Destruction of Cyanide and Removal of Copper from Waste Printed Circuit Boards Leach Solution Using Electro-Generated Hypochlorite Followed by Magnetite Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
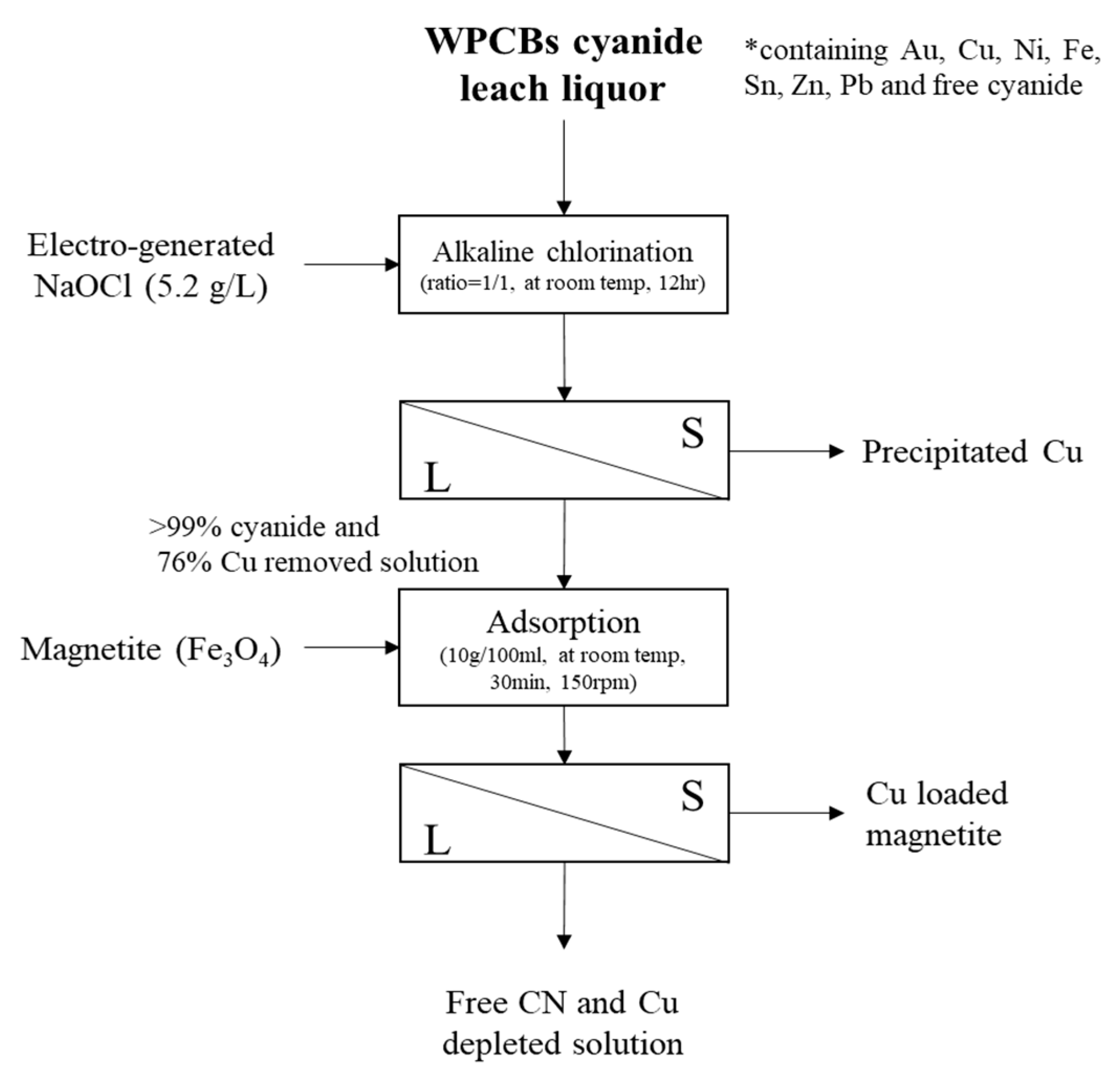
2.2. Methods
- ①
- add mixture of phenolphthalein and ethyl alcohol (0.5 W/V%).
- ②
- add acetic acid until the red color of the solution disappears.
- ③
- add phosphate-buffer and chloramine-T and waiting for 5 min.
- ④
- add pyridine and waiting for 30 min and then measure the wavelength.
