Prevalence and Patterns of Enteric Co-Infections Among Individuals Presenting with Cholera-like Diarrheal Disease During Seasonal Cholera Outbreaks
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Cholera Detection
2.3. Multi-Pathogen Amplification and Detection of Other Enteric Pathogens
2.4. Statistical Analysis
3. Results
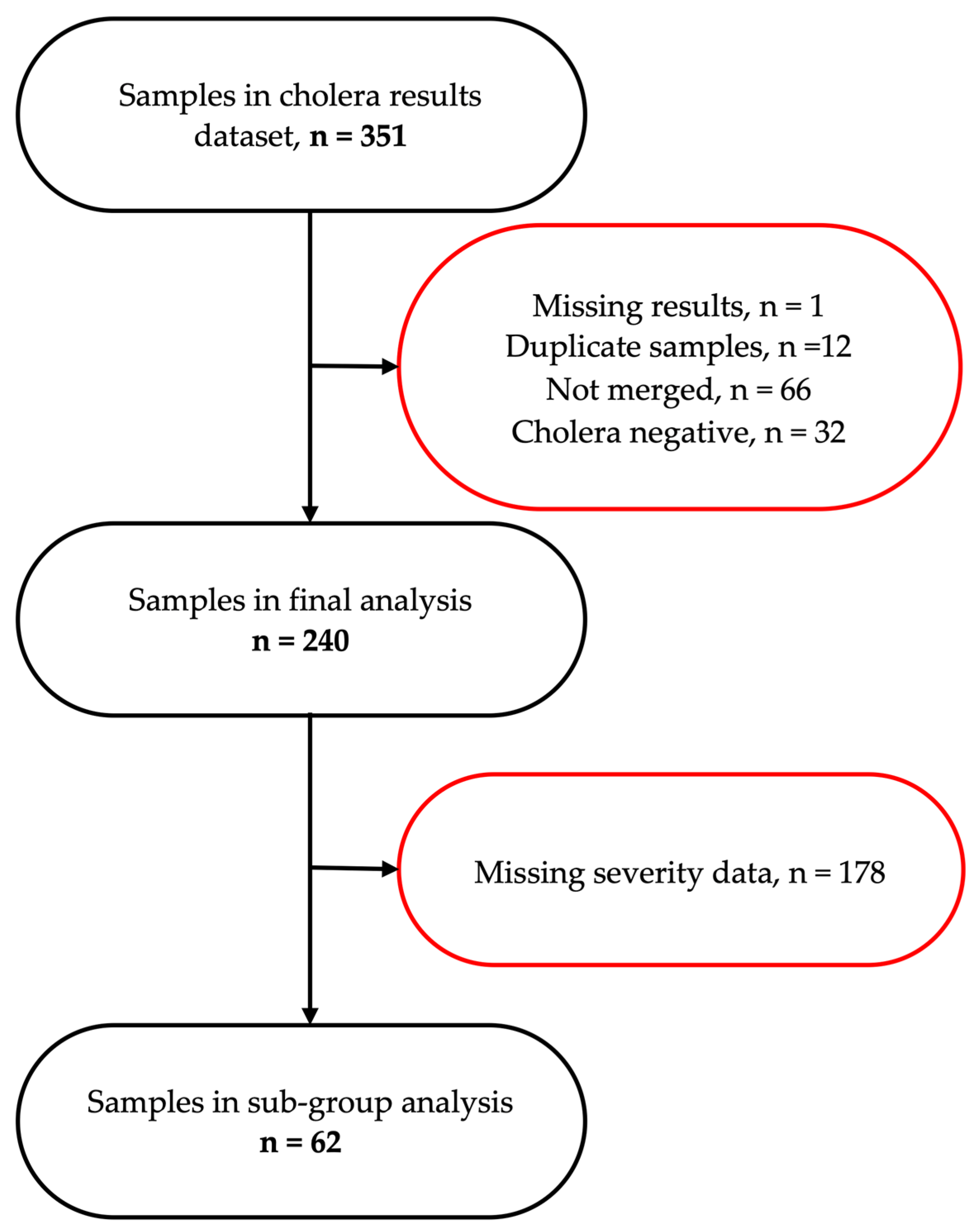
3.1. Participants’ Flow and Background Characteristics
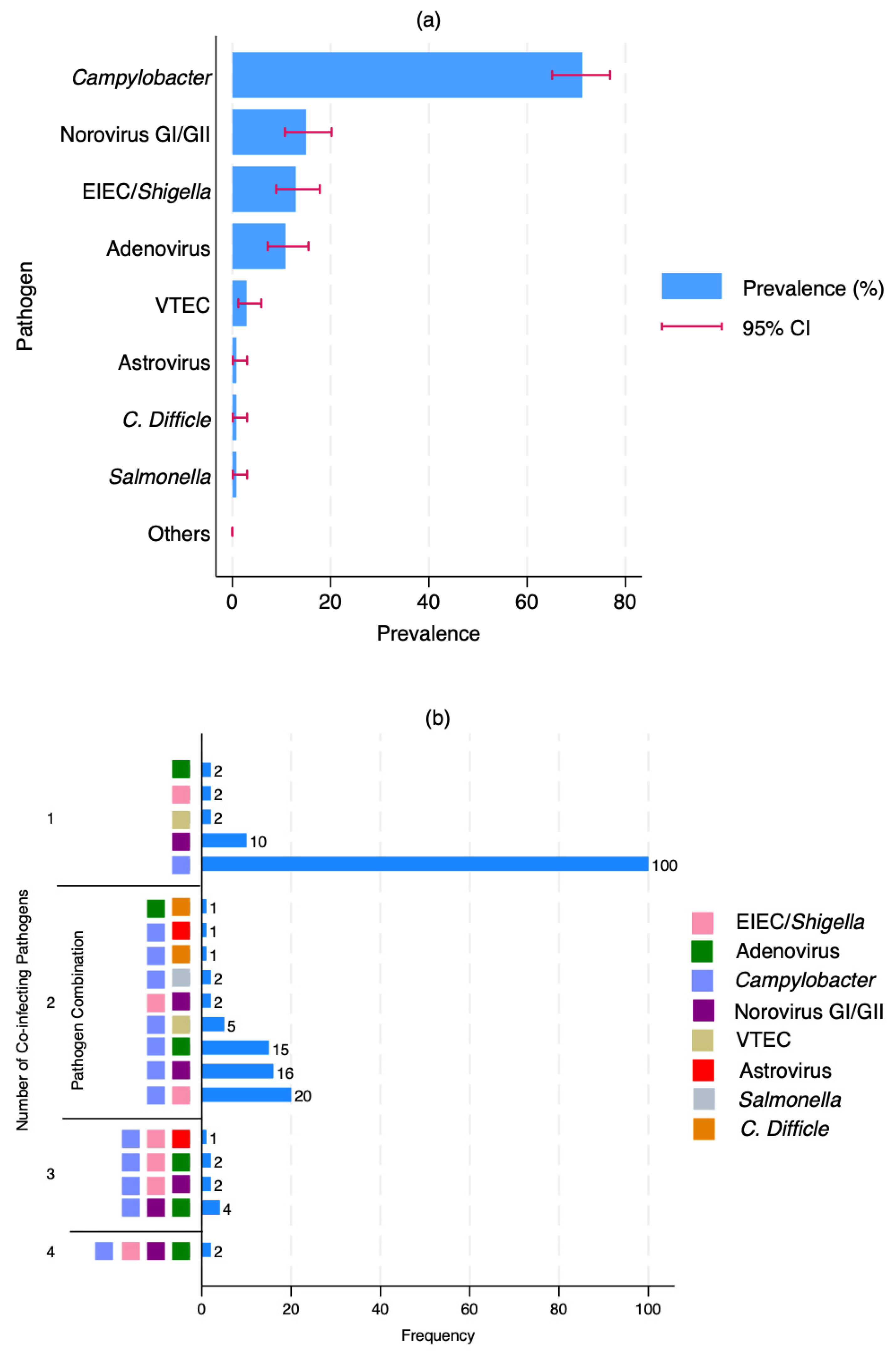
3.2. Prevalence and Patterns of Enteric Co-Infections
3.3. Risk Factors of Co-Infection
3.4. Relationship Between Infection Status and Disease Severity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation (WHO). Cholera Annual Report 2023; World Health Organisation: Geneva, Switzerland, 2024; pp. 481–496. [Google Scholar]
- World Health Organisation (WHO). Multi-Country Outbreak of Cholera, External Situation Report; World Health Organisation: Geneva, Switzerland, 2024. [Google Scholar]
- World Health Organization (WHO-AFRO). Monthly Regional Cholera Bulletin; World Health Organisation: Geneva, Switzerland, 2024. [Google Scholar]
- Amin, M.A.; Akhtar, M.; Khan, Z.H.; Islam, T.; Firoj, G.; Begum, Y.A.; Rahman, S.I.A.; Afrad, M.H.; Bhuiyan, T.R.; Chowdhury, F.; et al. Coinfection and Clinical Impact of Enterotoxigenic Escherichia coli Harboring Diverse Toxin Variants and Colonization Factors: 2017–2022. Int. J. Infect. Dis. 2025, 151, 107365. [Google Scholar] [CrossRef]
- Chen, W.-C.; Lin, Y.E. Recent Advances in the Epidemiology of Pathogenic Agents. Pathogens 2024, 13, 263. [Google Scholar] [CrossRef]
- Islam, A.; Labbate, M.; Djordjevic, S.P.; Alam, M.; Darling, A.; Melvold, J.; Holmes, A.J.; Johura, F.T.; Cravioto, A.; Charles, I.G.; et al. Indigenous Vibrio cholerae Strains from a Non-Endemic Region Are Pathogenic. Open Biol. 2013, 3, 120181. [Google Scholar] [CrossRef]
- Montero, D.A.; Vidal, R.M.; Velasco, J.; George, S.; Lucero, Y.; Gómez, L.A.; Carreño, L.J.; García-Betancourt, R.; O’Ryan, M. Vibrio cholerae, Classification, Pathogenesis, Immune Response, and Trends in Vaccine Development. Front. Med. 2023, 10, 1155751. [Google Scholar] [CrossRef]
