Study on Porous Mg-Zn-Zr ZK61 Alloys Produced by Laser Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
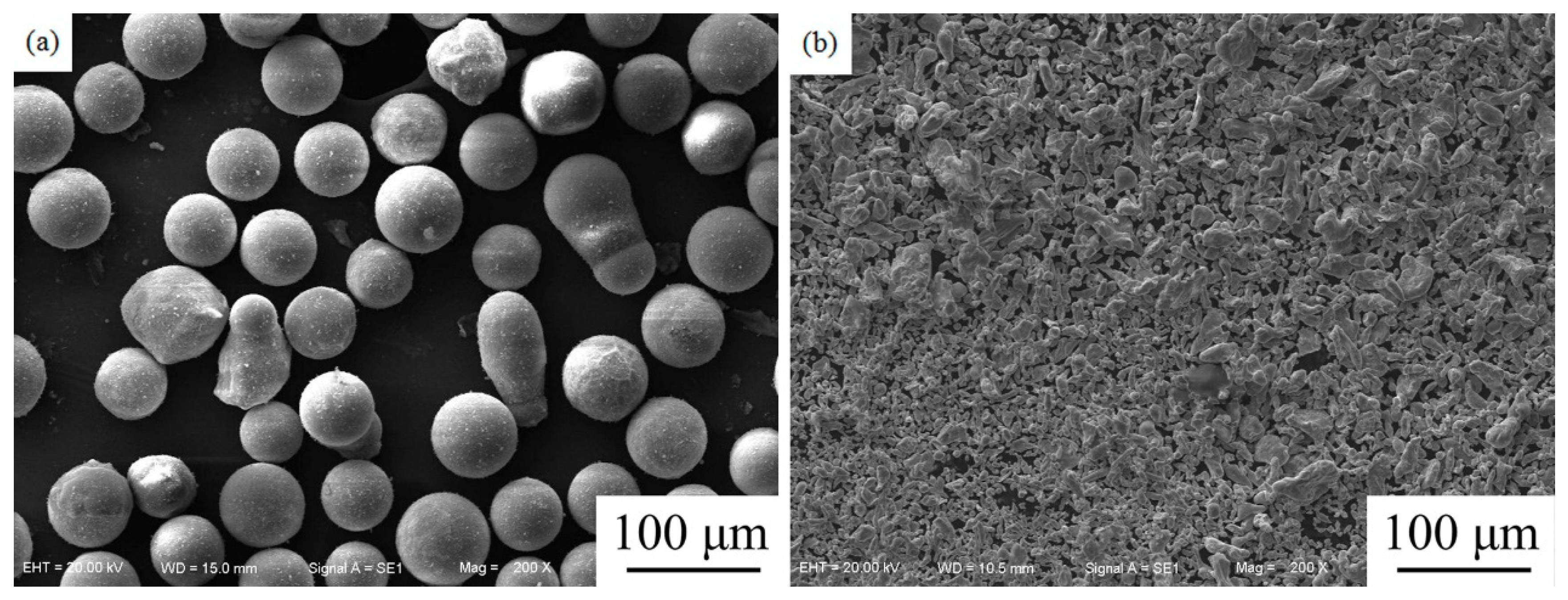
