Differential Contributions of the Complement Anaphylotoxin Receptors C5aR1 and C5aR2 to the Early Innate Immune Response against Staphylococcus aureus Infection
Abstract
:1. Introduction
2. Results
2.1. Expression of C5aR1 and C5aR2 by Murine Neutrophils
2.2. C5aR1 But not C5aR2 Contributes to Bacterial Clearance in a Murine Model S. Aureus Bloodstream Infection


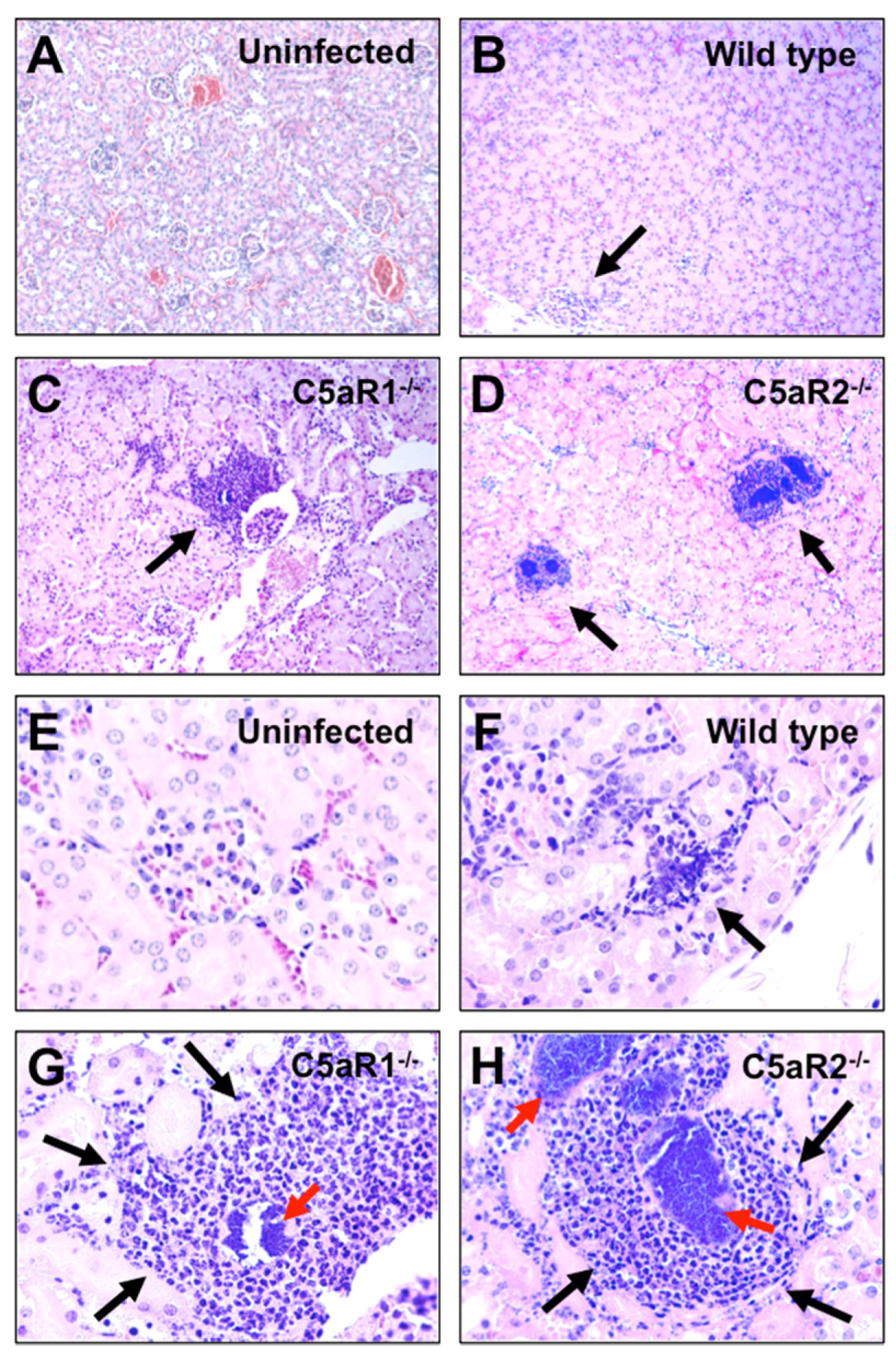
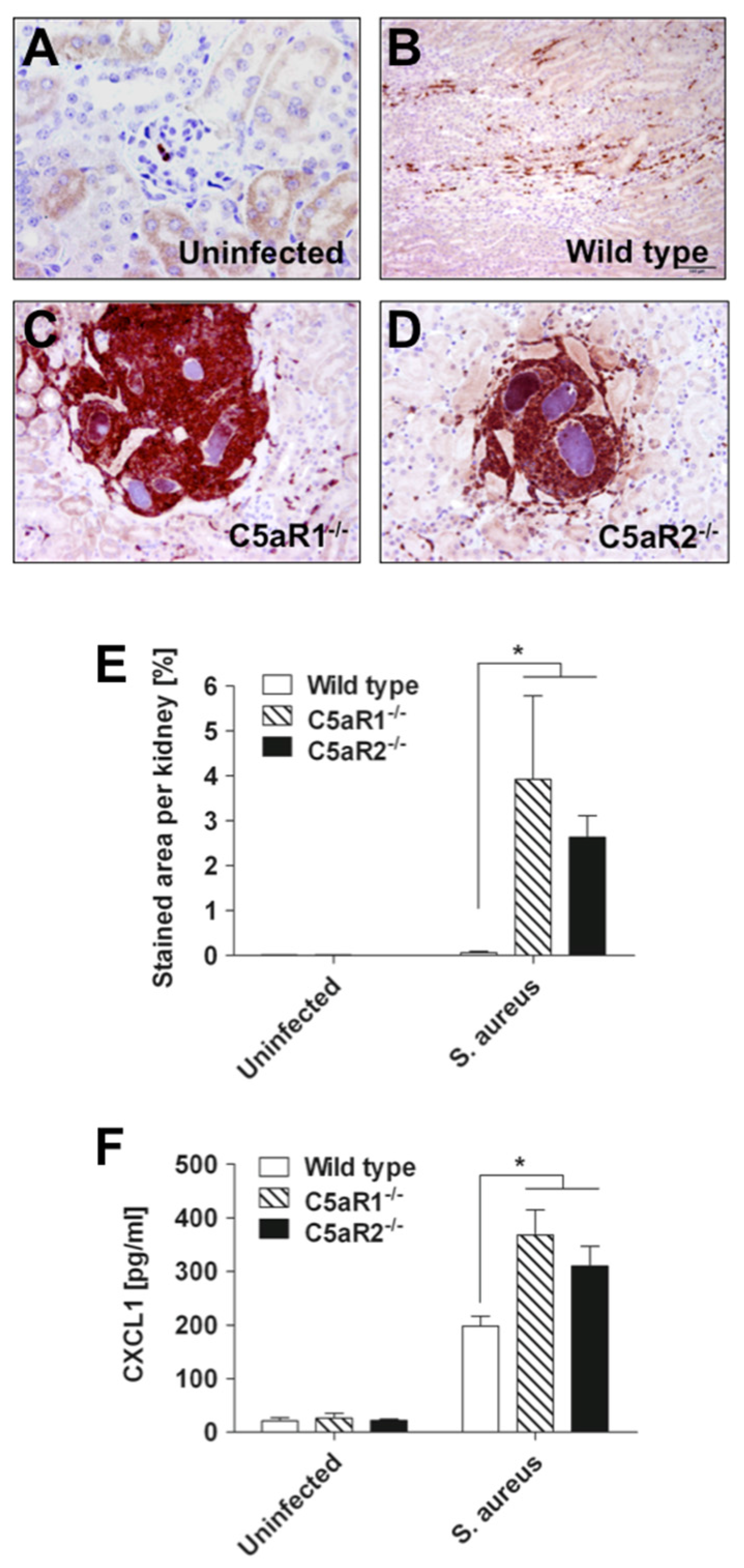
2.3. S. Aureus Abscess Formation is Accelerated in the Absence of C5aR1 or C5aR2
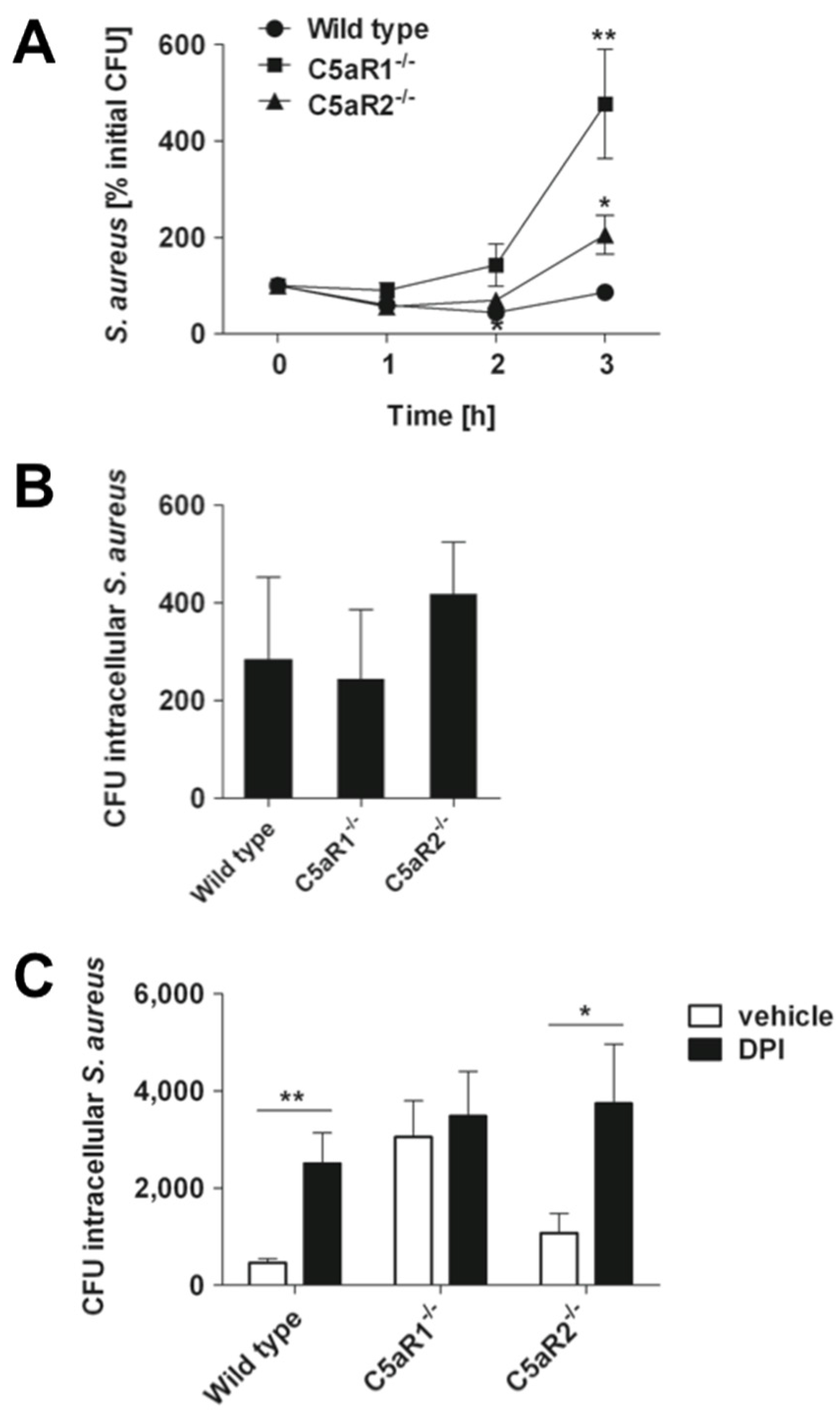
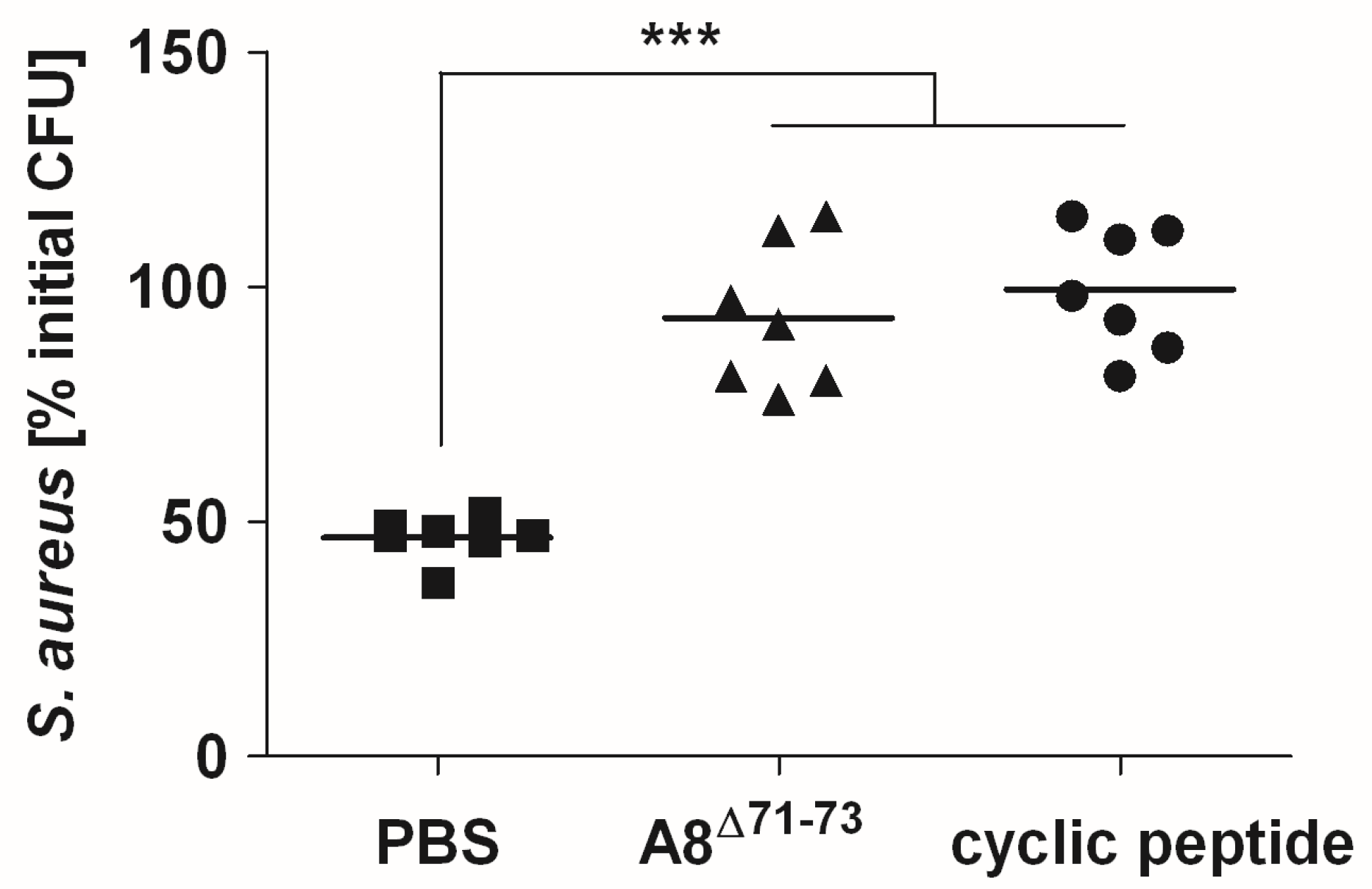
2.4. Signaling through C5aR1 is Required for Optimal Bactericidal Effect of Murine and Human Blood
3. Discussion
4. Experimental Section
4.1. Bacteria
4.2. Mice and Infection
4.3. Staining of C5aR1 and C5aR2
4.4. Cytokine Determination
4.5. Histology and Immunohistochemistry
4.6. Bactericidal Assay in Murine Blood
4.7. Bactericidal assay in Human Blood
4.8. Statistical analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement system part I—Molecular mechanisms of activation and regulation. Front. Immunol. 2015. [CrossRef] [PubMed]
- Figueroa, J.E.; Densen, P. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 1991, 4, 359–395. [Google Scholar] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Von Kockritz-Blickwede, M.; Konrad, S.; Foster, S.; Gessner, J.E.; Medina, E. Protective role of complement C5a in an experimental model of Staphylococcus aureus bacteremia. J. Innate Immun. 