- Arnaout, A.Y.; Nerabani, Y.; Sawas, M.N.; Alhejazi, T.J.; Farho, M.A.; Arnaout, K.; Alshaker, H.; Shebli, B.; Helou, M.; Mobaied, B.B.; et al. Acute Watery Diarrhoea Cases during Cholera Outbreak in Syria: A Cohort Study. BMJ Open 2024, 14, e082385. [Google Scholar] [CrossRef]
- Kuma, G.K.; Opintan, J.A.; Sackey, S.; Nyarko, K.M.; Opare, D.; Aryee, E.; Dongdem, A.Z.; Antwi, L.; Ofosu-Appiah, L.H.; Owusu-Okyere, G. Antibiotic Resistance Patterns amongst Clinical Vibrio cholerae O1 Isolates from Accra, Ghana. Int. J. Infect. Control 2014, 10. [Google Scholar]
- Phiri, T.M.; Imamura, T.; Mwansa, P.C.; Mathews, I.; Mtine, F.; Chanda, J.; Salasini, M.; Funaki, T.; Otridah, K.; Musonda, K.; et al. Increased Prevalence of Antimicrobial Resistance in Vibrio cholerae in the Capital and Provincial Areas of Zambia, January 2023–February 2024. Am. J. Trop. Med. Hyg. 2025, 112, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Chisenga, C.; Bosomprah, S.; Laban, N.M.; Mwila-K, M.J.; Simuyandi, M.; Chilengi, R. Aetiology of Diarrhoea in Children under Five in Zambia Detected Using Luminex xTAG Gastrointestinal Pathogen Panel. Pediatr. Infect. Dis. 2018, 3, 2573-0282. [Google Scholar] [CrossRef]
- Bliem, R.; Schauer, S.; Plicka, H.; Obwaller, A.; Sommer, R.; Steinrigl, A.; Alam, M.; Reischer, G.H.; Farnleitner, A.H.; Kirschner, A. A Novel Triplex Quantitative PCR Strategy for Quantification of Toxigenic and Nontoxigenic Vibrio cholerae in Aquatic Environments. Appl. Environ. Microbiol. 2015, 81, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Kırdar, S.; Başara, T.; Ömürlü, İ.K. Prevalence and Genetic Diversity of Norovirus in Acute Gastroenteritis Cases in the Southwest Province of Turkey. Balk. Med. J. 2022, 39, 153. [Google Scholar] [CrossRef]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L. Use of Quantitative Molecular Diagnostic Methods to Identify Causes of Diarrhoea in Children: A Reanalysis of the GEMS Case-Control Study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef]
- Fletcher, S.M.; Stark, D.; Ellis, J. Prevalence of Gastrointestinal Pathogens in Sub-Saharan Africa: Systematic Review and Meta-Analysis. J. Public Health Afr. 2011, 2, e30. [Google Scholar] [CrossRef]