2.1. Materials Preparation
2.2. LAM Process
2.3. Microstructure and Composition Characterizations
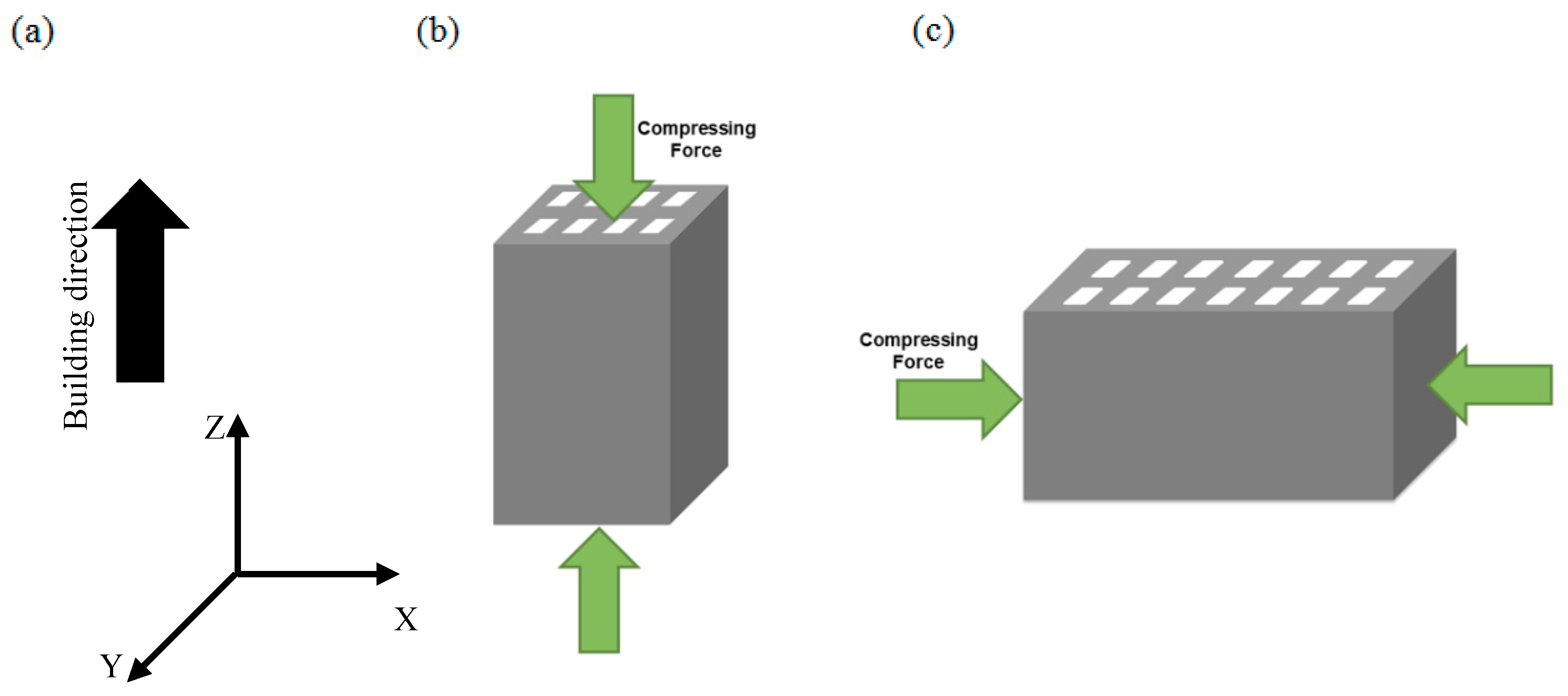
