Study on Non-Isothermal Transformation of Ti-6Al-4V in Solution Heating Stage
Abstract
:1. Introduction
2. Materials and Methods
3. Theoretical Model of Non-Isothermal Transformation
4. Results and Analysis
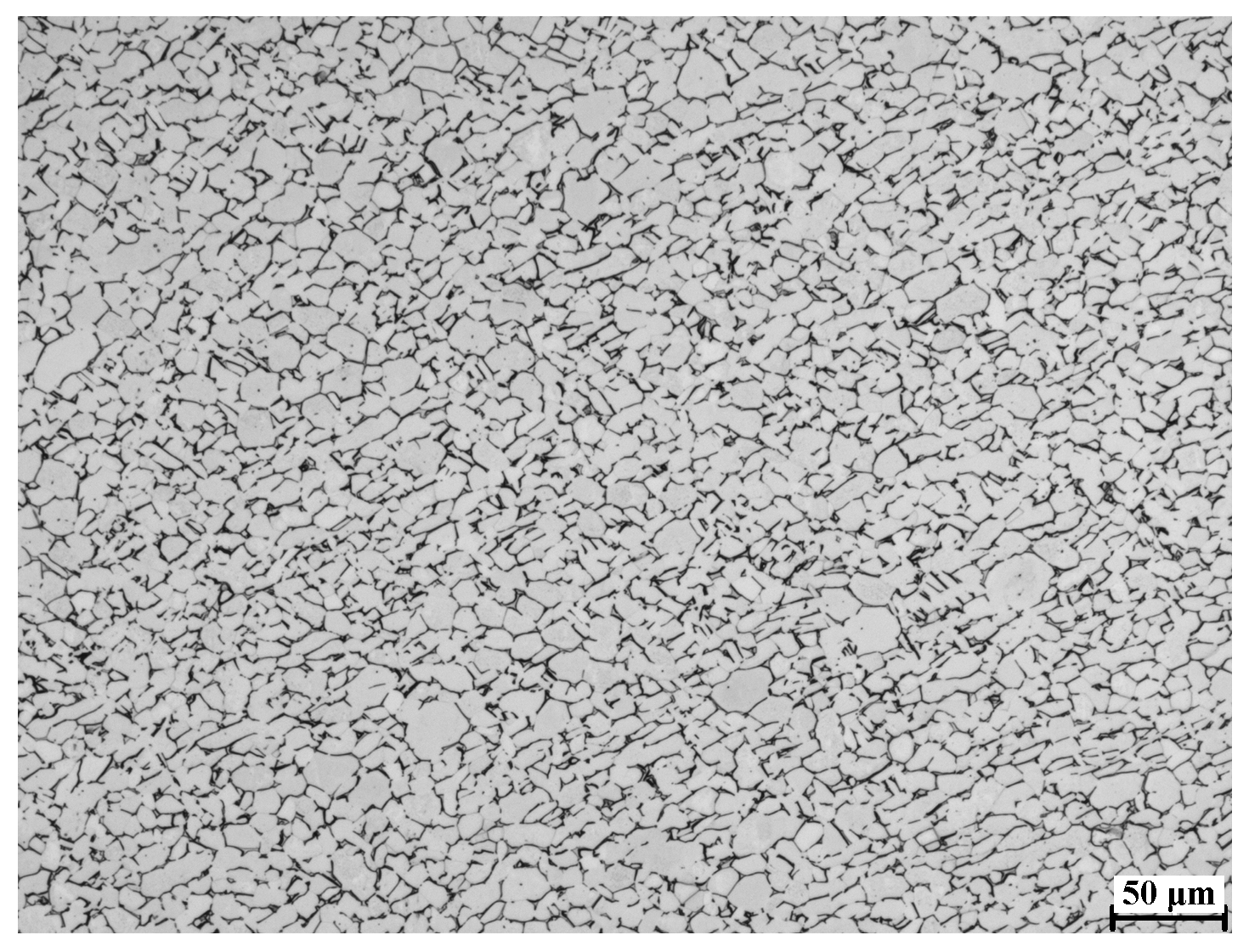
4.1. Microstructure Analysis
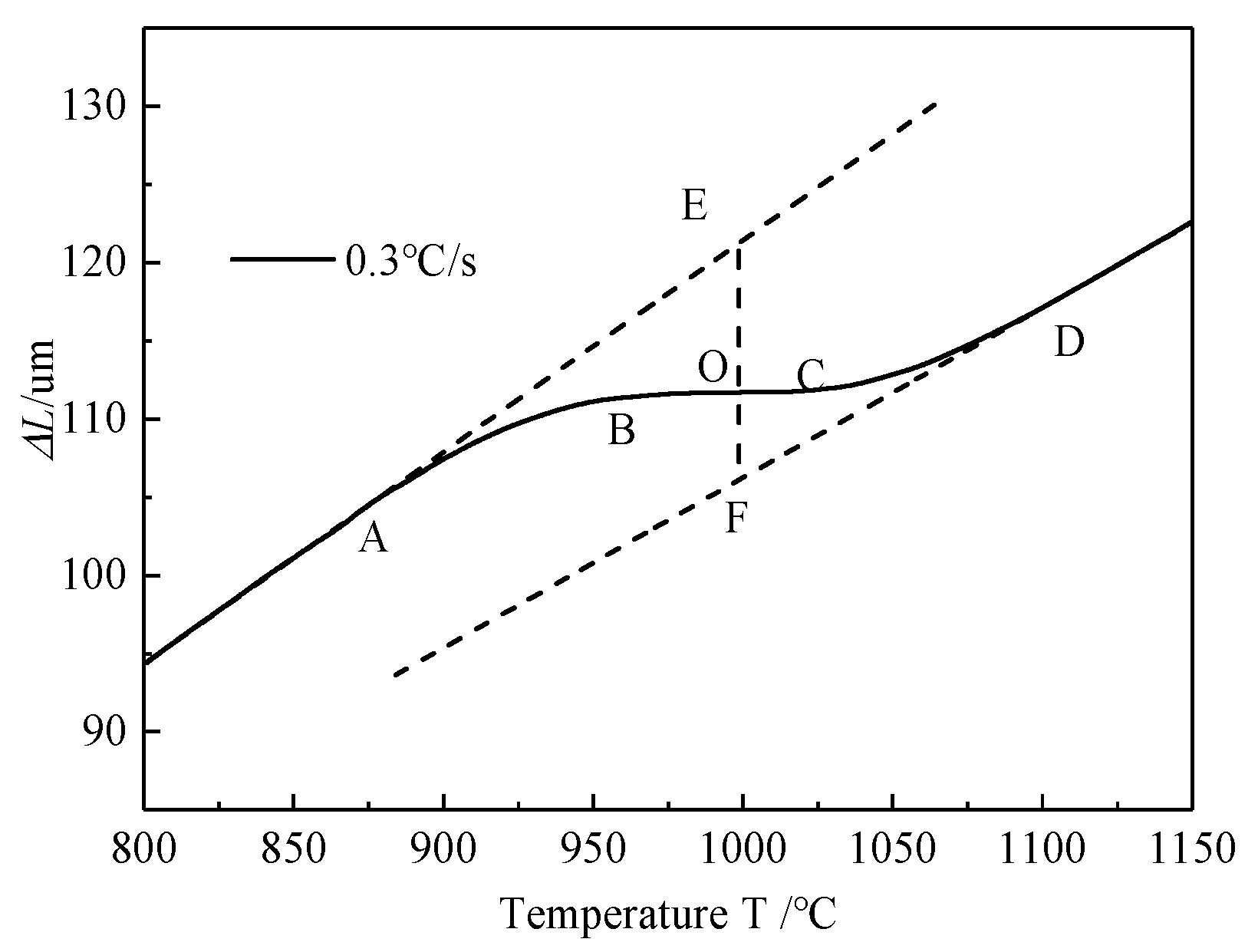
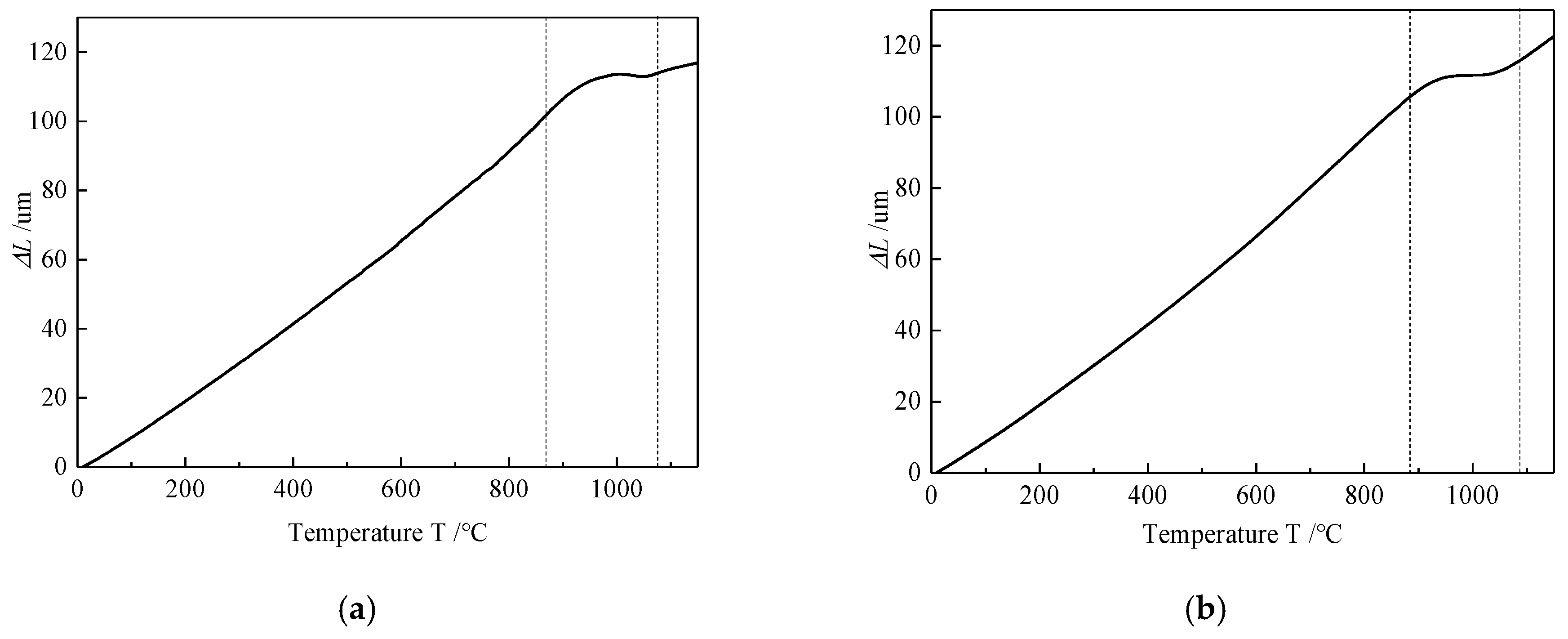