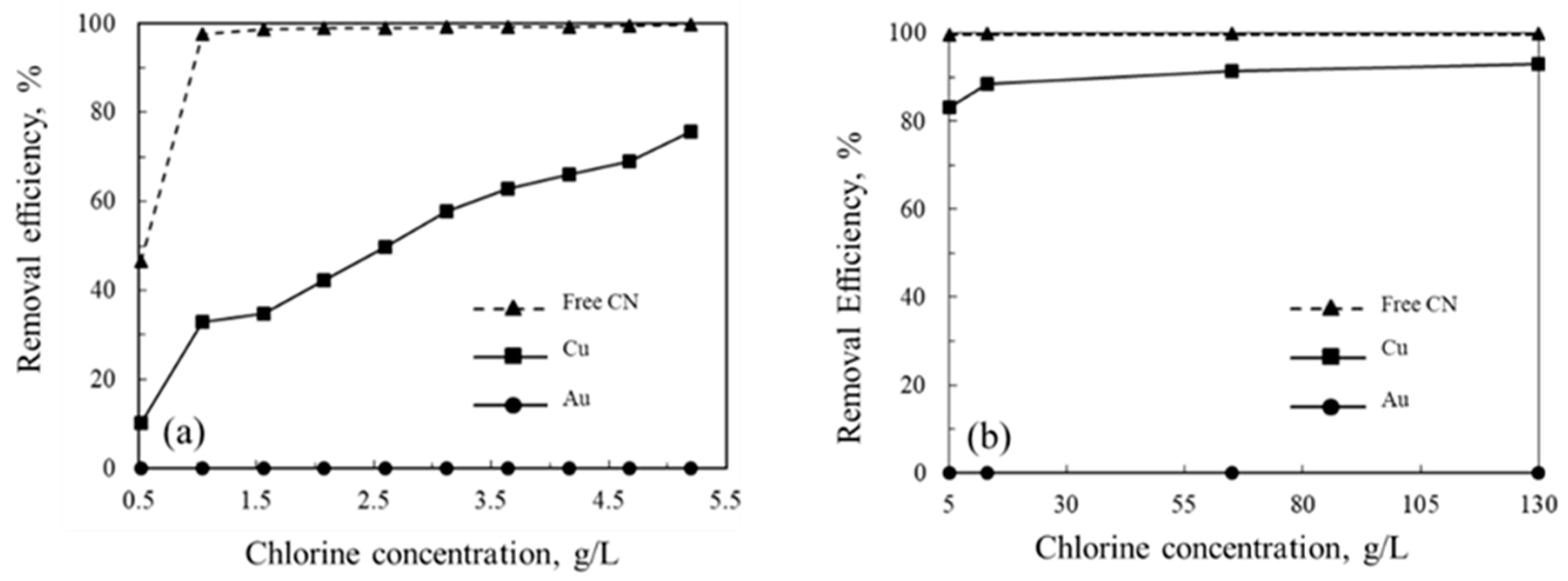
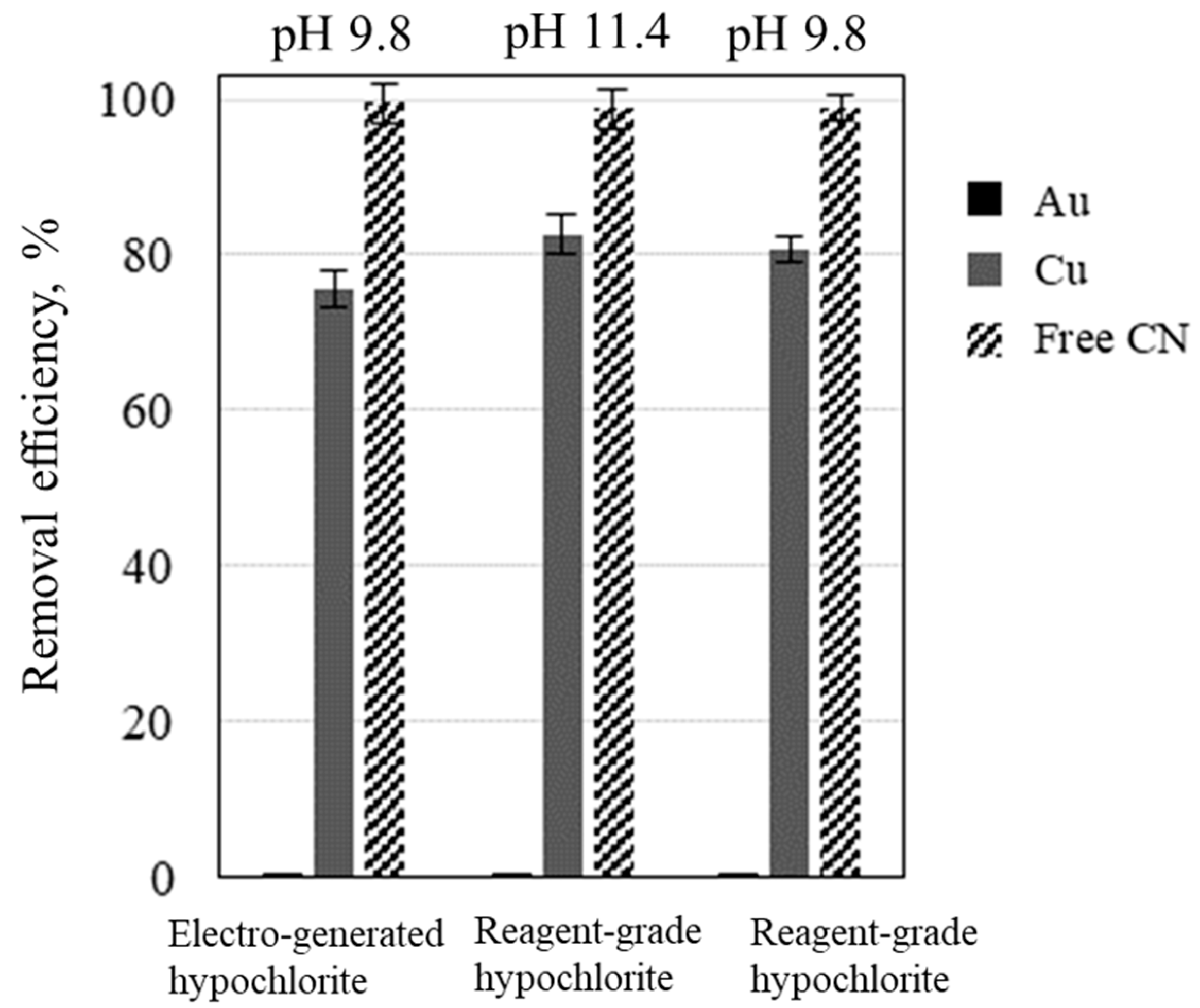
