Colombini, I.; Isernia, P.; Cuppari, I.; Baldanti, F. West Nile or Usutu Virus? A Three-Year Follow-Up of Humoral and Cellular Response in a Group of Asymptomatic Blood Donors. Viruses 2020, 12, 157. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.M.; Geervliet, M.; Verhagen, J.H.; Müskens, G.J.D.M.; Majoor, F.A.; Osterhaus, A.D.M.E.; Martina, B. Serologic evidence of West Nile virus and Usutu virus infections in Eurasian coots in the Netherlands. Zoonoses Public Health 2017, 65, 96–102. [Google Scholar] [CrossRef]
- Petrović, T.; Blazquez, A.; Lupulović, D.; Lazić, G.; Escribano-Romero, E.; Fabijan, D.; Kapetanov, M.; Lazić, S.; Sáiz, J.-C. Monitoring West Nile virus (WNV) infection in wild birds in Serbia during 2012: First isolation and characterisation of WNV strains from Serbia. Eurosurveillance 2013, 18, 20622. [Google Scholar] [CrossRef] [Green Version]
- Höfle, U.; Gamino, V.; Fernandez-De-Mera, I.G.; Mangold, A.J.; Ortíz, J.-A.; De La Fuente, J. Usutu Virus in Migratory Song Thrushes, Spain. Emerg. Infect. Dis. 2013, 19, 1173–1175. [Google Scholar] [CrossRef] [Green Version]
- Ferraguti, M.; La Puente, J.M.-D.; Soriguer, R.; Llorente, F.; Jiménez-Clavero, M.Á.; Figuerola, J. West Nile virus-neutralizing antibodies in wild birds from southern Spain. Epidemiol. Infect. 2016, 144, 1907–1911. [Google Scholar] [CrossRef] [Green Version]
- García-Bocanegra, I.; Paniagua, J.; Gutiérrez-Guzmán, A.V.; Lecollinet, S.; Boadella, M.; Arenas-Montes, A.; Cano-Terriza, D.; Lowenski, S.; Gortázar, C.; Höfle, U. Spatio-temporal trends and risk factors affecting West Nile virus and related flavivirus exposure in Spanish wild ruminants. BMC Vet. Res. 2016, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cano-Terriza, D.; Guerra, R.; Lecollinet, S.; Cerdà-Cuéllar, M.; Cabezón, Ó.; Almeria, S.; García-Bocanegra, I. Epidemiological survey of zoonotic pathogens in feral pigeons (Columba livia