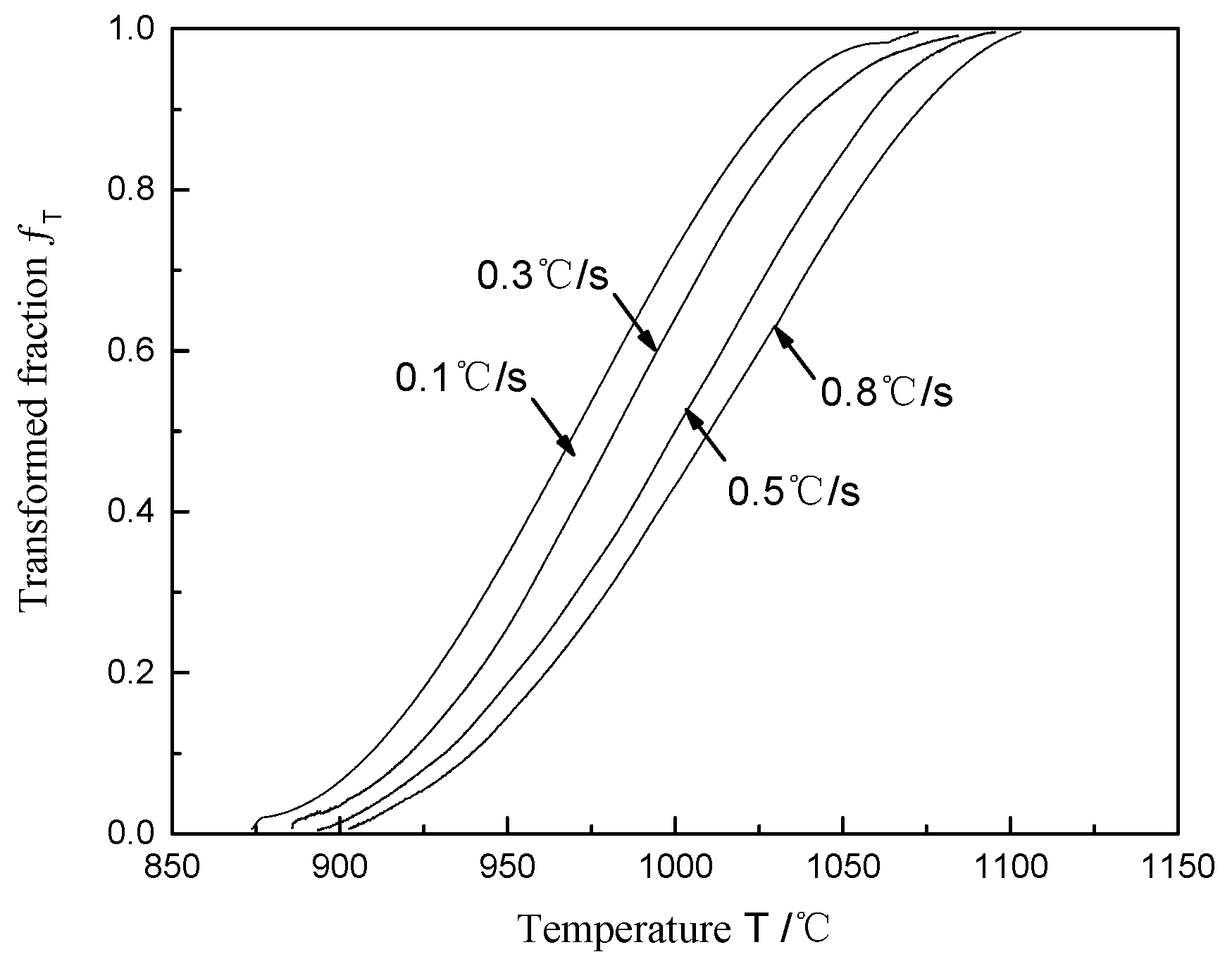
4.2. Phase Transformation Kinetics during the Heating Process
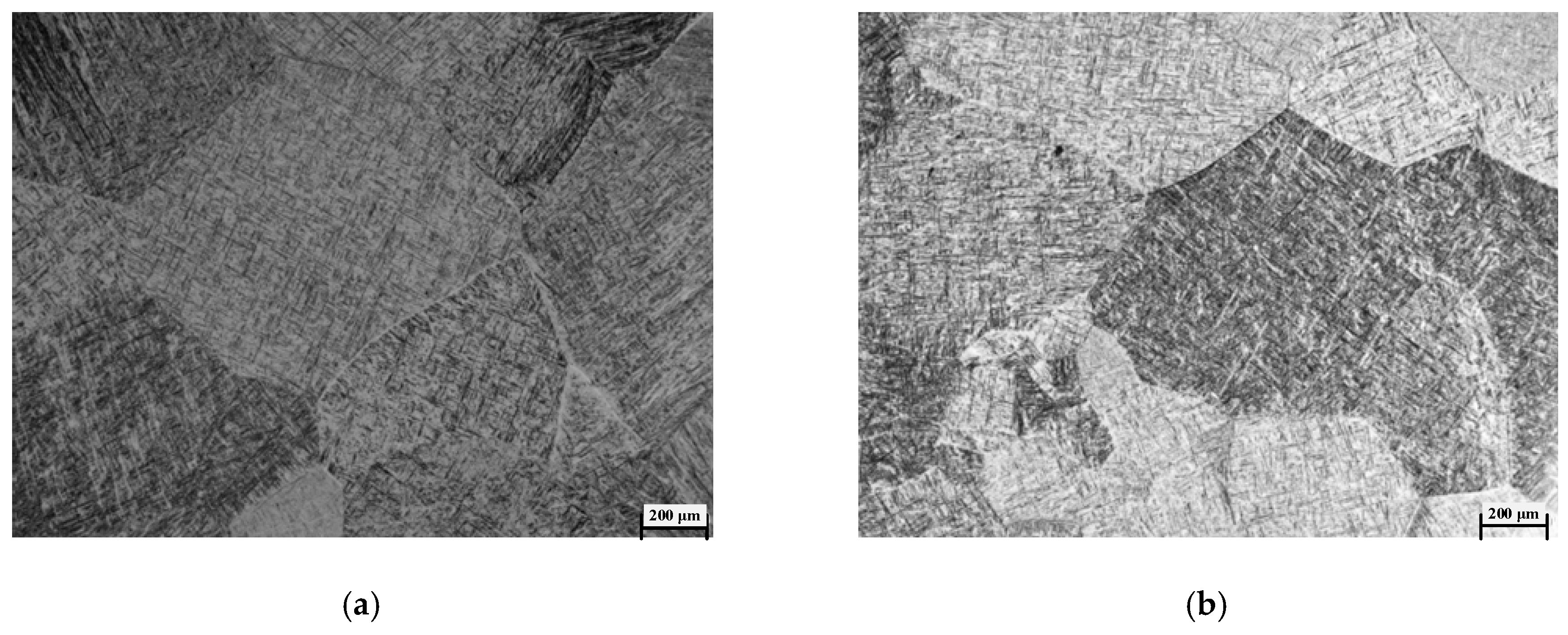