2010, 2, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Jongerius, I.; Kohl, J.; Pandey, M.K.; Ruyken, M.; van Kessel, K.P.; van Strijp, J.A.; Rooijakkers, S.H. Staphylococcal complement evasion by various convertase-blocking molecules. J. Exp. Med. 2007, 204, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Bestebroer, J.; Aerts, P.C.; Rooijakkers, S.H.; Pandey, M.K.; Kohl, J.; van Strijp, J.A.; de Haas, C.J. Functional basis for complement evasion by staphylococcal superantigen-like 7. Cell. Microbiol. 2010, 12, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.F.; Ward, P.A. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005, 23, 821–852. [Google Scholar] [CrossRef] [PubMed]
- Mollnes, T.E.; Brekke, O.L.; Fung, M.; Fure, H.; Christiansen, D.; Bergseth, G.; Videm, V.; Lappegard, K.T.; Kohl, J.; Lambris, J.D. Essential role of the C5a receptor in E. coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 2002, 100, 1869–1877. [Google Scholar] [PubMed]
- Monsinjon, T.; Gasque, P.; Chan, P.; Ischenko, A.; Brady, J.J.; Fontaine, M.C. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003, 17, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Shuster, D.E.; Kehrli, M.E., Jr.; Rainard, P.; Paape, M. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect. Immun. 1997, 65, 3286–3292. [Google Scholar] [PubMed]
- Bosmann, M.; Sarma, J.V.; Atefi, G.; Zetoune, F.S.; Ward, P.A. Evidence for anti-inflammatory effects of C5a on the innate Il-L7a/IL-23 axis. FASEB J. 2012, 26, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Gerard, N.P.; Gerard, C. The chemotactic receptor for human C5a anaphylatoxin. Nature 1991, 349, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Hirata, T.; Enomoto, M.; Araki, T.; Ishimaru, H.; Takahashi, T.A. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol. Immunol. 2000, 37, 407–412. [Google Scholar] [CrossRef]