- Hlashwayo, D.F.; Sigauque, B.; Noormahomed, E.V.; Afonso, S.M.; Mandomando, I.M.; Bila, C.G. A Systematic Review and Meta-Analysis Reveal That Campylobacter Spp. and Antibiotic Resistance Are Widespread in Humans in Sub-Saharan Africa. PLoS ONE 2021, 16, e0245951. [Google Scholar] [CrossRef]
- Gahamanyi, N.; Mboera, L.E.G.; Matee, M.I.; Mutangana, D.; Komba, E.V.G. Prevalence, Risk Factors, and Antimicrobial Resistance Profiles of Thermophilic Campylobacter Species in Humans and Animals in Sub-Saharan Africa: A Systematic Review. Int. J. Microbiol. 2020, 2020, 2092478. [Google Scholar] [CrossRef]
- Corcionivoschi, N.; Gundogdu, O. Foodborne Pathogen Campylobacter. Microorganisms 2021, 9, 1241. [Google Scholar] [CrossRef]
- Veronese, P.; Dodi, I. Campylobacter Jejuni/Coli Infection: Is It Still a Concern? Microorganisms 2024, 12, 2669. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, N.; Heine, L.; Ngandu, J.P.; Ledwaba, S.E.; Zitha, T.; Mudau, L.S.; Becker, P.; Traore, A.N.; Barnard, T.G. High Burden of Co-Infection with Multiple Enteric Pathogens in Children Suffering with Diarrhoea from Rural and Peri-Urban Communities in South Africa. Pathogens 2023, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Hugho, E.A.; Kumburu, H.H.; Amani, N.B.; Mseche, B.; Maro, A.; Ngowi, L.E.; Kyara, Y.; Kinabo, G.; Thomas, K.M.; Houpt, E.R.; et al. Enteric Pathogens Detected in Children under Five Years Old Admitted with Diarrhea in Moshi, Kilimanjaro, Tanzania. Pathogens 2023, 12, 618. [Google Scholar] [CrossRef]
- Nhampossa, T.; Mandomando, I.; Acacio, S.; Quintó, L.; Vubil, D.; Ruiz, J.; Nhalungo, D.; Sacoor, C.; Nhabanga, A.; Nhacolo, A. Diarrheal Disease in Rural Mozambique: Burden, Risk Factors and Etiology of Diarrheal Disease among Children Aged 0–59 Months Seeking Care at Health Facilities. PLoS ONE 2015, 10, e0119824. [Google Scholar] [CrossRef] [PubMed]
- Breurec, S.; Vanel, N.; Bata, P.; Chartier, L.; Farra, A.; Favennec, L.; Franck, T.; Giles-Vernick, T.; Gody, J.-C.; Luong Nguyen, L.B. Etiology and Epidemiology of Diarrhea in Hospitalized Children from Low Income Country: A Matched Case-Control Study in Central African Republic. PLoS Negl. Trop. Dis. 2016, 10, e0004283. [Google Scholar] [CrossRef]
