Intra-Palpebral Tuberculin Skin Test and Interferon Gamma Release Assay in Diagnosing Tuberculosis Due to Mycobacterium caprae in European Bison (Bison bonasus)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Material
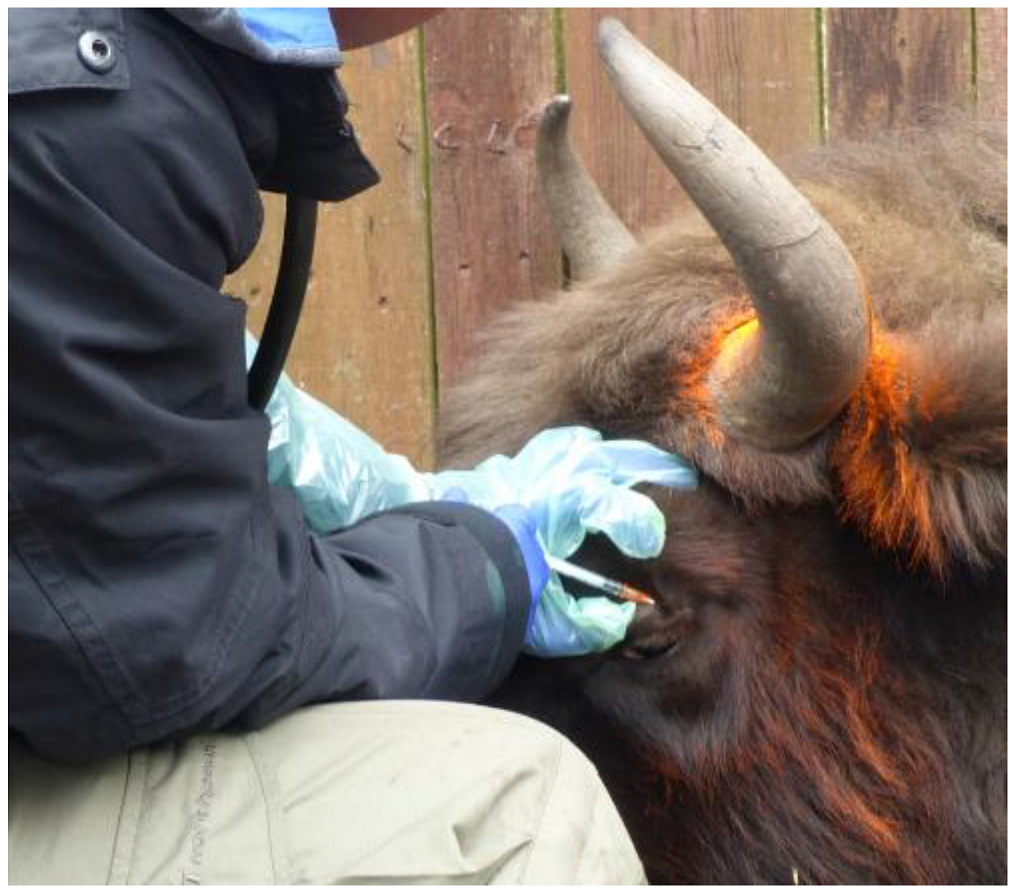
4.2. Tuberculin Skin Test (TST)
4.3. Interferon Gamma Release Assay (IGRA)
4.4. Microbiological and Molecular Examination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Krajewska-Wędzina, M.; Didkowska, A.; Sridhara, A.A.; Elahi, R.; Johnathan-Lee, A.; Radulski, Ł.; Lipiec, M.; Anusz, K.; Lyashchenko, K.P.; Miller, M.A.; et al. Transboundary tuberculosis: Importation of alpacas infected with Mycobacterium bovis from the United Kingdom to Poland and potential for serodiagnostic assays in detecting tuberculin skin test false-negative animals. Transbound. Emerg. Dis. 2020, 67, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, M.; Radulski, Ł.; Szulowski, K. A case of bovine tuberculosis in pigs in Poland-a country free from the disease. AAEM 2019, 26, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Orłowska, B.; Augustynowicz-Kopeć, E.; Krajewska, M.; Zabost, A.; Welz, M.; Kaczor, S.; Anusz, K. Mycobacterium caprae transmission to free-living grey wolves (Canis lupus) in the Bieszczady mountains in southern Poland. Eur. J. Wildl. Res. 2017, 63, 21. [Google Scholar] [CrossRef] [Green Version]
- Krajewska, M.; Lipiec, M.; Zabost, A.; Augustynowicz-Kopeć, E.; Szulowski, K. Bovine tuberculosis in a wild boar (Sus scrofa) in Poland. J. Wild. Dis. 2014, 50, 1001–1002. [Google Scholar] [CrossRef] [PubMed]
- Orłowska, B.; Krajewska-Wędzina, M.; Augustynowicz-Kopeć, E.; Kozińska, M.; Brzezińska, S.; Zabost, A.; Didkowska, A.; Welz, M.; Kaczor, S.; Żmuda, P.; et al. Epidemiological characterization of Mycobacterium caprae strains isolated from wildlife in the Bieszczady Mountains, on the border of Southeast Poland. BMC Vet. Res. 2020, 16, 362. [Google Scholar] [CrossRef] [PubMed]
- Krajewska-Wędzina, M.; Olech, W.; Kozińska, M.; Augustynowicz-Kopeć, E.M.; Weiner, M.; Szulowski, K. Bovine tuberculosis outbreak in farmed American bison (Bison bison) in Poland. Pol. J. Vet. Sci. 2017, 20, 819–821. [Google Scholar] [PubMed]