var. domestica) and sympatric zoo species in Southern Spain. Comp. Immunol. Microbiol. Infect. Dis. 2015, 43, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Vieille, G.; Turin, L.; Kaiser, L. Usutu virus in cerebrospinal fluid: A 2-year survey in a Tertiary Care Hospital, Geneva, Switzerland. J. Med. Virol. 2017, 90, 609–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, O.; Savini, G.; Papa, A.; Figuerola, J.; Groschup, M.H.; Kampen, H.; Medlock, J.M.; Vaux, A.; Wilson, A.J.; Werner, D.; et al. European Surveillance for West Nile Virus in Mosquito Populations. Int. J. Environ. Res. Public Health 2013, 10, 4869–4895. [Google Scholar] [CrossRef] [Green Version]
- Wipf, N.C.; Guidi, V.; Tonolla, M.; Ruinelli, M.; Muller, P.; Engler, O. Evaluation of honey-baited FTA cards in combination with different mosquito traps in an area of low arbovirus prevalence. Parasites Vectors 2019, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Buckley, A.; Dawson, A.; Gould, E.A. Detection of seroconversion to West Nile virus, Usutu virus and Sindbis virus in UK sentinel chickens. Virol. J. 2006, 3, 71. [Google Scholar] [CrossRef] [Green Version]
- Horton, D.L.; Lawson, B.; Egbetade, A.; Jeffries, C.L.; Johnson, N.; Cunningham, A.A.; Fooks, A.R. Targeted surveillance for Usutu virus in British birds (2005–2011). Vet. Rec. 2012, 172, 17. [Google Scholar] [CrossRef] [Green Version]
- Vaux, A.G.C.; Gibson, G.; Hernández-Triana, L.M.; Cheke, R.A.; McCracken, F.; Jeffries, C.L.; Horton, D.L.; Springate, S.; Johnson, N.; McElhinney, L.M.; et al. Enhanced West Nile virus surveillance in the North Kent marshes, UK. Parasites Vectors 2015, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Mannasse, B.; Mendelson, E.; Orshan, L.; Mor, O.; Shalom, U.; Yeger, T.; Lustig, Y. Usutu Virus RNA in Mosquitoes, Israel, 2014–2015. Emerg. Infect. Dis. 2017, 23, 1699–1702. [Google Scholar] [CrossRef]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van De Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, C.; Napoli, C.; Venturi, G.; Pupella, S.; Lombardini, L.; Calistri, P.; Monaco, F.; Cagarelli, R.; Angelini, P.; Bellini, R.; et al. West Nile virus transmission: Results from the integrated surveillance system in Italy, 2008 to 2015. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.J.; Coulombier, D.; Domanović, D.; Zeller, H.; Gossner, C.M.; European Union West Nile Fever Working Group. One Health approach for West Nile virus surveillance in the European Union: Relevance of equine data for blood safety. Eurosurveillance 2019, 24, 1800349. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Vidanović, D.; Barbić, L.; Jeličić, P.; Lazić, S.; Radmanić, L.; Lupulović, D.; Janev-Holcer, N.; Tešović, B.; Milošević, V.; et al. Importance of Multidisciplinary and Regional Collaboration in Integrated West Nile Virus Surveillance—The “One Health” Concept. Infektološki Glasn. 2019, 39, 40–47. [Google Scholar] [CrossRef]
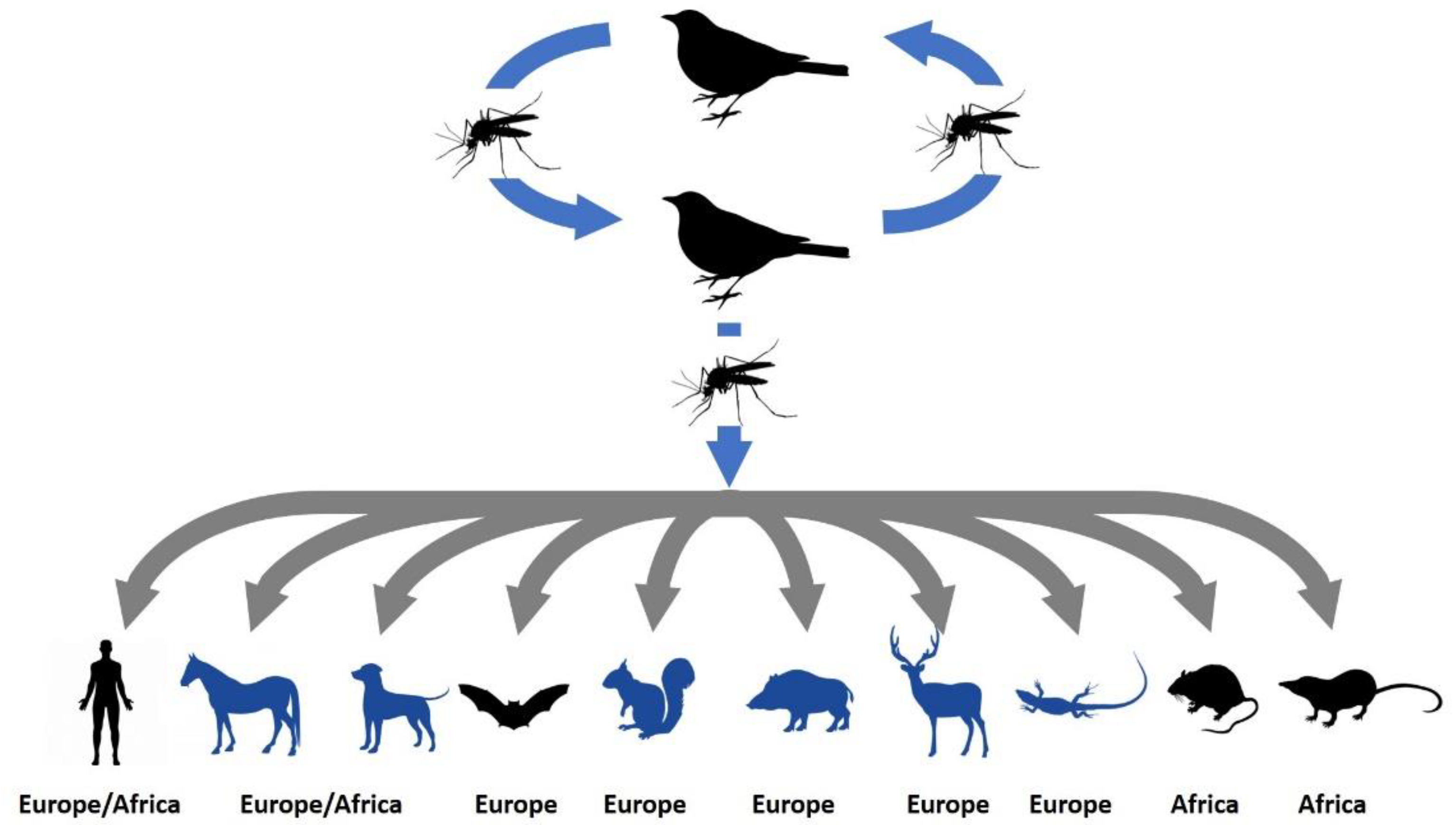

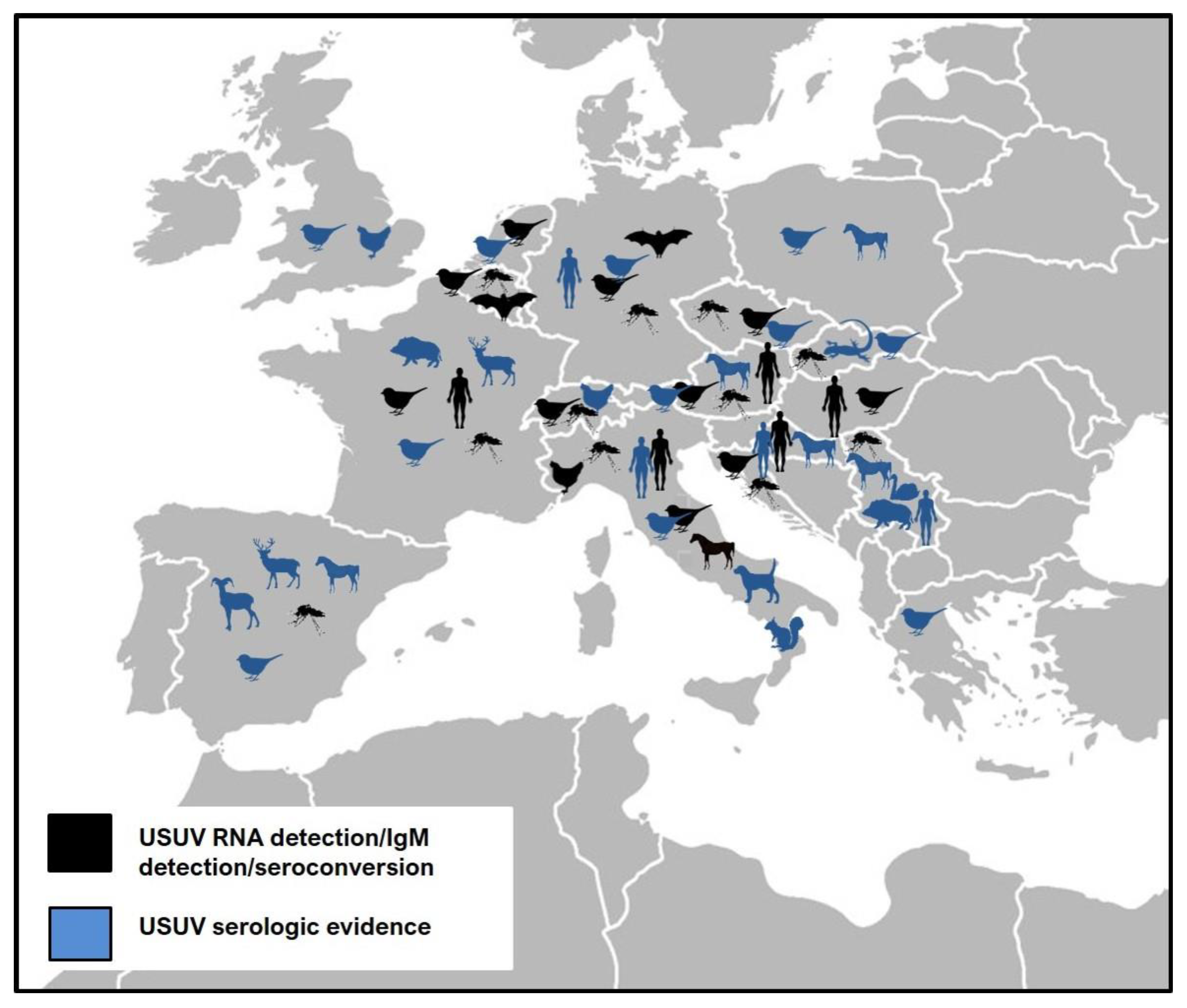
| Country |  |  |  |  |  | Reference |
|---|---|---|---|---|---|---|
| Austria | 2016* | 2001 | 2017 | [8,17,37,38,39] | ||
| Belgium | 2012 | 2016 | 2017 | [1,20,40] | ||
| Croatia | 2012 | 2018 | 2011 | 2016 | [15,22,30,32] | |
| Czech Republic | 2004 | 2013 | [41,42] | |||
| France | 2016 | 2015 | 2015 | [31,43,44] | ||
| Germany | 2012* | 2011 | 2010 | 2013 | [9,13,19,45] | |
| Greece | 2010 | [46] | ||||
| Hungary | 2018 | 2005 | [34,47] | |||
| Italy | 2009 | 1996 | 2008 | 2009 | [7,21,28,29,48] | |
| Netherlands | 2016 | [49] | ||||
| Poland | 2006 | 2012 | [50,51] | |||
| Serbia | 2015 | 2012 | 2009 | 2014 | [52,53,54] | |
| Slovakia | 2010 | [23,55] | ||||
| Spain | 2011 | 2011 | 2006 | [56,57,58,59] | ||
| Switzerland | 2006 | [60] | ||||
| United Kingdom | 2001 | [61] |
 Clinical cases/RNA detection/seroconversion;