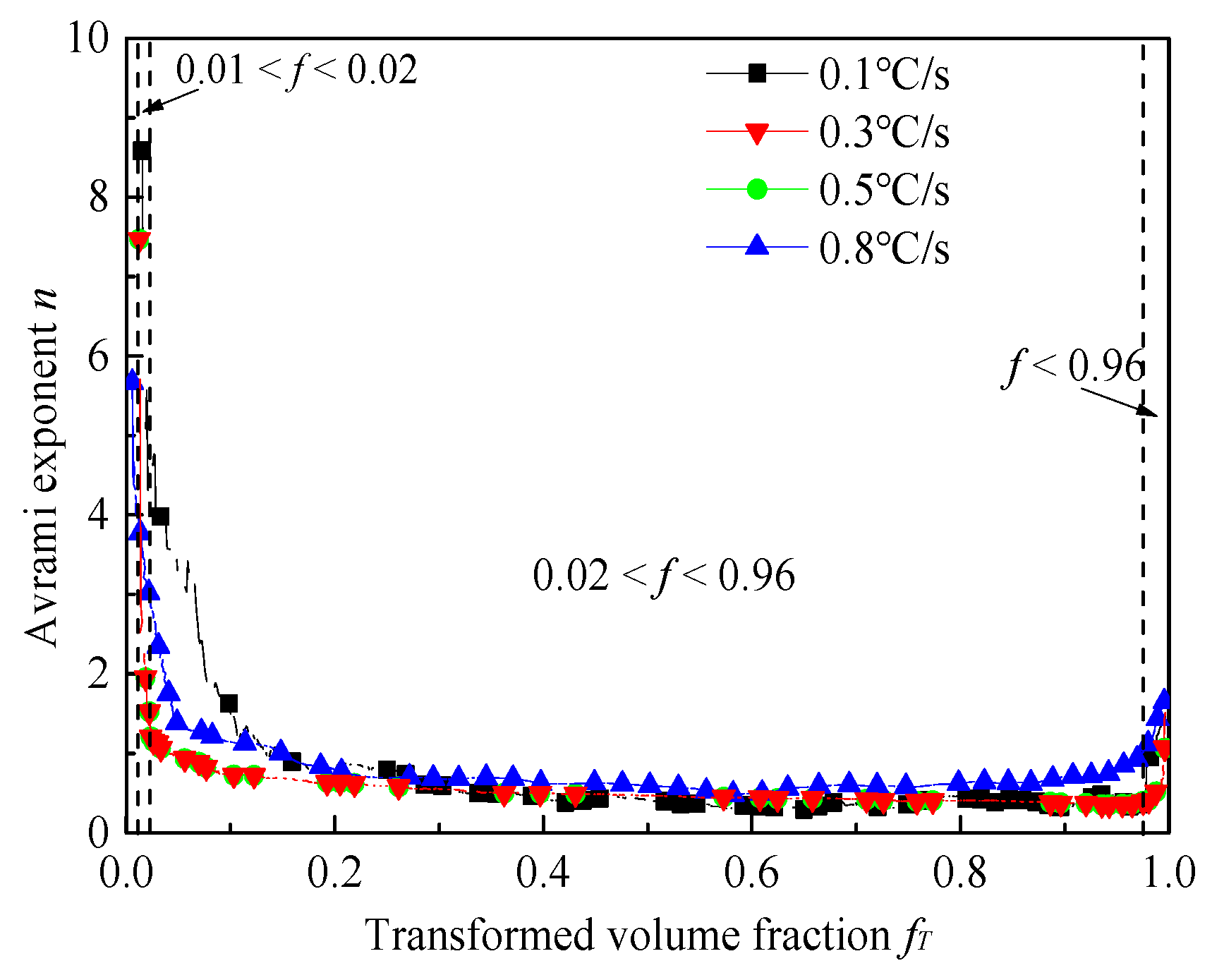
4.3. Transformation Mechanism during the Heating Process
5. Conclusions
- (1)
- The thermal dilatometry test is an effective method to study the phase transformation process. The expansion curve and its first derivative curve can directly reflect the starting, developing and finishing of phase transformation.