- Krajewska-Wędzina, M.; Kozińska, M.; Orłowska, B.; Weiner, M.; Szulowski, K.; Augustynowicz-Kopeć, E.; Anusz, K.; Smith, N.H. Molecular characterisation of Mycobacterium caprae strains isolated in Poland. Vet. Rec. 2018, 182, 292. [Google Scholar] [CrossRef] [PubMed]
- Didkowska, A.; Orłowska, B.; Witkowski, L.; Olbrych, K.; Brzezińska, S.; Augustynowicz-Kopeć, E.; Krajewska-Wędzina, M.; Bereznowski, A.; Bielecki, W.; Krzysiak, M.; et al. Biopsy and Tracheobronchial Aspirates as Additional Tools for the Diagnosis of Bovine Tuberculosis in Living European Bison (Bison bonasus). Animals 2020, 10, 2017. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M.; Załuski, M.; Zabost, A.; Orłowska, B.; Augustynowicz-Kopeć, E.; Anusz, K.; Lipiec, M.; Weiner, M.; Szulowski, K. Tuberculosis in Antelopes in a Zoo in Poland–Problem of Public Health. Pol. J. Microbiol. 2015, 64, 395–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didkowska, A. Improved Algorithm for Clinical and Laboratory Diagnostics of Bovine Tuberculosis in European Bison (Bison bonasus). Ph.D. Thesis, Warsaw University of Life Science, Warsaw, Poland, 2 July 2020. (In Polish). [Google Scholar]
- Didkowska, A.; Krajewska-Wędzina, M.; Bielecki, W.; Brzezińska, S.; Augustynowicz-Kopeć, E.; Olech, W.; Anusz, K.; Sridhara, A.A.; Johnathan-Lee, A.; Elahi, R.; et al. Antibody responses in European bison (Bison bonasus) naturally infected with Mycobacterium caprae. Vet. Microb. 2021, 253, 108952. [Google Scholar] [CrossRef]
- Didkowska, A.; Dziekan, P.; Czujkowska, A.; Bereznowski, A.; Witkowski, L.; Orłowska, B.; Wiśniewski, J.; Krzysiak, M.; Krajewska-Wędzina, M.; Bruczyńska, M.; et al. The first visually-guided bronchoscopy in European bison (Bison bonasus)-An additional tool in the diagnosis of bovine tuberculosis? Vet. Anim. Sci. 2021, 12, 100174. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2021-3. 2021. Available online: https://www.iucnredlist.org (accessed on 24 January 2022).