Clinical cases/RNA detection/seroconversion;  serologic evidence; *asymptomatic blood donors/blood donations.
serologic evidence; *asymptomatic blood donors/blood donations.| Country |  |  |  |  | Reference |
|---|---|---|---|---|---|
| Austria | Europe 2* Africa 3* | Europe 1,2 Africa 3 | Europe 2** | [34,35,62,63] | |
| Belgium | Europe 1,3 Africa 3 | Europe 3 | Europe 3 | [20,36,62,63] | |
| Croatia | Europe 2 | Europe 2 | Europe 2 | [32] | |
| Czech Republic | Europe 1,2,3 Africa 3 | Europe 2 | [18,42] | ||
| France | Africa 2 | Europe 3 | Europe 2 Africa 2,3 | [31,44] | |
| Germany | Europe 3 | Europe 2,3,5 Africa 2,3 Africa 3-like | Europe 3 Africa 3 | Europe 3 | [9,19,63,64,65,66] |
| Hungary | Europe 2 | Europe 1,2 | [34,39,47,67] | ||
| Italy | Europe 1,2*,3*,4* | Europe 2,4 | Europe 2,4 | [16,29,63,68,69] | |
| Netherlands | Europe 3 | Europe 3 Africa 3 | [70,71] | ||
| Serbia | Europe 1,2 | [72,73] | |||
| Slovakia | Europe 2 | [55] | |||
| Spain | Africa 2 | Africa 2 | [1,74] | ||
| Switzerland | Europe 1 | [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilibic-Cavlek, T.; Petrovic, T.; Savic, V.; Barbic, L.; Tabain, I.; Stevanovic, V.; Klobucar, A.; Mrzljak, A.; Ilic, M.; Bogdanic, M.; et al. Epidemiology of Usutu Virus: The European Scenario. Pathogens 2020, 9, 699. https://doi.org/10.3390/pathogens9090699
Vilibic-Cavlek T, Petrovic T, Savic V, Barbic L, Tabain I, Stevanovic V, Klobucar A, Mrzljak A, Ilic M, Bogdanic M, et al. Epidemiology of Usutu Virus: The European Scenario. Pathogens. 2020; 9(9):699. https://doi.org/10.3390/pathogens9090699
Chicago/Turabian StyleVilibic-Cavlek, Tatjana, Tamas Petrovic, Vladimir Savic, Ljubo Barbic, Irena Tabain, Vladimir Stevanovic, Ana Klobucar, Anna Mrzljak, Maja Ilic, Maja Bogdanic, and et al. 2020. "Epidemiology of Usutu Virus: The European Scenario" Pathogens 9, no. 9: 699. https://doi.org/10.3390/pathogens9090699