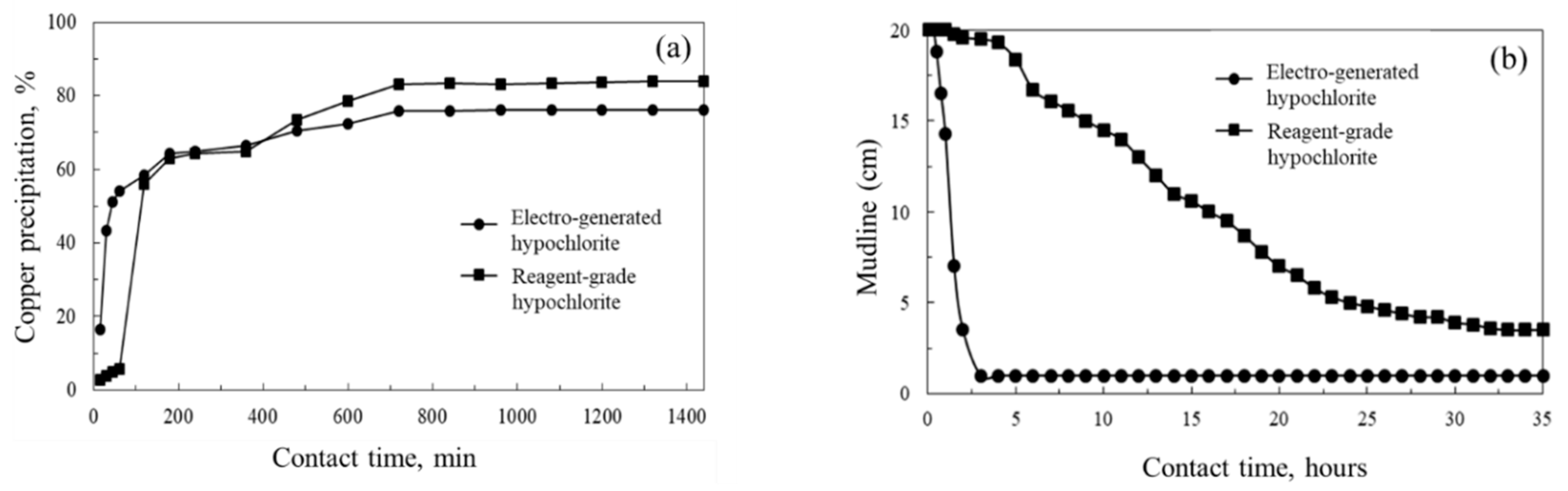
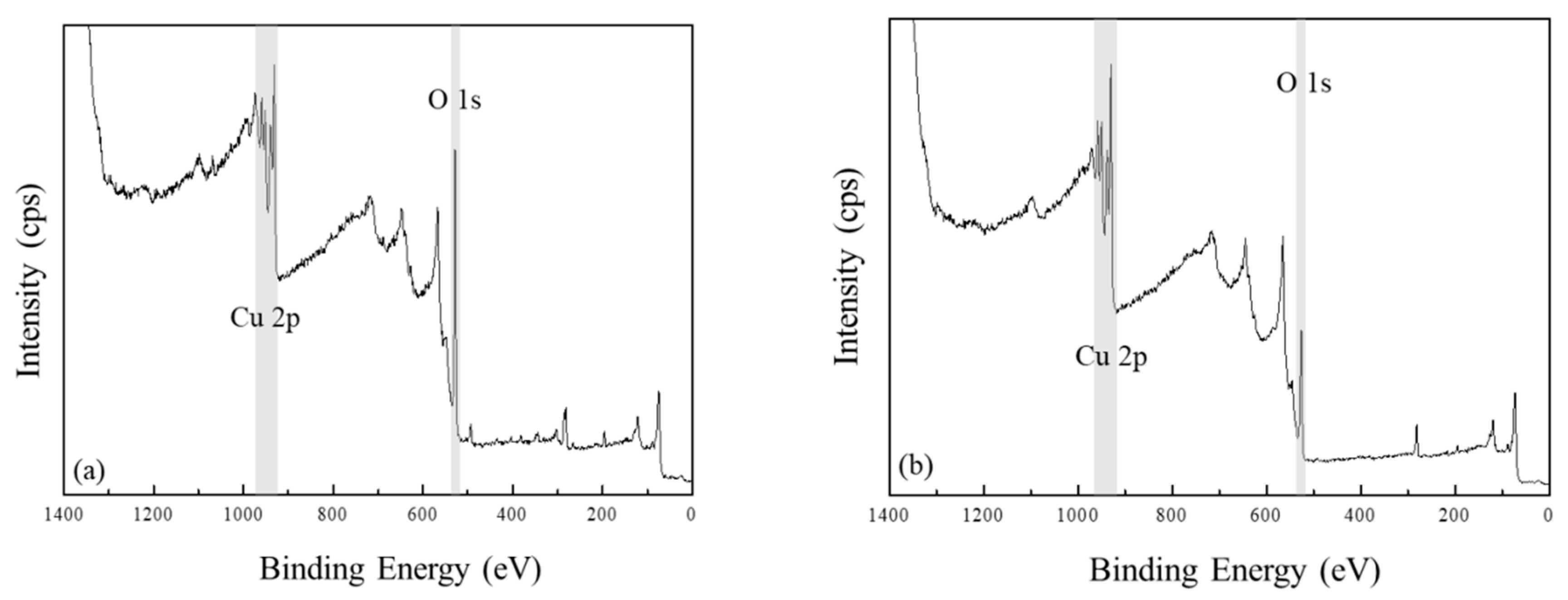
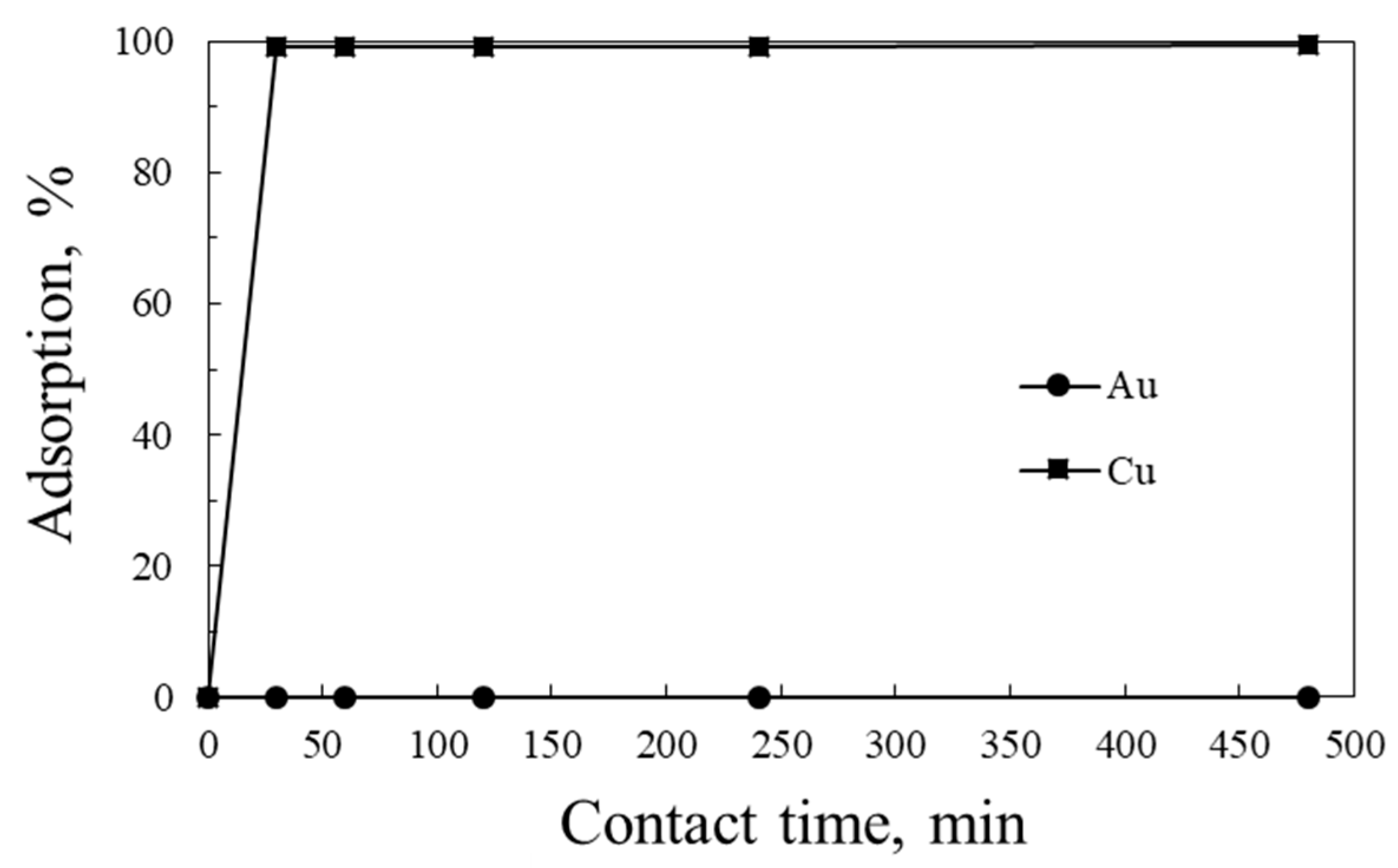