- Ward, P.A. Functions of C5a receptors. J. Mol. Med. (Berl) 2009, 87, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Monk, P.N.; Scola, A.M.; Madala, P.; Fairlie, D.P. Function, structure and therapeutic potential of complement C5a receptors. Br. J. Pharmacol. 2007, 152, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Okinaga, S.; Slattery, D.; Humbles, A.; Zsengeller, Z.; Morteau, O.; Kinrade, M.B.; Brodbeck, R.M.; Krause, J.E.; Choe, H.R.; Gerard, N.P.; et al. C5L2, a nonsignaling C5a binding protein. Biochemistry 2003, 42, 9406–9415. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.A.; Monk, P.N. The orphan receptor C5AR2 has high affinity binding sites for complement fragments C5a and C5a des-arg(74). J. Biol. Chem. 2002, 277, 7165–7169. [Google Scholar] [CrossRef] [PubMed]
- Johswich, K.; Martin, M.; Thalmann, J.; Rheinheimer, C.; Monk, P.N.; Klos, A. Ligand specificity of the anaphylatoxin C5L2 receptor and its regulation on myeloid and epithelial cell lines. J. Biol. Chem. 2006, 281, 39088–39095. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Neff, T.A.; Guo, R.F.; Speyer, C.L.; Sarma, J.V.; Tomlins, S.; Man, Y.; Riedemann, N.C.; Hoesel, L.M.; Younkin, E.; et al. Evidence for a functional role of the second C5a receptor C5AR2. FASEB J. 2005, 19, 1003–1005. [Google Scholar] [PubMed]
- Gerard, N.P.; Lu, B.; Liu, P.; Craig, S.; Fujiwara, Y.; Okinaga, S.; Gerard, C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J. Biol. Chem. 2005, 280, 39677–39680. [Google Scholar] [CrossRef] [PubMed]