- François, R.; Yori, P.P.; Rouhani, S.; Siguas Salas, M.; Paredes Olortegui, M.; Rengifo Trigoso, D.; Pisanic, N.; Burga, R.; Meza, R.; Meza Sanchez, G.; et al. The Other Campylobacters: Not Innocent Bystanders in Endemic Diarrhea and Dysentery in Children in Low-Income Settings. PLoS Negl. Trop. Dis. 2018, 12, e0006200. [Google Scholar] [CrossRef]
- Das, R.; Nasrin, S.; Palit, P.; Sobi, R.A.; Sultana, A.-A.; Khan, S.H.; Haque, M.A.; Nuzhat, S.; Ahmed, T.; Faruque, A.S.G.; et al. Vibrio cholerae in Rural and Urban Bangladesh, Findings from Hospital-Based Surveillance, 2000–2021. Sci. Rep. 2023, 13, 6411. [Google Scholar] [CrossRef]
- Moyo, S.J.; Kommedal, Ø.; Blomberg, B.; Hanevik, K.; Tellevik, M.G.; Maselle, S.Y.; Langeland, N. Comprehensive Analysis of Prevalence, Epidemiologic Characteristics, and Clinical Characteristics of Monoinfection and Coinfection in Diarrheal Diseases in Children in Tanzania. Am. J. Epidemiol. 2017, 186, 1074–1083. [Google Scholar] [CrossRef]
- Imbrea, A.-M.; Balta, I.; Dumitrescu, G.; McCleery, D.; Pet, I.; Iancu, T.; Stef, L.; Corcionivoschi, N.; Liliana, P.-C. Exploring the Contribution of Campylobacter Jejuni to Post-Infectious Irritable Bowel Syndrome: A Literature Review. Appl. Sci. 2024, 14, 3373. [Google Scholar] [CrossRef]
- Kemper, L.; Hensel, A. Campylobacter Jejuni: Targeting Host Cells, Adhesion, Invasion, and Survival. Appl. Microbiol. Biotechnol. 2023, 107, 2725–2754. [Google Scholar] [CrossRef] [PubMed]
- Tikhomirova, A.; McNabb, E.R.; Petterlin, L.; Bellamy, G.L.; Lin, K.H.; Santoso, C.A.; Daye, E.S.; Alhaddad, F.M.; Lee, K.P.; Roujeinikova, A. Campylobacter Jejuni Virulence Factors: Update on Emerging Issues and Trends. J. Biomed. Sci. 2024, 31, 45. [Google Scholar] [CrossRef] [PubMed]
- Makimaa, H.; Ingle, H.; Baldridge, M.T. Enteric Viral Co-Infections: Pathogenesis and Perspective. Viruses 2020, 12, 904. [Google Scholar] [CrossRef]
- Ahmed, N.H.; Chowdhary, A. Pattern of Co-Infection by Enteric Pathogenic Parasites among HIV Sero-Positive Individuals in a Tertiary Care Hospital, Mumbai, India. Indian J. Sex. Transm. Dis. AIDS 2015, 36, 40–47. [Google Scholar]
- Tay, S.C.; Aryee, E.N.O.; Badu, K. Intestinal Parasitemia and HIV/AIDS Co-Infections at Varying CD4+ T-Cell Levels. HIVAIDS Res. Treat.-Open J. 2017, 4, 40–48. [Google Scholar] [CrossRef]