3. Results and Discussion
- (i)
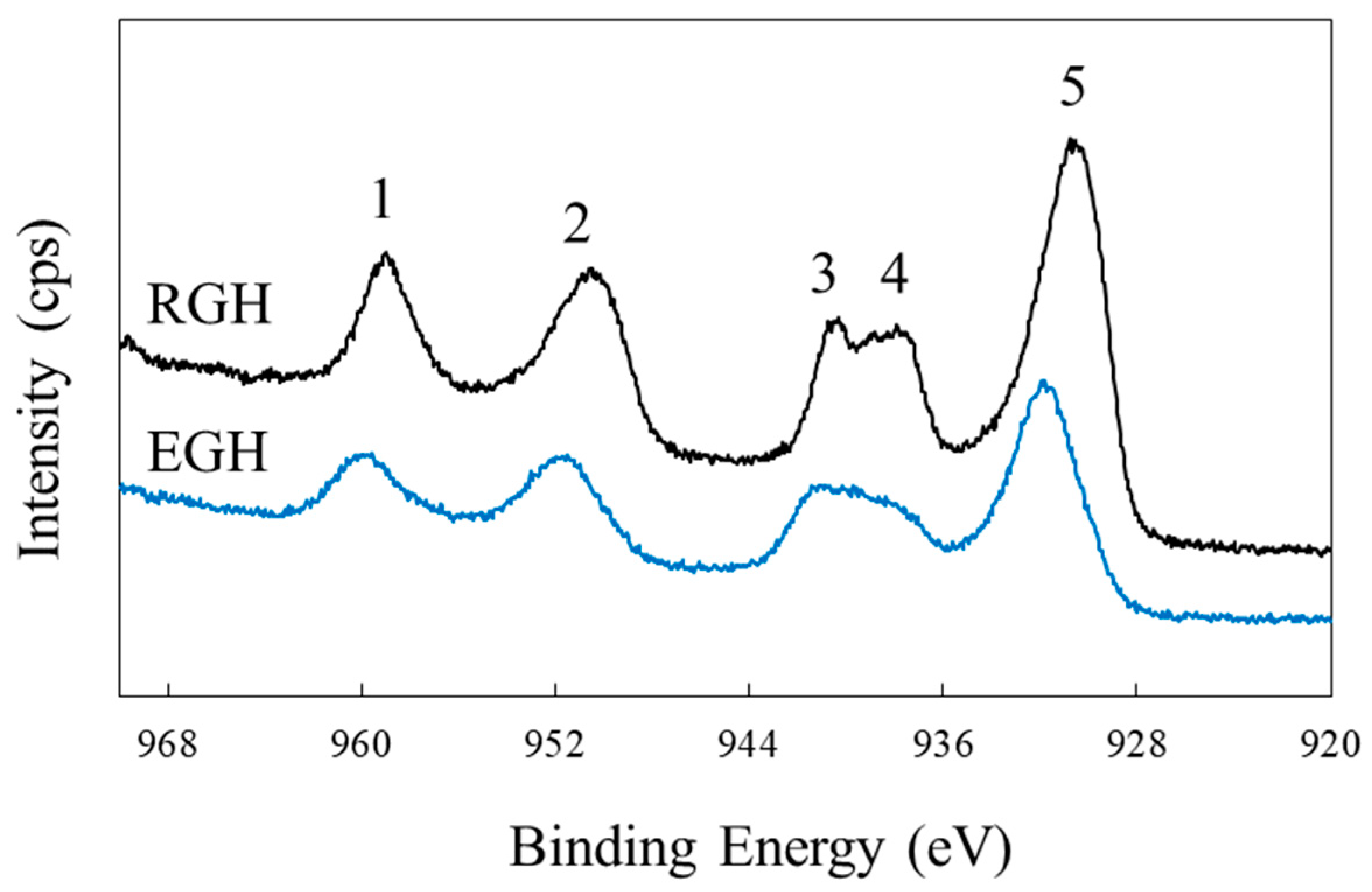
- Peak-shape and main peak to shake-up peak separation is quite different for CuO and Cu(OH)2.