- Pucken, V.B.; Knubben-Schweizer, G.; Döpfer, D.; Groll, A.; Hafner-Marx, A.; Hörmansdorfer, S.; Sauter-Louis, C.; Straubinger, R.K.; Zimmermann, P.; Hartnack, S. Evaluating diagnostic tests for bovine tuberculosis in the southern part of Germany: A latent class analysis. PLoS ONE 2017, 12, e0179847. [Google Scholar] [CrossRef]
- Pollock, J.M.; Neill, S.D. Mycobacterium bovis infection and tuberculosis in cattle. Vet. J. 2002, 163, 115–127. [Google Scholar] [CrossRef]
- Clifton-Hadley, R.S.; Goodchild, A.V. The Fall and Rise of Bovine Tuberculosis in Great Britan: Second Edition. In Mycobacterium Bovis Infection in Animals and Humans (110–116); Thoen, C.O., Steele, J.H., GliADorf, M.F., Eds.; Blackwell Publishers: Boston, MA, USA, 2005. [Google Scholar]
- de la Rua-Domenech, R.; Goodchild, A.T.; Vordermeier, H.M.; Hewinson, R.G.; Christiansen, K.H.; Clifton-Hadley, R.S. Ante mortem diagnosis of tuberculosis in cattle: A review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci. 2006, 81, 190–210. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Tellechea, J.; García Marín, J.F. Evaluation of cellular and serological diagnostic tests for the detection of Mycobacterium bovis-infected goats. Vet. Microbiol. 1998, 62, 281–290. [Google Scholar] [CrossRef]
- Palmer, M.V.; Waters, W.R.; Thacker, T.C.; Greenwald, R.; Esfandiari, J.; Lyashchenko, K.P. Effects of different tuberculin skin-testing regimens on gamma interferon and antibody responses in cattle experimentally infected with Mycobacterium bovis. Clin. Vaccine Immunol. 2006, 13, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Coad, M.; Clifford, D.; Rhodes, S.G.; Hewinson, R.G.; Vordermeier, H.M.; Whelan, A.O. Repeat tuberculin skin testing leads to desensitisation in naturally infected tuberculous cattle which is associated with elevated interleukin-10 and decreased interleukin-1 beta responses. Vet. Res. 2010, 41, 14. [Google Scholar] [CrossRef] [Green Version]
- Blanco, F.C.; Bianco, M.V.; Meikle, V.; Garbaccio, S.; Vagnoni, L.; Forrellad, M.; Klepp, L.I.; Cataldi, A.A.; Bigi, F. Increased IL-17 expression is associated with pathology in a bovine model of tuberculosis. Tuberculosis 2011, 91, 57–63. [Google Scholar] [CrossRef]
- Waters, W.R.; Maggioli, M.F.; Palmer, M.V.; Thacker, T.C.; McGill, J.L.; Vordermeier, H.M.; Berney-Meyer, L.; Jacobs, W.R., Jr.; Larsen, M.H. Interleukin-17A as a Biomarker for Bovine Tuberculosis. Clin. Vaccine Immunol. 2016, 23, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Panarella, M.L.; Bimes, R.S. A Naturally Occurring Outbreak of Tuberculosis in a Group of Imported Cynomolgus Monkeys (Macaca fascicularis). J. Am. Assoc. Lab Anim. Sci. 2010, 49, 221–225. [Google Scholar]
- Breed, D.; Meyer, L.C.R.; Steyl, J.C.A.; Goddard, A.; Burroughs, R.; Kohn, T.A. Conserving wildlife in a changing world: Understanding capture myopathy-a malignant outcome of stress during capture and translocation. Conserv. Physiol. 2019, 7, coz027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corti, P.; Arnemo, J.M. Partially Reversible Immobilization of Free-Ranging Huemul Deer (Hippocamelus bisulcus) with Medetomidine-Ketamine and Atipamezole. J. Wildl. Dis. 2021, 57, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, F.M.; Burr, P.D.; Swift, B.M.C.; Rees, C.E.D.; Masters, N. Conservation challenges: The limitations of antemortem tuberculosis testing in captive Asiatic lions (Panthera leo persica). J. Zoo Wildl. Med. 2020, 51, 426–432. [Google Scholar] [CrossRef]