2.4. Mechanical Properties
3. Results and Discussion
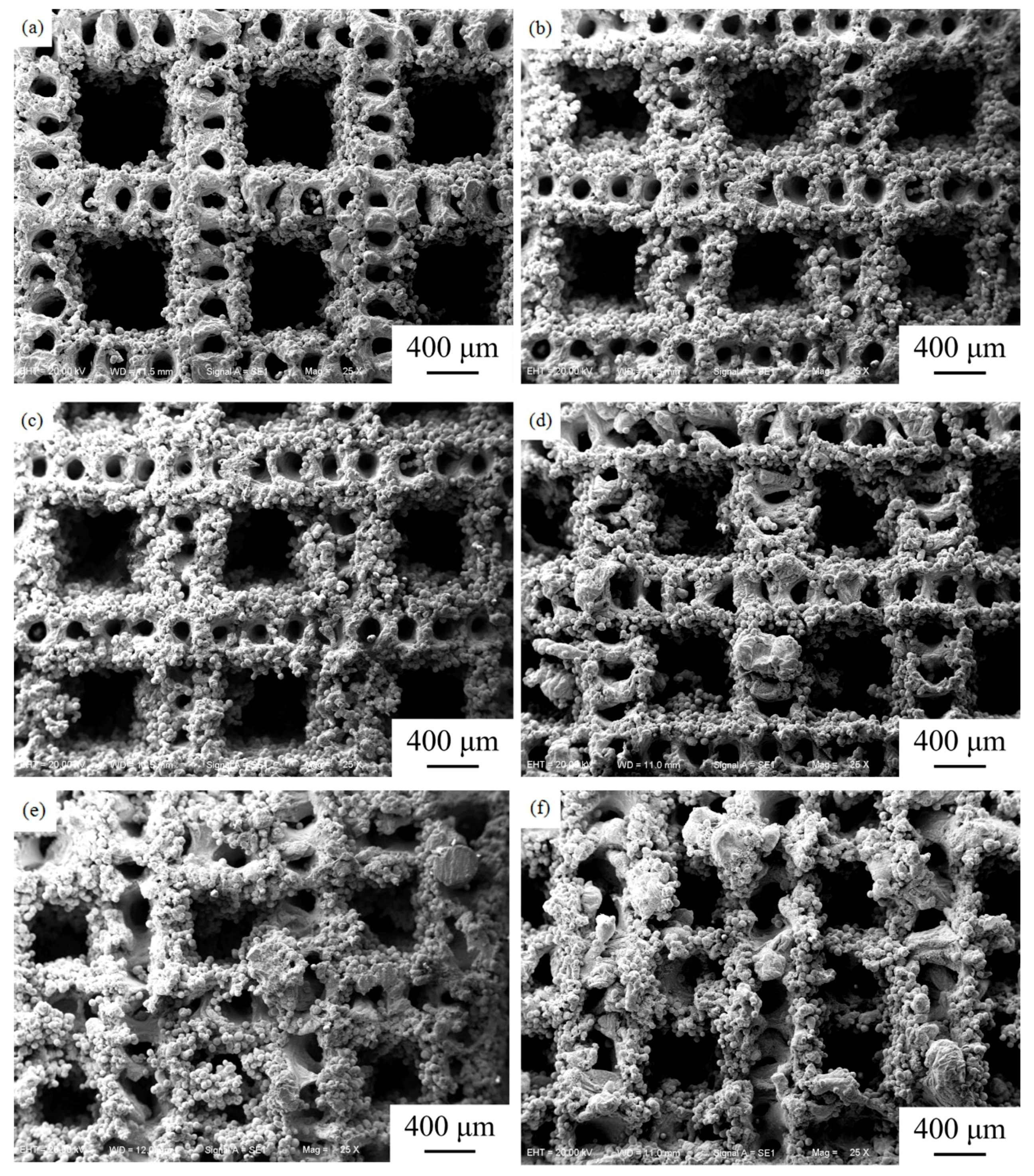
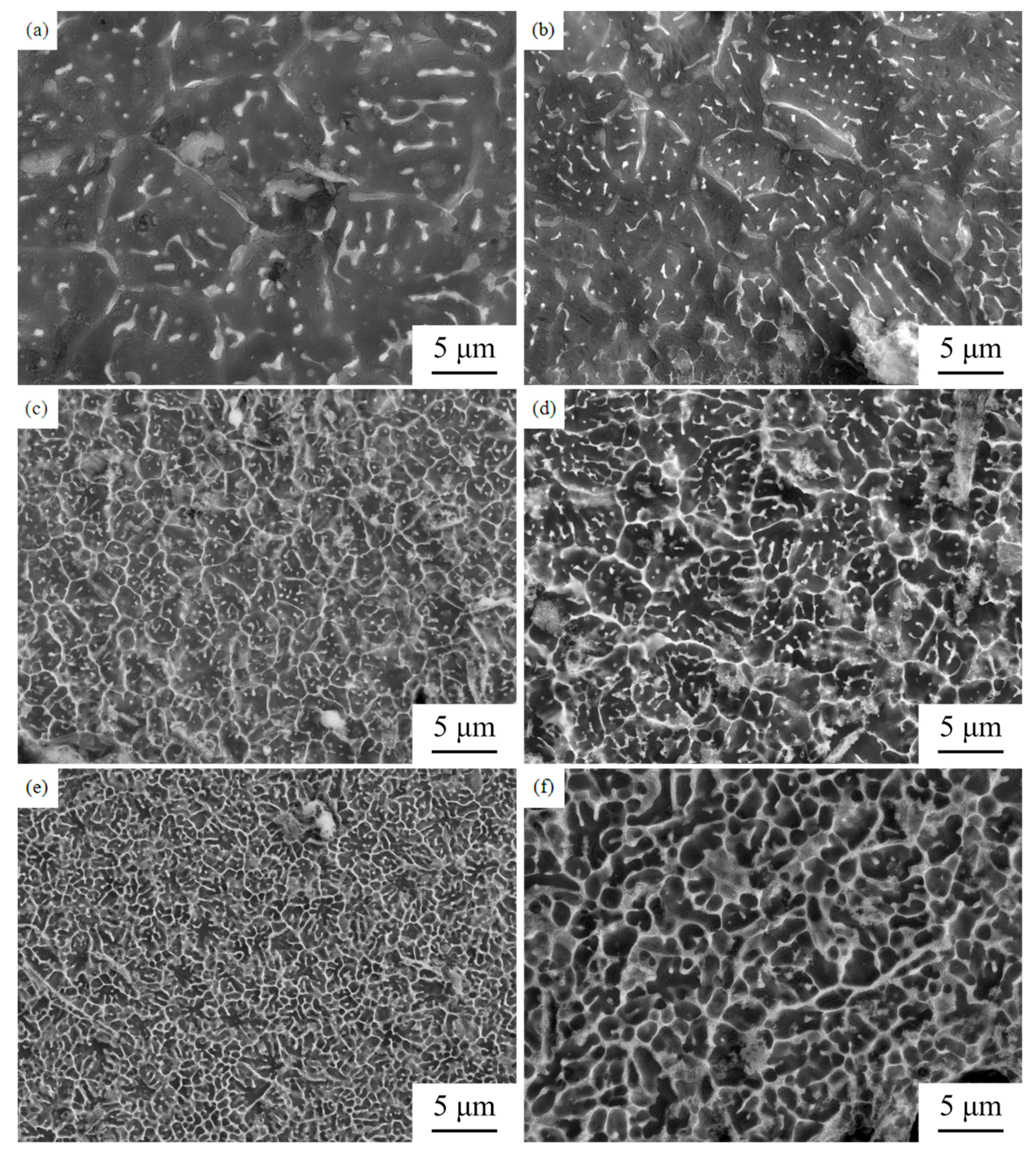