- (ii)
- O 1 s spectra must show the presence or absence of hydroxyl group or lattice oxide peak.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aram, S. Gold 2048: The Next 30 Years for Gold; World Gold Council: London, UK, 2018; p. 51. [Google Scholar]
- Kim, E.; Kim, M.; Lee, J.; Pandey, B.D. Selective recovery of gold from waste mobile phone PCBs by hydrometallurgical process. J. Hazard. Mater. 2011, 198, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, J.; Choi, J. Characterization of Metal Composition in Spent Printed Circuit Boards of Mobile Phones. J. Korean Inst. Resour. Recycl. 2015, 24, 76–80. [Google Scholar] [Green Version]
- Syed, S. The Recovery of Gold from Secondary Sources; Imperial College Press: London, UK, 2016; ISBN 978-1-78326-989-1. [Google Scholar]
- Kim, B.-C.; Chae, S.; Kim, J.; Yoo, K. Oversea Production Status of Gold, Silver, Platinum and Palladium from Scrap. J. Korean Inst. Resour. Recycl. 2018, 27, 76–83. [Google Scholar]
- Jeon, S.; Ito, M.; Tabelin, C.B.; Pongsumrankul, R.; Kitajima, N.; Park, I.; Hiroyoshi, N. Gold recovery from shredder light fraction of E-waste recycling plant by flotation-ammonium thiosulfate leaching. Waste Manag. 2018, 77, 195–202. [Google Scholar] [CrossRef] [PubMed]
- You, D.-S.; Park, C.-Y. The Leaching and Recovery of Au from Scrap of PCBs. J. Korean Earth Sci. Soc. 2014, 35, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, B.; Ghosh, M.K.; Parhi, P.; Mukherjee, P.S.; Mishra, B.K. Waste Printed Circuit Boards recycling: an extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Ning, C.; Lin, C.S.K.; Hui, D.C.W.; McKay, G. Waste Printed Circuit Board (PCB) Recycling Techniques. Top. Curr. Chem. 2017, 375, 43. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, I.; Oh, J.; Yoo, K. Behaviors of Cyanide Leaching of Gold in Tailings and Adsorption of Gold Ions on Activated Carbon. Korean Soc. Miner. Energy Resour. Eng. 2018, 55, 414–420. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Xie, H.; Zeng, X.; Li, J. Current Status on Leaching Precious Metals from Waste Printed Circuit Boards. Procedia Environ. Sci. 2012, 16, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Robbins, G.H. Historical development of the INCO SO2/AIR cyanide destruction process. CIM Bull. 1996, 89, 63–69. [Google Scholar]
- Dai, X.; Simons, A.; Breuer, P. A review of copper cyanide recovery technologies for the cyanidation of copper containing gold ores. Miner. Eng. 2012, 25, 1–13. [Google Scholar] [CrossRef]
- Gurol, M.D.; Bremen, W.M. Kinetics and mechanism of ozonation of free cyanide species in water. Environ. Sci. Technol. 1985, 19, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Chamberlin, N.S.; Snyder, H.B. Technology of treating plating wastes. In Proceedings of the 10th Purdue Industrial Waste Conference, West Lafayette, IN, USA, 9–11 May 1955; p. 277. [Google Scholar]
- Clark, D.P.; Poulter, L.W.; Wilson, O.W.; Christensen, W.N. The Treatment and Analysis of Cyanide Wastewater; Report 253 No. AFCEC-TR-74-5; Thiokol Corporation: Ogden, UT, USA; Tyndall AFB: Bay, FL, USA, 1975; p. 131. [Google Scholar]
- David, A.D.; Rajat, S.G.; George, M.W.-C. Cyanide in Water and Soil: Chemistry, Risk, and Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-1-56670-666-7. [Google Scholar]
- Adrian, S.; Terry, M. Chemistry and Treatment of Cyanidation Wastes; Mining Journal Books LTD.: London, UK, 1991; ISBN 0-900117-51-6. [Google Scholar]
- Environmental Protection Agency of USA. Clean Water Act Methods Update Rule for the Analysis of Effluent; Authenticated U.S. Government Information: Washington, DC, USA, 2017.