- (2)
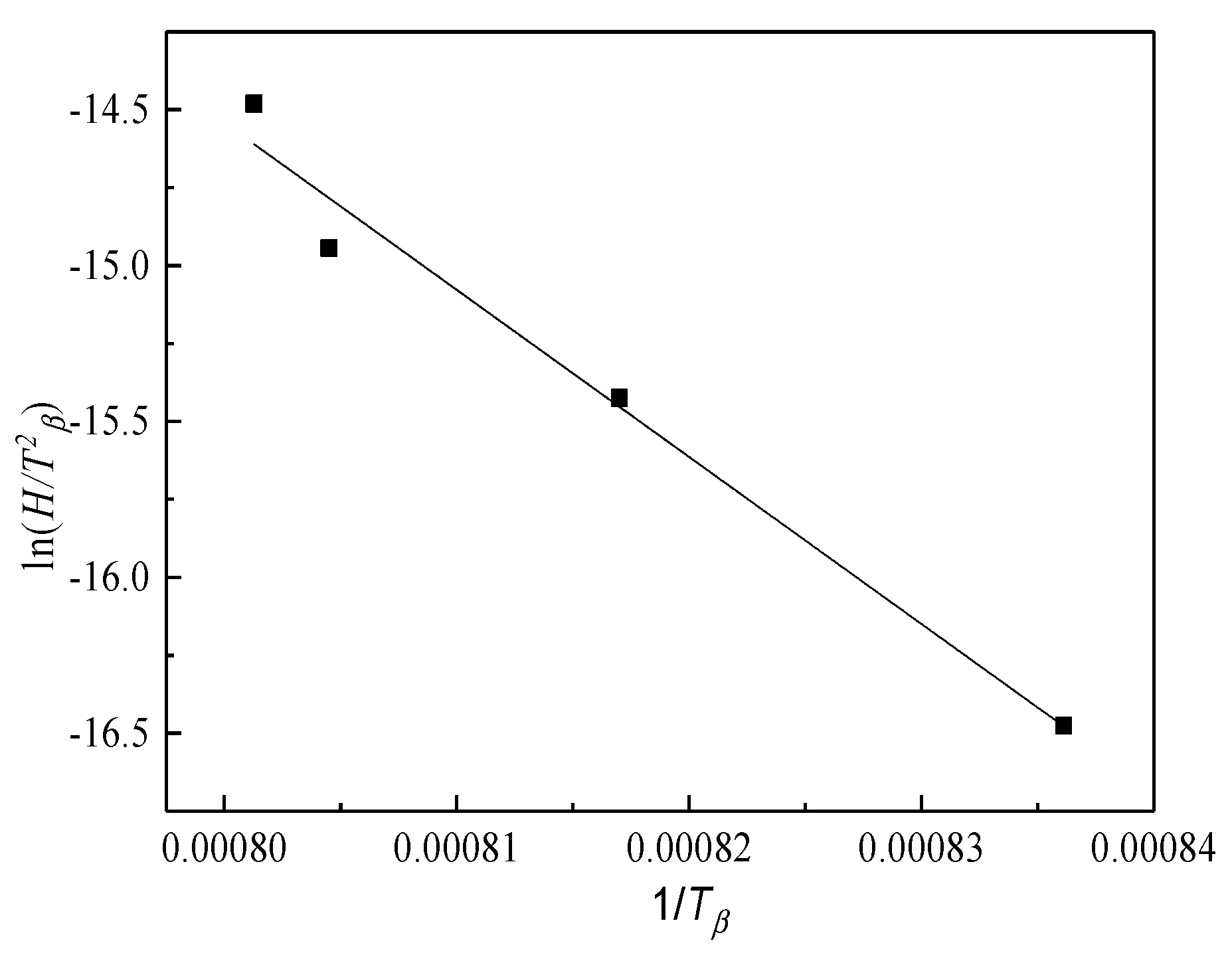
- The phase transformation kinetics curve of Ti-6Al-4V during the continuous heating process presents a typical “S” pattern, indicating that the phase transformation mechanism is the nucleation and growth of grains controlled by elemental diffusion. The activation energy Q of the diffusion transformation during the continuous heating process is 445.5 kJ·mol−1.