- Chapinal, N.; Schumaker, B.A.; Joly, D.O.; Elkin, B.T.; Stephen, C. Bayesian analysis to evaluate tests for the detection of Mycobacterium bovis infection in free-ranging wild bison (Bison bison athabascae) in the absence of a gold standard. J. Wildl. Dis. 2015, 51, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Himsworth, C.G.; Elkin, B.T.; Nishi, J.S.; Epp, T.; Lyashchenko, K.P.; Surujballi, O.; Turcotte, C.; Esfandiari, J.; Greenwald, R.; Leighton, F.A. Comparison of test performance and evaluation of novel immunoassays for tuberculosis in a captive herd of wood bison naturally infected with Mycobacterium bovis. J. Wildl. Dis. 2010, 46, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gcebe, N.; Hlokwe, T.M. Non-tuberculous Mycobacteria in South African Wildlife: Neglected Pathogens and Potential Impediments for Bovine Tuberculosis Diagnosis. Front. Cell Infect. Microbiol. 2017, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, A.O.; Gormley, E.; Gcebe, N.; Fosgate, G.T.; Conan, A.; Aagaard, C.; Michel, A.L.; Rutten, V.P.M.G. Cross reactive immune responses in cattle arising from exposure to Mycobacterium bovis and non-tuberculous mycobacteria. Prev. Vet. Med. 2018, 152, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, T.J.; Buddle, B.M.; De Lisle, G.W. An evaluation of the gamma interferon test for detecting bovine tuberculosis in cattle 8 to 28 days after tuberculin skin testing. Res. Vet. Sci. 2000, 69, 57–61. [Google Scholar] [CrossRef]
- Schiller, I.; Vordermeier, H.M.; Waters, W.R.; Whelan, A.O.; Coad, M.; Gormley, E.; Buddle, B.M.; Palmer, M.; Thacker, T.; McNair, J.; et al. Bovine tuberculosis: Effect of the tuberculin skin test on in vitro interferon gamma responses. Vet. Immunol. Immunopathol. 2010, 136, 1–11. [Google Scholar] [CrossRef]
- Jones, G.J.; Coad, M.; Khatri, B.; Bezos, J.; Parlane, N.A.; Buddle, B.M.; Villarreal-Ramos, B.; Hewinson, R.G.; Vordermeier, H.M. Tuberculin Skin Testing Boosts Interferon Gamma Responses to DIVA Reagents in Mycobacterium bovis-Infected Cattle. Clin. Vaccine Immunol. 2017, 24, e00551-16. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.; Cooper, D.; Goosen, W.J.; McFadyen, R.; Warren, R.M.; van Helden, P.D.; Parsons, S.D.C.; Miller, M.A. Antigen-specific interferon-gamma release is decreased following the single intradermal comparative cervical skin test in African buffaloes (Syncerus caffer). Vet. Immunol. Immunopathol. 2018, 201, 12–15. [Google Scholar] [CrossRef]
- Gormley, E.; Corner, L.A.; Costello, E.; Rodriguez-Campos, S. Bacteriological diagnosis and molecular strain typing of Mycobacterium bovis and Mycobacterium caprae. Res. Vet. Sci. 2014, 97, S30–S43. [Google Scholar] [CrossRef]
- Cousins, D.V. Chapter 3.4.6. Bovine Tuberculosis. In OIE Terrestrial Manual 2018; OIE: Paris, France, 2018; pp. 1058–1074. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.06_BOVINE_TB.pdf (accessed on 24 January 2022).