- Caputo, V.; Libera, M.; Sisti, S.; Giuliani, B.; Diotti, R.A.; Criscuolo, E. The Initial Interplay between HIV and Mucosal Innate Immunity. Front. Immunol. 2023, 14, 1104423. [Google Scholar] [CrossRef]
- Mogensen, T.H.; Melchjorsen, J.; Larsen, C.S.; Paludan, S.R. Innate Immune Recognition and Activation during HIV Infection. Retrovirology 2010, 7, 54. [Google Scholar] [CrossRef]
- Desai, S.N.; Cravioto, A.; Sur, D.; Kanungo, S. Maximizing Protection from Use of Oral Cholera Vaccines in Developing Country Settings. Hum. Vaccines Immunother. 2014, 10, 1457–1465. [Google Scholar] [CrossRef]
- Leung, D.T.; Chowdhury, F.; Calderwood, S.B.; Qadri, F.; Ryan, E.T. Immune Responses to Cholera in Children. Expert Rev. Anti Infect. Ther. 2012, 10, 435–444. [Google Scholar] [CrossRef]
- Qadri, F.; Bhuiyan, T.R.; Sack, D.A.; Svennerholm, A.-M. Immune Responses and Protection in Children in Developing Countries Induced by Oral Vaccines. Vaccine 2013, 31, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Sack, D.A.; Qadri, F.; Svennerholm, A.-M. Determinants of Responses to Oral Vaccines in Developing Countries. Ann. Nestlé Engl. Ed. 2008, 66, 71–79. [Google Scholar] [CrossRef]
- Burke, R.M.; Ramani, S.; Lynch, J.; Cooper, L.V.; Cho, H.; Bandyopadhyay, A.S.; Kirkwood, C.D.; Steele, A.D.; Kang, G. Geographic Disparities Impacting Oral Vaccine Performance: Observations and Future Directions. Clin. Exp. Immunol. 2025, 219, uxae124. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Crane, M.; Zhou, J.; Mina, M.; Post, J.J.; Cameron, B.A.; Lloyd, A.R.; Jaworowski, A.; French, M.A.; Lewin, S.R. HIV and Co-Infections. Immunol. Rev. 2013, 254, 114–142. [Google Scholar] [CrossRef]
- Lawn, S. AIDS in Africa: The Impact of Coinfections on the Pathogenesis of HIV-1 Infection. J. Infect. 2004, 48, 1–12. [Google Scholar] [CrossRef]
- Ferreira, R.B.R.; Antunes, L.C.M.; Sal-Man, N. Pathogen-Pathogen Interactions during Co-Infections. ISME J. 2025, 19, wraf104. [Google Scholar] [CrossRef]