- Bamberg, C.E.; Mackay, C.R.; Lee, H.; Zahra, D.; Jackson, J.; Lim, Y.S.; Whitfeld, P.L.; Craig, S.; Corsini, E.; Lu, B.; et al. The C5a receptor (C5aR1) C5L2 is a modulator of C5aR1-mediated signal transduction. J. Biol. Chem. 2010, 285, 7633–7644. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.J.; Mirtsos, C.; Suh, D.; Lu, Y.C.; Lin, W.J.; McKerlie, C.; Lee, T.; Baribault, H.; Tian, H.; Yeh, W.C. C5AR2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 2007, 446, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Flierl, M.A.; Nadeau, B.A.; Day, D.E.; Huber-Lang, M.; Mackay, C.R.; Zetoune, F.S.; Gerard, N.P.; Cianflone, K.; Kohl, J.; et al. Functional roles for C5a receptors in sepsis. Nat. Med. 2008, 14, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Coulthard, L.G.; Wu, M.C.; Taylor, S.M.; Woodruff, T.M. C5L2: A controversial receptor of complement anaphylatoxin, C5a. FASEB J. 2013, 27, 855–864. [Google Scholar] [CrossRef] [PubMed]
- von Kockritz-Blickwede, M.; Rohde, M.; Oehmcke, S.; Miller, L.S.; Cheung, A.L.; Herwald, H.; Foster, S.; Medina, E. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am. J. Pathol. 2008, 173, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Bozic, C.R.; Kolakowski, L.F., Jr.; Gerard, N.P.; Garcia-Rodriguez, C.; von Uexkull-Guldenband, C.; Conklyn, M.J.; Breslow, R.; Showell, H.J.; Gerard, C. Expression and biologic characterization of the murine chemokine KC. J. Immunol. 1995, 154, 6048–6057. [Google Scholar] [PubMed]