- Smith, K.; Bernitz, N.; Cooper, D.; Kerr, T.J.; de Waal, C.R.; Clarke, C.; Goldswain, S.; McCall, W.; McCall, A.; Cooke, D.; et al. Optimisation of the tuberculin skin test for detection of Mycobacterium bovis in African buffaloes (Syncerus caffer). Prev. Vet. Med. 2021, 188, 105254. [Google Scholar] [CrossRef]
- Bernitz, N.; Goosen, W.J.; Clarke, C.; Kerr, T.J.; Higgitt, R.; Roos, E.O.; Cooper, D.V.; Warren, R.M.; van Helden, P.D.; Parsons, S.D.C.; et al. Parallel testing increases detection of Mycobacterium bovis-infected African buffaloes (Syncerus caffer). Vet. Immunol. Immunopathol. 2018, 204, 40–43. [Google Scholar] [CrossRef]
- Bernitz, N.; Clarke, C.; Roos, E.O.; Goosen, W.J.; Cooper, D.; van Helden, P.D.; Parsons, S.D.C.; Miller, M.A. Detection of Mycobacterium bovis infection in African buffaloes (Syncerus caffer) using QuantiFERON®-TB Gold (QFT) tubes and the Qiagen cattletype® IFN-gamma ELISA. Vet Immunol. Immunopathol. 2018, 196, 48–52, Erratum in Vet. Immunol. Immunopathol. 2018, 201, 88. [Google Scholar] [CrossRef]
- Bernitz, N.; Kerr, T.J.; Goosen, W.J.; Clarke, C.; Higgitt, R.; Roos, E.O.; Cooper, D.V.; Warren, R.M.; van Helden, P.D.; Parsons, S.D.C.; et al. Parallel measurement of IFN-γ and IP-10 in QuantiFERON®-TB Gold (QFT) plasma improves the detection of Mycobacterium bovis infection in African buffaloes (Syncerus caffer). Prev. Vet. Med. 2019, 169, 104700. [Google Scholar] [CrossRef] [PubMed]
- Goosen, W.J.; Kerr, T.J.; Kleynhans, L.; Buss, P.; Cooper, D.; Warren, R.M.; van Helden, P.D.; Schröder, B.; Parsons, S.D.C.; Miller, M.A. The VetMAX™ M. tuberculosis complex PCR kit detects MTBC DNA in antemortem and postmortem samples from white rhinoceros (Ceratotherium simum), African elephants (Loxodonta africana) and African buffaloes (Syncerus caffer). BMC Vet. Res. 2020, 16, 220. [Google Scholar] [CrossRef]
- Klich, D.; Łopucki, R.; Stachniuk, A.; Sporek, M.; Fornal, E.; Wojciechowska, M.; Olech, W. Pesticides and conservation of large ungulates: Health risk to European bison from plant protection products as a result of crop depredation. PLoS ONE 2020, 15, e0228243. [Google Scholar] [CrossRef] [Green Version]
- Didkowska, A.; Klich, D.; Hapanowicz, A.; Orłowska, B.; Gałązka, M.; Rzewuska, M.; Olech, W.; Anusz, K. Pathogens with potential impact on reproduction in captive and free-ranging European bison (Bison bonasus) in Poland-a serological survey. BMC Vet. Res. 2021, 17, 345. [Google Scholar] [CrossRef]
- Department for Environment, Food & Rural Affairs; Beef cattle and dairy cows: Health regulations. 2012. Available online: https://www.gov.uk/guidance/cattle-health (accessed on 6 February 2022).
- British Cattle Movement Service; Guidance on keeping cattle, bison and buffalo in Great Britain. 2022. Available online: https://www.gov.uk/government/collections/guidance-on-keeping-cattle-bison-and-buffalo-in-great-britain (accessed on 6 February 2022).