3.1. Surface Morphology and Porosity
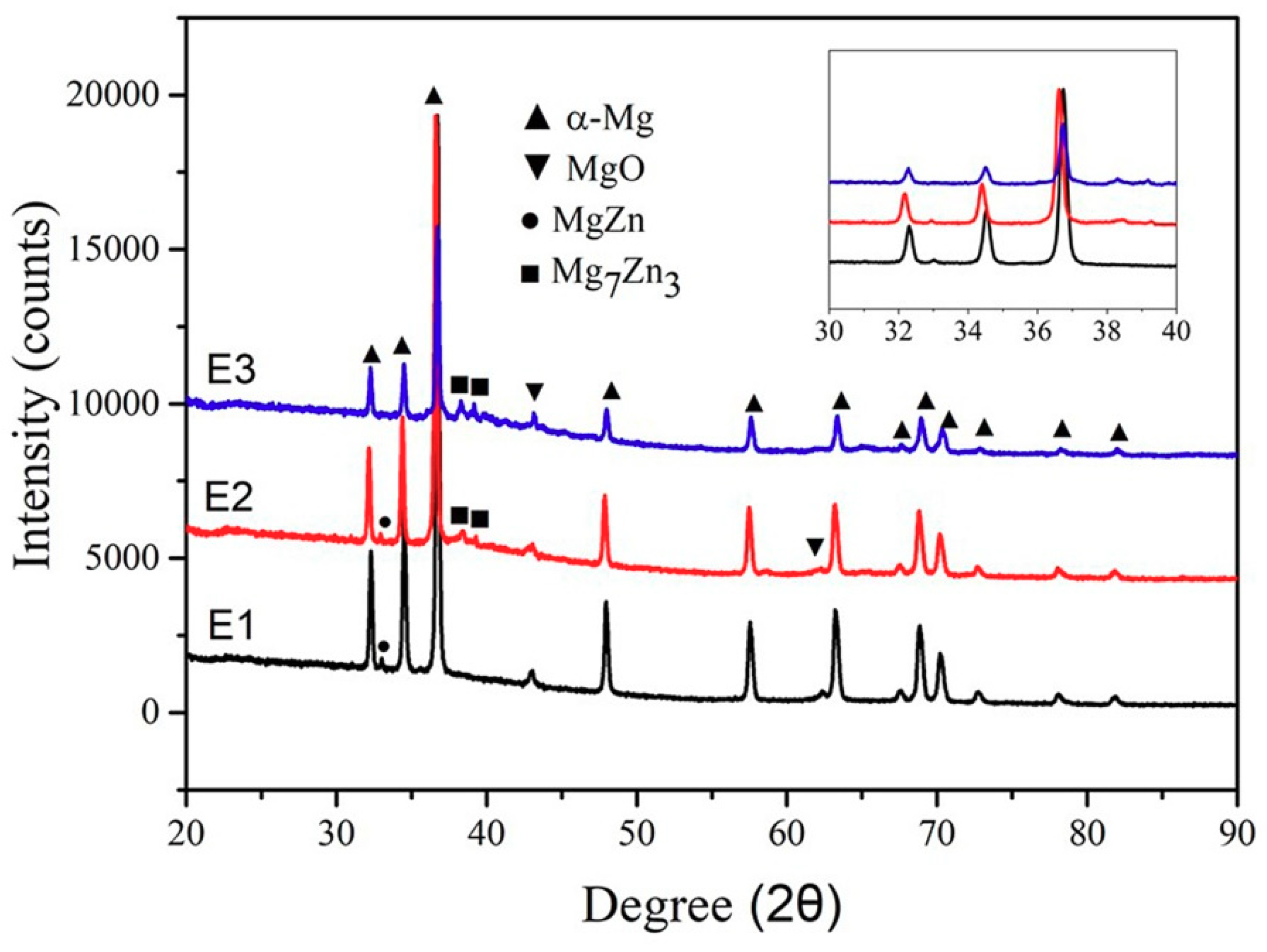
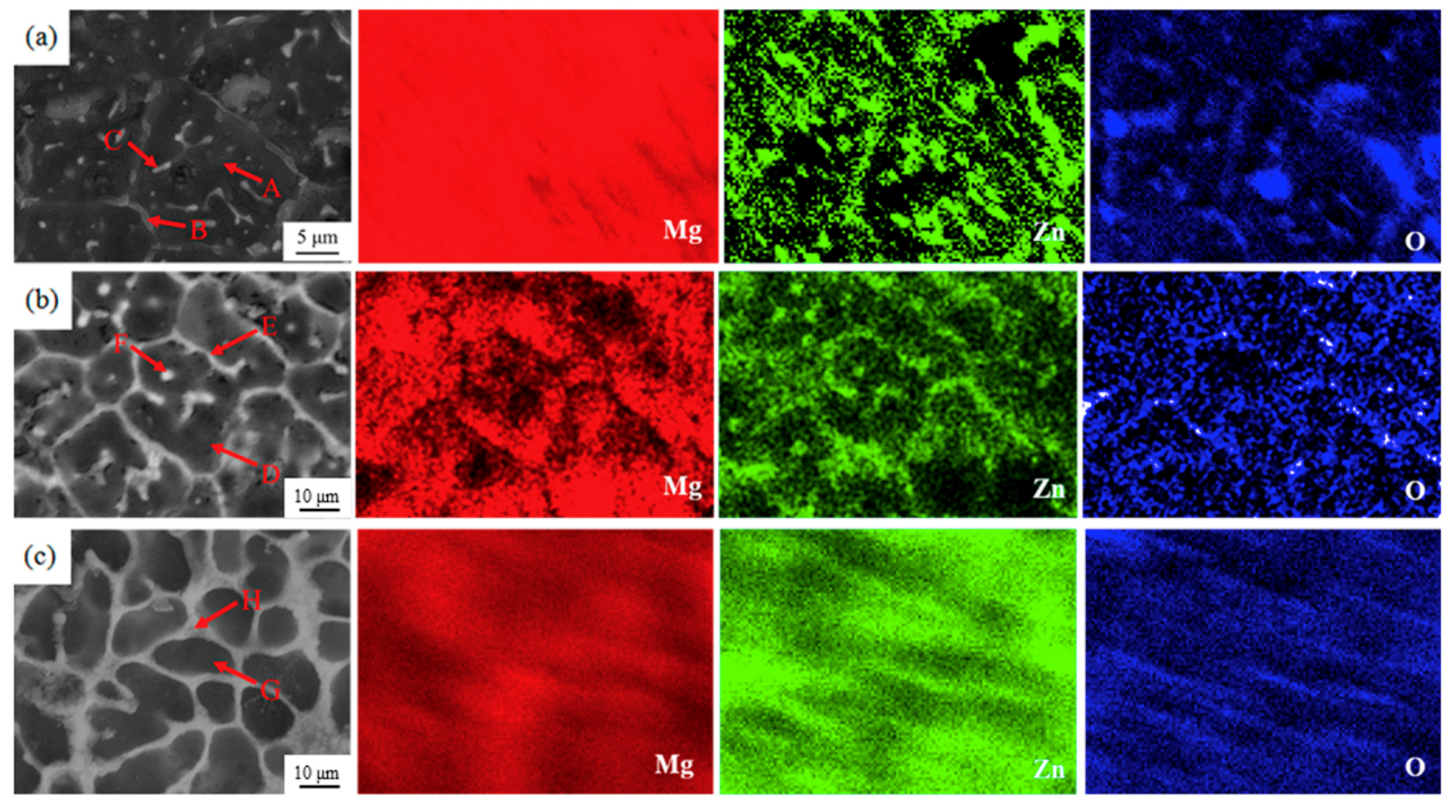
3.2. Microstructure, Phase and Composition