- Borders, C.W.; Courtney, A.; Ronen, K.; Pilar Laborde-Lahoz, M.; Guidry, T.V.; Hwang, S.A.; Olsen, M.; Hunter, R.L., Jr.; Hollmann, T.J.; Wetsel, R.A.; et al. Requisite role for complement C5 and the C5a receptor in granulomatous response to mycobacterial glycolipid trehalose 6,6′-dimycolate. Scand. J. Immunol. 2005, 62, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.S.; Riedeman, N.C.; Sarma, J.V.; Younkin, E.M.; McGuire, S.R.; Laudes, I.J.; Lu, K.T.; Guo, R.F.; Neff, T.A.; Padgaonkar, V.A.; et al. Protection of innate immunity by C5aR1 antagonist in septic mice. FASEB J. 2002, 16, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Ehrengruber, M.U.; Geiser, T.; Deranleau, D.A. Activation of human neutrophils by C3a and C5a. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett. 1994, 346, 181–184. [Google Scholar] [CrossRef]
- Seow, V.; Lim, J.; Iyer, A.; Suen, J.Y.; Ariffin, J.K.; Hohenhaus, D.M.; Sweet, M.J.; Fairlie, D.P. Inflammatory responses induced by lipopolysaccharide are amplified in primary human monocytes but suppressed in macrophages by complement protein C5a. J. Immunol. 2013, 191, 4308–4316. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lambris, J.D. Microbial manipulation of receptor crosstalk in innate immunity. Nat. Rev. Immunol. 2011, 11, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.; van Kessel, K.P.; Vandenesch, F.; et al. The staphylococcal toxin panton-valentine leukocidin targets human C5a receptors. Cell Host Microbe. 2013, 13, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Schiepers, A.; de Haas, C.J.; van Hooijdonk, D.D.; Badiou, C.; Contamin, H.; Vandenesch, F.; Lina, G.; Gerard, N.P.; Gerard, C.; et al. Differential interaction of the staphylococcal toxins Panton-Valentine leukocidin and gamma-hemolysin CB with human C5a receptors. J. Immunol. 