- Act of 15 January 2015 on the Protection of Animals Used for Scientific or Educational Purposes Sets out the Principles and Conditions for the Protection of Animals Used for Scientific or Educational Purposes; Polish Sejm: Warsaw, Poland, 5 January 2015; Dz. U. 2015 poz. 266. (In Polish)
- Krzysiak, M.K.; Larska, M. Pharmacological immobilization of European bison (Bison bonasus). Med. Weter. 2014, 70, 172–175. [Google Scholar]
- Rozporządzenie Ministra Rolnictwa i Rozwoju Wsi z dnia 4 lipca 2017 r. W sprawie Sposobu Prowadzenia Dokumentacji związanej ze Zwalczaniem Chorób Zakaźnych Zwierząt; Polish Sejm: Warsaw, Poland, 4 July 2017; Dz.U. 2017 poz. 1388. (In Polish)
- Anusz, K.; Orłowska, B.; Krajewska-Wędzina, M.; Augustynowicz-Kopeć, E.M.; Krzysiak, M.K.; Bielecki, W.; Witkowski, L.; Welz, M.; Kita, J. Ante-mortem and post-mortem tuberculosis diagnostics in three European Bison from the enclosure in Bukowiec in the Bieszczady National Park in Poland. Med. Wet. 2017, 73, 642–646. [Google Scholar] [CrossRef] [Green Version]

| Animal ID (Age in Years) | TST | IGRA | Lesions | Culture |
|---|---|---|---|---|
| 1 (3) | Not tested | − | ||
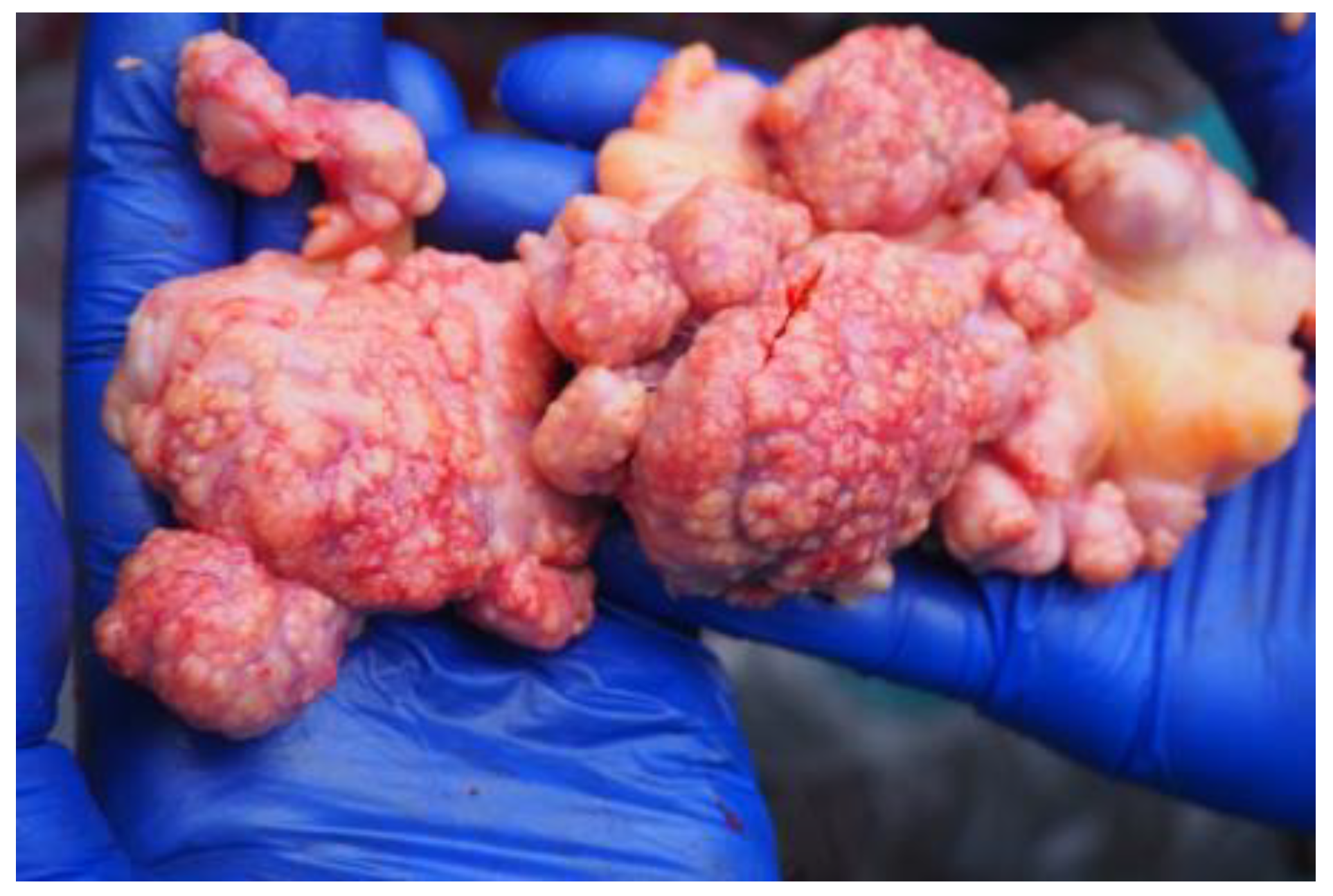
| 2 (17) | + | + | caseous necrosis in retropharyngeal and mesenteric lymph nodes | + |
| 3 (3) | − | − | − | |
| 4 (5) | − | − | − | |
| 5 (7) | − | − | − | |
| 6 (6) | (±) | + | caseous necrosis in retropharyngeal, tracheobronchial, and mediastinal lymph nodes | + |
| 7 (10) | (±) | + | generalized tuberculous lesions | + |
| 8 (7) | not tested | + | caseous necrosis in retropharyngeal, tracheobronchial, and mediastinal lymph nodes, enlarged duodenal and mesenteric lymph nodes | + |