- Walch, P.; Broz, P. Viral-Bacterial Co-Infections Screen in Vitro Reveals Molecular Processes Affecting Pathogen Proliferation and Host Cell Viability. Nat. Commun. 2024, 15, 8595. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F. Burden and Aetiology of Diarrhoeal Disease in Infants and Young Children in Developing Countries (the Global Enteric Multicenter Study, GEMS): A Prospective, Case-Control Study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Munoz, G.A.; Riveros-Ramirez, M.D.; Chea-Woo, E.; Ochoa, T.J. Clinical Course of Children with Campylobacter Gastroenteritis with and without Co-Infection in Lima, Peru. Am. J. Trop. Med. Hyg. 2022, 106, 1384. [Google Scholar] [CrossRef]
- Valentini, D.; Vittucci, A.; Grandin, A.; Tozzi, A.; Russo, C.; Onori, M.; Menichella, D.; Bartuli, A.; Villani, A. Coinfection in Acute Gastroenteritis Predicts a More Severe Clinical Course in Children. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 909–915. [Google Scholar] [CrossRef]
- Lindsay, B.; Ramamurthy, T.; Gupta, S.S.; Takeda, Y.; Rajendran, K.; Nair, G.B.; Stine, O.C. Diarrheagenic Pathogens in Polymicrobial Infections. Emerg. Infect. Dis. 2011, 17, 606. [Google Scholar] [CrossRef]
- Parker, C.T.; Schiaffino, F.; Huynh, S.; Paredes Olortegui, M.; Peñataro Yori, P.; Garcia Bardales, P.F.; Pinedo Vasquez, T.; Curico Huansi, G.E.; Manzanares Villanueva, K.; Shapiama Lopez, W.V.; et al. Shotgun Metagenomics of Fecal Samples from Children in Peru Reveals Frequent Complex Co-Infections with Multiple Campylobacter Species. PLoS Negl. Trop. Dis. 2022, 16, e0010815. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- McArdle, A.J.; Turkova, A.; Cunnington, A.J. When Do Co-Infections Matter? Curr. Opin. Infect. Dis. 2018, 31, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K. Wrong Diagnosis—Wrong Antimicrobials: Rise in Antimicrobial Resistance in Developing Countries. IDCases 2025, 41, e02313. [Google Scholar] [CrossRef]
- Ayomide, I.T.; Promise, L.O.; Christopher, A.A.; Okikiola, P.P.; Esther, A.D.; Favour, A.C.; Agbo, O.S.; Sandra, O.-A.; Chiagozie, O.J.; Precious, A.C. The Impact of Antimicrobial Resistance on Co-INFECTIONS: Management Strategies for HIV, TB and Malaria. Int. J. Pathog. Res. 2024, 13, 117–128. [Google Scholar] [CrossRef]