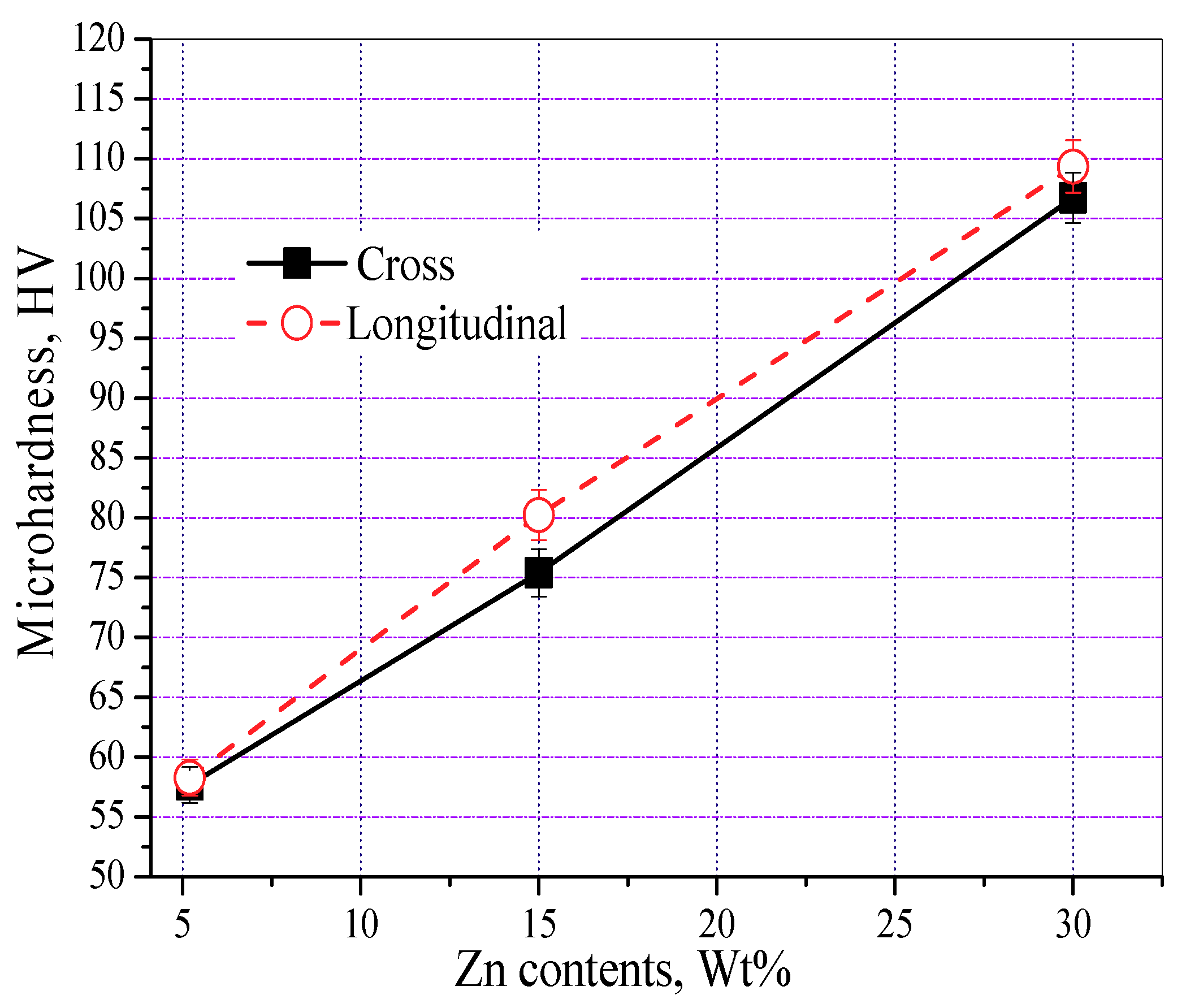
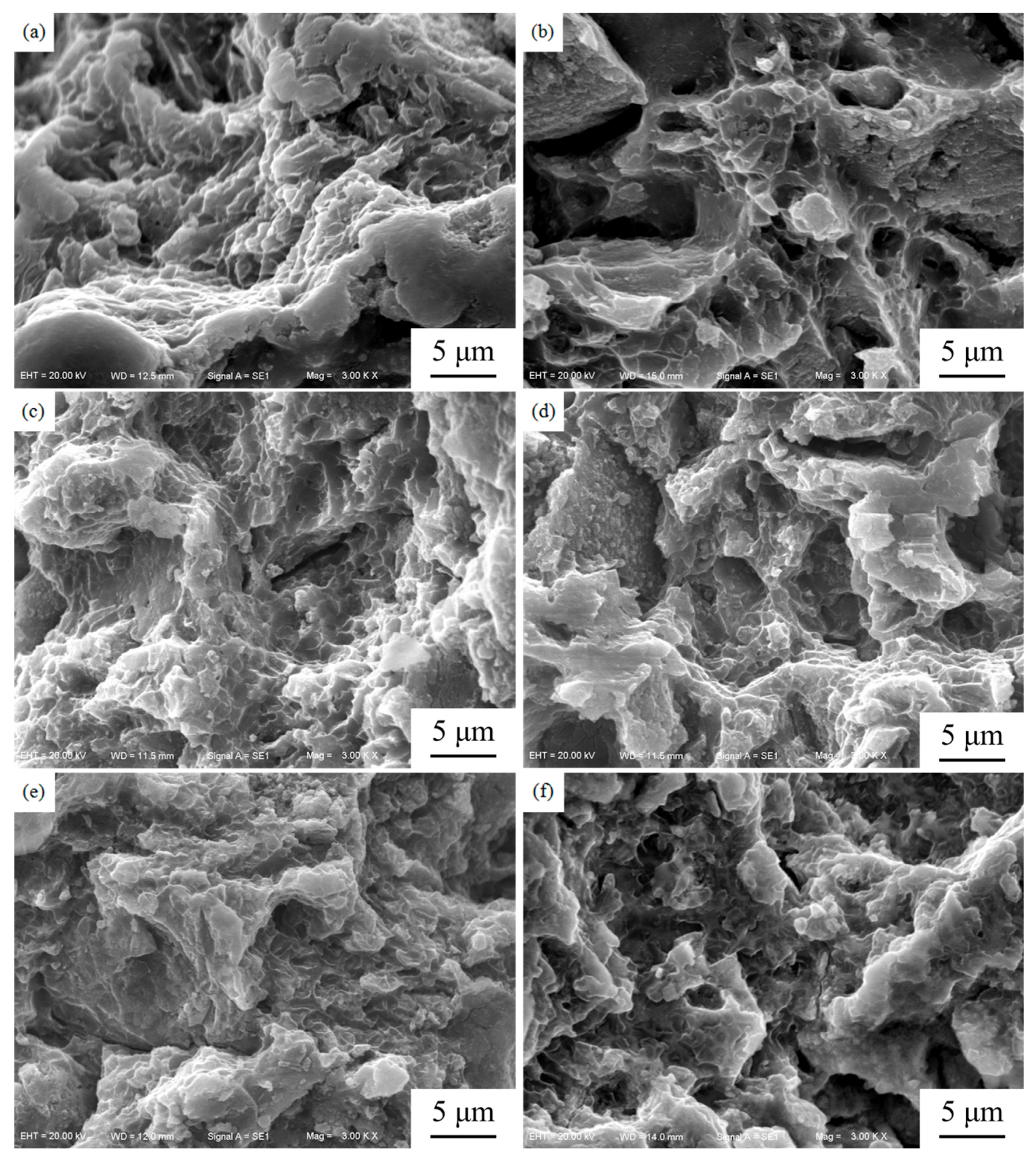
3.3. Mechanical Properties
4. Conclusions
- The surface morphology and porosity of the LAMed porous Mg-Zn-Zr alloys depend on the laser energy input during LAM process. ZK61 samples with higher quality could be acquired between 1019 J/mm3 and 1146 J/mm3, and with increasing E, the porosity of porous ZK61 alloys decreases gradually.