2015, 195, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Vrieling, M.; Wallet, P.; Badiou, C.; Reyes-Robles, T.; Ohneck, E.A.; Benito, Y.; de Haas, C.J.; Day, C.J.; Jennings, M.P.; et al. The staphylococcal toxins gamma-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 2014, 5, 5438. [Google Scholar] [CrossRef] [PubMed]
- de Haas, C.J.; Veldkamp, K.E.; Peschel, A.; Weerkamp, F.; Van Wamel, W.J.; Heezius, E.C.; Poppelier, M.J.; Van Kessel, K.P.; van Strijp, J.A. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 2004, 199, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, K.E.; Heezius, H.C.; Verhoef, J.; van Strijp, J.A.; van Kessel, K.P. Modulation of neutrophil chemokine receptors by Staphylococcus aureus supernate. Infect. Immun. 2000, 68, 5908–5913. [Google Scholar] [CrossRef] [PubMed]
- Postma, B.; Poppelier, M.J.; van Galen, J.C.; Prossnitz, E.R.; van Strijp, J.A.; de Haas, C.J.; van Kessel, K.P. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J. Immunol. 2004, 172, 6994–7001. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, I.M.; Arvidson, S.; Foster, S.; Tarkowski, A. Sigma factor b and rsbu are required for virulence in staphylococcus aureus-induced arthritis and sepsis. Infect. Immun 2004, 72, 6106–6111. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, M.J.; Aish, J.L.; White, I.J.; Shaw, L.; Lithgow, J.K.; Foster, S.J. Sigmab modulates virulence determinant expression and stress resistance: Characterization of a functional rsbu strain derived from staphylococcus aureus 8325-4. J. Bacteriol. 