- Birger, R.B.; Kouyos, R.D.; Cohen, T.; Griffiths, E.C.; Huijben, S.; Mina, M.J.; Volkova, V.; Grenfell, B.; Metcalf, C.J.E. The Potential Impact of Coinfection on Antimicrobial Chemotherapy and Drug Resistance. Trends Microbiol. 2015, 23, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Rossolini, G.M.; Schultsz, C.; Tacconelli, E.; Murthy, S.; Ohmagari, N.; Holmes, A.; Bachmann, T.; Goossens, H.; Canton, R. Key Considerations on the Potential Impacts of the COVID-19 Pandemic on Antimicrobial Resistance Research and Surveillance. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total N = 240 |
|---|---|
| n (% of Total) | |
| Sex | |
| Male | 112 (46.7) |
| Female | 77 (32.1) |
| Missing | 51 (21.2) |
| Age (Years), Median (IQI *) | 26 (14–38) |
| Age group (Years) | |
| <15 | 47 (19.6) |
| 15–24 | 41 (17.1) |
| 25–34 | 41 (17.1) |
| 35+ | 59 (24.6) |
| Missing | 52 (21.7) |
| Facility | |
| Chipata | 26 (10.8) |
| George | 62 (25.8) |
| Heroes | 54 (22.5) |
| Levy | 8 (3.3) |
| Matero | 90 (37.5) |
| Vaccinated against cholera | |
| No | 18 (7.5) |
| Yes | 4 (1.7) |
| Missing | 218 (90.8) |
| HIV Status | |
| Negative | 215 (89.6) |
| Positive | 19 (7.9) |
| Missing | 6 (2.5) |
| Characteristic | Co-Infection (n = 190, 79.2%) | p-Value | Unadjusted PR * | p-Value | Adjusted PR | p-Value |
|---|---|---|---|---|---|---|
| n (% of Row Total) | Ratio (95% CI) | Ratio (95% CI) | ||||
| Sex | ||||||
| Male | 87/112 (77.7) | 0.865 a | Reference | 0.866 | ||
| Female | 59/77 (76.6) | 0.99 (0.84, 1.16) | ||||
| Age group (Years) | ||||||
| <15 | 41/47 (87.2) | 0.080 a | Reference | 0.098 | Reference | 0.068 |
| 15–24 | 28/41 (68.3) | 0.78 (0.62, 0.99) | 0.75 (0.58, 0.96) | |||
| 25–34 | 29/41 (70.7) | 0.81 (0.65, 1.02) | 0.80 (0.64, 1.00) | |||
| 35+ | 49/59 (83.1) | 0.95 (0.81, 1.12) | 0.92 (0.78, 1.09) | |||
| Vaccinated against cholera | ||||||
| No | 14/18 (77.8) | 1.000 b | Reference | 0.910 | ||
| Yes | 3/4 (75.0) | 0.96 (0.51, 1.81) | ||||
| HIV Status | ||||||
| Negative | 166/215 (77.2) | 0.084 b | Reference | 0.002 | Reference | |
| Positive | 18/19 (94.7) | 1.23 (1.08, 1.40) | 1.27 (1.07, 1.51) | 0.008 |
| Characteristic | Total N = 62 |
|---|---|
| n (% of Total) | |
| Sex | |
| Male | 30 (48.4) |
| Female | 32 (51.6) |
| Age, Median (IQI *) | 27 (20–40) |
| Age group | |
| <15 | 1 (1.6) |
| 15–24 | 9 (14.5) |
| 25–34 | 15 (24.2) |
| 35+ | 16 (25.8) |
| Vaccinated against cholera | |
| No | 14 (22.6) |
| Yes | 4 (6.5) |
| Missing | 44 (71.0) |