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra: Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Gong, S.; Wu, X.; Zhang, J.; Han, N.; Chen, Y. Facile solution synthesis of Cu2O–CuO–Cu(OH)2 hierarchical nanostructures for effective catalytic ozone decomposition. CrystEngComm 2018, 20, 3096–3104. [Google Scholar] [CrossRef]
- Alorro, R.D.; Hiroyoshi, N.; Kijitani, H.; Ito, M.; Tsunekawa, M. On the Use of Magnetite for Gold Recovery From Chloride Solution. Miner. Process. Extr. Metall. Rev. 2010, 31, 201–213. [Google Scholar] [CrossRef]
- Oh, J.; Yoo, K.; Bae, M.; Kim, S.; Alorro, R.D. The Adsorption Behaviors of Gold Ions in Simulated Leachate Using Magnetite. J. Korean Soc. Miner. Energy Resour. Eng. 2019, 56, 79–85. [Google Scholar] [CrossRef]
- Bas, A.D.; Ghali, E.; Choi, Y. A review on electrochemical dissolution and passivation of gold during cyanidation in presence of sulphides and oxides. Hydrometallurgy 2017, 172, 30–44. [Google Scholar] [CrossRef]
- Seisko, S.; Lampinen, M.; Aromaa, J.; Laari, A.; Koiranen, T.; Lundström, M. Kinetics and mechanisms of gold dissolution by ferric chloride leaching. Miner. Eng. 2018, 115, 131–141. [Google Scholar] [CrossRef]
- Frankenthal, R.P. The Anodic Corrosion of Gold in Concentrated Chloride Solutions. J. Electrochem. Soc. 1982, 129, 1192. [Google Scholar] [CrossRef]
- Hayashi, K.; Matsuyama, T.; Ida, J. A simple magnetite nanoparticle immobilized thermoresponsive polymer synthesis for heavy metal ion recovery. Powder Technol. 2019, 355, 183–190. [Google Scholar] [CrossRef]
- Andelescu, A.; Nistor, M.A.; Muntean, S.G.; Rădulescu-Grad, M.E. Adsorption studies on copper, cadmium, and zinc ion removal from aqueous solution using magnetite/carbon nanocomposites. Sep. Sci. Technol. 2018, 53, 2352–2364. [Google Scholar] [CrossRef]
- Tamez, C.; Hernandez, R.; Parsons, J.G. Removal of Cu (II) and Pb (II) from aqueous solution using engineered iron oxide nanoparticles. Microchem. J. 2016, 125, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kalpakli, Y. Removal of Cu (II) from aqueous solutions using magnetite: A kinetic, equilibrium study. Adv. Environ. Res. 2015, 4, 119–133. [Google Scholar] [CrossRef]