| 9 (6) | (±) | + | caseous necrosis in retropharyngeal, tracheobronchial, and mediastinal lymph nodes | + |
| 10 (19) | − | − | − |
| Animal ID | Sample Period | TST | IGRA | Culture |
|---|---|---|---|---|
| 6 | A | − | − | + |
| B | + | − | ||
| C | − | − | ||
| D | − | + | ||
| E | ± | + | ||
| 7 | A | + | − | + |
| B | + | − | ||
| C | − | − | ||
| D | − | ± | ||
| E | ± | + | ||
| 8 | A | − | − | + |
| B | − | − | ||
| C | − | − | ||
| D | Not tested | Not tested | ||
| E | Not tested | + | ||
| 9 | A | − | + | + |
| B | + | − | ||
| C | − | − | ||
| D | − | + | ||
| E | ± | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didkowska, A.; Orłowska, B.; Krajewska-Wędzina, M.; Krzysiak, M.; Bruczyńska, M.; Wiśniewski, J.; Klich, D.; Olech, W.; Anusz, K. Intra-Palpebral Tuberculin Skin Test and Interferon Gamma Release Assay in Diagnosing Tuberculosis Due to Mycobacterium caprae in European Bison (Bison bonasus). Pathogens 2022, 11, 260. https://doi.org/10.3390/pathogens11020260
Didkowska A, Orłowska B, Krajewska-Wędzina M, Krzysiak M, Bruczyńska M, Wiśniewski J, Klich D, Olech W, Anusz K. Intra-Palpebral Tuberculin Skin Test and Interferon Gamma Release Assay in Diagnosing Tuberculosis Due to Mycobacterium caprae in European Bison (Bison bonasus). Pathogens. 2022; 11(2):260. https://doi.org/10.3390/pathogens11020260
Chicago/Turabian StyleDidkowska, Anna, Blanka Orłowska, Monika Krajewska-Wędzina, Michał Krzysiak, Małgorzata Bruczyńska, Jan Wiśniewski, Daniel Klich, Wanda Olech, and Krzysztof Anusz. 2022. "Intra-Palpebral Tuberculin Skin Test and Interferon Gamma Release Assay in Diagnosing Tuberculosis Due to Mycobacterium caprae in European Bison (Bison bonasus)" Pathogens 11, no. 2: 260. https://doi.org/10.3390/pathogens11020260