| HIV Status | |
| Negative | 53 (85.5) |
| Positive | 9 (14.5) |
| Infection status | |
| Mono-infection | 14 (22.6) |
| Co-infection | 48 (77.4) |
| Characteristic | Moderate-to-Severe (n = 33, 53.2%) | p-Value | Unadjusted PR * | p-Value | Unadjusted PR * | p-Value |
|---|---|---|---|---|---|---|
| n (% of Row Total) | Ratio (95% CI) | Ratio (95% CI) | ||||
| Infection status | ||||||
| Mono-infection | 10 (71.4) | 0.121 | Reference | 0.080 | Reference | 0.014 |
| Co-infection | 23 (47.9) | 0.67 (0.43, 1.05) | 0.59 (0.38, 0.90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuntawala, D.H.; Bosomprah, S.; Phiri, B.; Ng’ombe, H.; Liswaniso, F.; Muchimba, M.; Silwamba, S.; Chibesa, K.; Nzangwa, B.T.; Luchen, C.C.; et al. Prevalence and Patterns of Enteric Co-Infections Among Individuals Presenting with Cholera-like Diarrheal Disease During Seasonal Cholera Outbreaks. Pathogens 2025, 14, 1224. https://doi.org/10.3390/pathogens14121224
Kuntawala DH, Bosomprah S, Phiri B, Ng’ombe H, Liswaniso F, Muchimba M, Silwamba S, Chibesa K, Nzangwa BT, Luchen CC, et al. Prevalence and Patterns of Enteric Co-Infections Among Individuals Presenting with Cholera-like Diarrheal Disease During Seasonal Cholera Outbreaks. Pathogens. 2025; 14(12):1224. https://doi.org/10.3390/pathogens14121224
Chicago/Turabian StyleKuntawala, Dhvani H., Samuel Bosomprah, Bernard Phiri, Harriet Ng’ombe, Fraser Liswaniso, Mutinta Muchimba, Suwilanji Silwamba, Kennedy Chibesa, Bertha T. Nzangwa, Charlie C. Luchen, and et al. 2025. "Prevalence and Patterns of Enteric Co-Infections Among Individuals Presenting with Cholera-like Diarrheal Disease During Seasonal Cholera Outbreaks" Pathogens 14, no. 12: 1224. https://doi.org/10.3390/pathogens14121224
APA StyleKuntawala, D. H., Bosomprah, S., Phiri, B., Ng’ombe, H., Liswaniso, F., Muchimba, M., Silwamba, S., Chibesa, K., Nzangwa, B. T., Luchen, C. C., Mwape, I., Tigere, S. F., Simuyandi, M., Mbewe, N., Chilengi, R., Debes, A. K., Thomson, N. R., Sack, D. A., & Chisenga, C. C. (2025). Prevalence and Patterns of Enteric Co-Infections Among Individuals Presenting with Cholera-like Diarrheal Disease During Seasonal Cholera Outbreaks. Pathogens, 14(12), 1224. https://doi.org/10.3390/pathogens14121224