- Steyn, J.; Sandenbergh, R.F. A study of the influence of copper on the gold electrowinning process. J. S. Afr. Inst. Min. Metall. 2004, 104, 177–182. [Google Scholar]
- Bae, M.; Kim, S.; Lee, J. Gold Recovery from Waste Solutions of PCBs Gold Plating Process using Hydro Cyclone Reactor for Demonstration Study. In Proceedings of the 3rd Pan-American Materials Congress; Springer: New York, NY, USA, 2017; ISBN 978-3-319-52131-2. [Google Scholar]
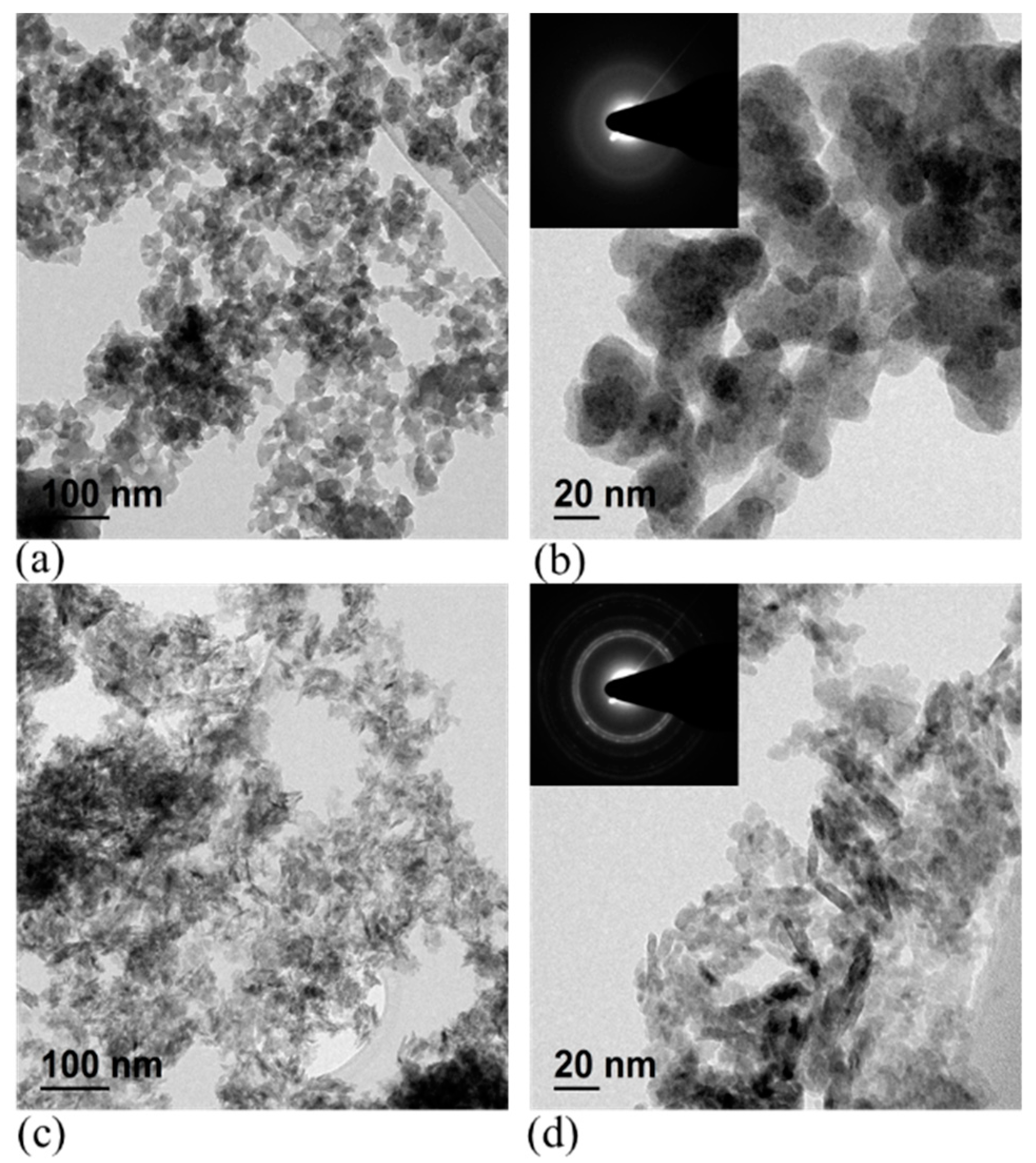
| Elements | Au | Ag | Cu | Ni | Fe | Sn | Zn | Pb | Free Cyanide |
|---|---|---|---|---|---|---|---|---|---|
| mg/L | 60.90 | 0.08 | 421.00 | 1.80 | 0.10 | 0.06 | 0.41 | 0.07 | 105.00 |
| Sample | Initial | 1 Cycle | 2 Cycle | 3 Cycle | 4 Cycle | 5 Cycle |
|---|---|---|---|---|---|---|
| Time [min] | 5 | 20 | 30 | 40 | 50 | 60 |
| Chlorine conc. [g/L] | 1.600 | 3.368 | 4.224 | 5.215 | 5.250 | 5.252 |
| Compound | Cu | O | ||||
|---|---|---|---|---|---|---|
| Peak1 | Peak2 | Peak3 | Peak4 | Peak5 | ||
| Reagent-grade NaOCl | 958.6 | 950.7 | 940.6 | 937.5 | 930.3 | 526.4 |
| Electro-generated NaOCl | 959.6 | 951.2 | 940.5 | - | 932.0 | 528.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.; Lee, H.; Kim, S.; Yoo, K. Destruction of Cyanide and Removal of Copper from Waste Printed Circuit Boards Leach Solution Using Electro-Generated Hypochlorite Followed by Magnetite Adsorption. Metals 2019, 9, 963. https://doi.org/10.3390/met9090963
Bae M, Lee H, Kim S, Yoo K. Destruction of Cyanide and Removal of Copper from Waste Printed Circuit Boards Leach Solution Using Electro-Generated Hypochlorite Followed by Magnetite Adsorption. Metals. 2019; 9(9):963. https://doi.org/10.3390/met9090963
Chicago/Turabian StyleBae, Mooki, Hyunju Lee, Sookyung Kim, and Kyoungkeun Yoo. 2019. "Destruction of Cyanide and Removal of Copper from Waste Printed Circuit Boards Leach Solution Using Electro-Generated Hypochlorite Followed by Magnetite Adsorption" Metals 9, no. 9: 963. https://doi.org/10.3390/met9090963