2002, 184, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- Hopken, U.E.; Lu, B.; Gerard, N.P.; Gerard, C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature 1996, 383, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.; Seeliger, F.; Kreutzer, M.; Germann, P.G.; Baumgartner, W. Limited remyelination in theiler’s murine encephalomyelitis due to insufficient oligodendroglial differentiation of nerve/glial antigen 2 (ng2)-positive putative oligodendroglial progenitor cells. Neuropathol. Appl. Neurobiol. 2008, 34, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Kummerfeld, M.; Seehusen, F.; Klein, S.; Ulrich, R.; Kreutzer, R.; Gerhauser, I.; Herder, V.; Baumgartner, W.; Beineke, A. Periventricular demyelination and axonal pathology is associated with subependymal virus spread in a murine model for multiple sclerosis. Intervirology 2012, 55, 401–416. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horst, S.A.; Itzek, A.; Klos, A.; Beineke, A.; Medina, E. Differential Contributions of the Complement Anaphylotoxin Receptors C5aR1 and C5aR2 to the Early Innate Immune Response against Staphylococcus aureus Infection. Pathogens 2015, 4, 722-738. https://doi.org/10.3390/pathogens4040722
Horst SA, Itzek A, Klos A, Beineke A, Medina E. Differential Contributions of the Complement Anaphylotoxin Receptors C5aR1 and C5aR2 to the Early Innate Immune Response against Staphylococcus aureus Infection. Pathogens. 2015; 4(4):722-738. https://doi.org/10.3390/pathogens4040722
Chicago/Turabian StyleHorst, Sarah A., Andreas Itzek, Andreas Klos, Andreas Beineke, and Eva Medina. 2015. "Differential Contributions of the Complement Anaphylotoxin Receptors C5aR1 and C5aR2 to the Early Innate Immune Response against Staphylococcus aureus Infection" Pathogens 4, no. 4: 722-738. https://doi.org/10.3390/pathogens4040722