- (3)
- The phase transformation process of Ti-6Al-4V in the continuous heating stage is mainly divided into three stages: (1) Three-dimensional nucleation and growth, and the rate of nucleation increases. (2) Consumption of β stable element leads to a decrease in the nucleation rate. (3) Nucleation saturation and β grains growth.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nabil, K.; Mabrouk, B.; Riad, B. Beta to alpha transformation kinetics and microstructure of Ti-6Al-4V alloy during continuous cooling. Mater. Chem. Phys. 2016, 181, 462–469. [Google Scholar]
- Tarzimoghadam, Z.; Sandlöbes, S.; Pradeep, K.G. Microstructure design and mechanical properties in a near-α Ti-4Mo alloy. Acta Mater. 2015, 97, 291–304. [Google Scholar] [CrossRef]
- Terlinde, G.T.; Duerig, T.W.; Williams, J.C. Microstructure, tensile deformation, and fracture in aged Ti-10V-2Fe-3Al. Metall. Trans. A 1983, 14, 2101–2115. [Google Scholar] [CrossRef]
- Li, C.L.; Zou, L.N.; Fu, Y.Y. Effect of heat treatments on microstructure and property of a high strength/toughness Ti-8V-1.5Mo-2Fe-3Al Alloy. Mater. Sci. Eng. A 2014, 616, 207–213. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Li, W. Study on the relationship between microstructure and mechanical property in a metastable β titanium alloy. J. Alloys Compd. 2015, 627, 222–230. [Google Scholar] [CrossRef]
- Yu, W.X.; Li, M.Q.; Jiao, L. Effect of deformation parameters on the precipitation mechanism of secondary phase under high temperature isothermal compression of Ti–6Al–4V alloy. Mater. Sci. Eng. A 2010, 527, 4210–4217. [Google Scholar] [CrossRef]
- Jie, F.; Hua, D.; Yi, H. Influence of phase volume fraction on the grain refining of a Ti-6Al-4V alloy by high-pressure torsion. J. Mater. Res. Technol. 2014, 4, 1–6. [Google Scholar]
- Peng, X.N.; Guo, H.Z.; Wang, T. Effects of β treatments on microstructures and mechanical properties of Ti-6Al-4V-DT titanium alloy. Mater. Sci. Eng. A 2012, 533, 55–63. [Google Scholar] [CrossRef]
- Shah, A.K.; Kulkarni, G.J.; Gopinathan, V. Determination of activation energy for α + β→ β transformation in Ti-6AI-4V alloy by dilatometry. Scr. Metall. Mater. 1995, 32, 1353–1356. [Google Scholar] [CrossRef]
- Ming, L.C.; Manghnani, M.H.; Katahara, K.W. Phase Transformations in the Ti-V system under high pressure up to 25 GPa. Acta Metall. 1981, 29, 479–485. [Google Scholar] [CrossRef]
- Katzarov, I.; Malinov, S.; Sha, W. Finite element modeling of the morphology of β to α phase transformation in Ti-6Al-4V alloy. Metall. Mater. Trans. A 2002, 33, 1027–1040. [Google Scholar] [CrossRef]
- Elmer, J.W.; Palmer, T.A.; Babu, S.S. In situ observations of lattice expansion and transformation rates of α and β phases in Ti-6Al-4V. Mater. Sci. Eng. A 2005, 391, 104–113. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Lai, M.J.; Bin, T. Non-isothermal phase transformation kinetics of phase in TB-13 titanium alloys. Mater. Sci. Eng. A 2010, 527, 5400–5104. [Google Scholar] [CrossRef]
- Hui, Q.; Xue, X.; Kou, H. Kinetics of the ω phase transformation of Ti-7333 titanium alloy during continuous heating. J. Mater. Sci. 2013, 48, 1966–1972. [Google Scholar] [CrossRef]
- Elmer, J.W.; Palmer, T.A.; Babu, S.S. Low temperature relaxation of residual stress in Ti-6Al-4V. Scr. Mater. 2005, 52, 1051–1056. [Google Scholar] [CrossRef]
- Feng, S.; Li, J.; Kou, H. β phase transformation kinetics in Ti60 alloy during continuous cooling. J. Alloys Compd. 2013, 576, 108–113. [Google Scholar]
- Charpentier, M.; Hazotte, A.; Daloz, D. Lamellar transformation in near-γ TiAl alloys—Quantitative analysis of kinetics and microstructure. Mater. Sci. Eng. A 2008, 491, 321–330. [Google Scholar] [CrossRef]
- Blazquez, J.S.; Conde, C.F.; Conde, A. Non-isothermal approach to isokinetic crystallization processes: Application to the nanocrystallization of HITPERM alloys. Acta Mater. 2005, 53, 2305–2311. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, H.; Chang, H. Phase transformation in TC21 alloy during continuous heating. J. Alloys Compd. 2009, 472, 252–256. [Google Scholar] [CrossRef]
- Budrugeac, P.; MusAt, V.; Segal, E. Non-isothermal kinetic study on the decomposition of Zn acetate-based sol-gel precursor. J. Therm. Anal. Calorim. 2007, 88, 699–702. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Ahmed, T.; Rack, H.J. Phase transformations during cooling in α+β titanium alloys. Mater. Sci. Eng. A 1998, 243, 206–211. [Google Scholar] [CrossRef]
- Christian, J.W. The theory of transformations in metals and alloys. Mater. Today 2003, 6, 53–53. [Google Scholar] [CrossRef]
- Jiang, X.D.; Zhang, H.W.; Wen, Q.Y. Crystallization kinetics of CoNbZr amorphous alloys thin films. Mater. Chem. Phys. 2004, 88, 197–201. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.A. Growth order and activation energies for grain growth of Ti6Al4V alloy in β phase. Scr. Metall. Mater. 1991, 25, 2843–2848. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.A. Behaviour of normal grain growth kinetics in single phase titanium and titanium alloys. Mater. Sci. Eng. A 2000, 283, 17–24. [Google Scholar] [CrossRef]


| Heating Rate (°C/s) | 0.1 | 0.3 | 0.5 | 0.8 |
|---|---|---|---|---|
| Starting temperature (°C) | 873 | 885 | 893 | 902 |
| Finishing temperature (°C) | 1072 | 1086 | 1095 | 1103 |
| Heating Rate (°C/s) | 0.1 | 0.3 | 0.5 | 0.8 |
|---|---|---|---|---|
| Tβ (°C) | 925 | 951 | 970 | 975 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Li, W.; Zou, H.; Li, S.; Zhai, T.; Liu, L. Study on Non-Isothermal Transformation of Ti-6Al-4V in Solution Heating Stage. Metals 2019, 9, 968. https://doi.org/10.3390/met9090968
Yu H, Li W, Zou H, Li S, Zhai T, Liu L. Study on Non-Isothermal Transformation of Ti-6Al-4V in Solution Heating Stage. Metals. 2019; 9(9):968. https://doi.org/10.3390/met9090968
Chicago/Turabian StyleYu, Hui, Wei Li, Haibei Zou, Songsong Li, Tongguang Zhai, and Ligang Liu. 2019. "Study on Non-Isothermal Transformation of Ti-6Al-4V in Solution Heating Stage" Metals 9, no. 9: 968. https://doi.org/10.3390/met9090968