- With the increasing Zn content, the surface morphology gets worse and the grains of the alloys are significantly refined. Zk61 (E1) alloy mainly contains α-Mg, MgO, MgZn phase and lesser Mg7Zn3 phase. The amount of particle/rod-like MgZn phase and grain boundary Mg7Zn3 phase first increase with the increase of Zn content. However, When the Zn content reaches 30 wt.%, the particle/rod-like MgZn phases inside the grains almost completely disappeared and grain boundary Mg7Zn3 phase further increases and becomes coarsen.
- The microhardness of the LAMed porous Mg-Zn-Zr alloys increases obviously with the increasing Zn content, which ranges from 57.67 HV to 109.36 HV. The increase of microhardness is mainly attributed to the fine grain strengthening, solution strengthening and precipitation strengthening of MgZn phase and Mg7Zn3 phase.
- The UCS and elastic modulus of the LAMed porous Mg-Zn-Zr alloys are slightly lower than those of the compact bone but much higher than those of the cancellous bone. The fracture mode of the LAMed porous Mg-Zn-Zr alloys exhibits a quasi-cleavage and the longitudinal compression performance is better than the horizontal compression performance. The LAMed porous Mg-Zn-Zr alloys are promising biodegradable materials for bone tissue engineering.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Locci, P.; Marinucci, L.; Lilli, C.; Belcastro, S.; Staffolani, N.; Bellocchio, S.; Damiani, F.; Becchetti, E. Biocompatibility of alloys used in orthodontics evaluated by cell culture tests. J. Biomed. Mater. Res. 2000, 51, 561–568. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: a review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology. Heart 2003, 89, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Haefeli-Bleuer, B.; Weibei, E.R. Morphometry of the human pulmonary acinus. Anat. Rec. 1988, 220, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J. The mechanical behavior of cancellous bone. J. Biomech. 1985, 18, 317–328. [Google Scholar] [CrossRef]
- Yamada, Y.; Shimojima, K.; Sakaguchi, Y.; Mabuchi, M.; Nakamura, M.; Asahina, T.; Mukai, T.; Kanahashi, H.; Higashi, K. Processing of an open-cellular AZ91 magnesium alloy with a low density of 0.05 g/cm3. J. Mater. Sci. Lett. 1999, 18, 1477–1480. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Shimojima, K.; Chino, Y.; Hosokawa, H.; Mabuchi, M. Compressibility of porous magnesium foam: Dependency on porosity and pore size. Mater. Lett. 2004, 58, 357–360. [Google Scholar] [CrossRef]
- Park, S.-H.; Um, Y.-S.; Kum, C.-H.; Hur, B.-Y. Thermophysical properties of Al and Mg alloys for metal foam fabrication. Coll. Surf. A 2005, 263, 280–283. [Google Scholar] [CrossRef]
- Qin, L.; Chen, C.; Zhang, M.; Yan, K.; Cheng, G.; Jing, H.; Wang, X. The microstructure and mechanical properties of deposited-IN625 by laser additive manufacturing. Rapid Prototyp. J. 2017, 23, 1119–1129. [Google Scholar] [CrossRef]
- Li, G.C.; Li, J.; Tian, X.J.; Cheng, X.; He, B.; Wang, H.M. microstructure and properties of a novel titanium alloy Ti-6Al-2V-1.5Mo-0.5Zr-0.3Si manufactured by laser additive manufacturing. Mater. Sci. Eng. A 2017, 684, 233–238. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Zheng, H.; Tang, K.; Ding, L.; Li, H.; Gong, S. microstructure and mechanical properties of LMD-SLM hybrid forming Ti-6Al-4V alloy. Mater. Sci. Eng. A 2016, 660, 24–33. [Google Scholar] [CrossRef]
- Wang, L.; Felicelli, S.; Gooroochurn, Y.; Wang, P.T.; Horstemeyer, M.F. Optimization of LENS process for steady molten pool size. Mater. Sci. Eng. A 2008, 474, 148–156. [Google Scholar] [CrossRef]
- Vilaro, T.; Colin, C.; Bartout, J.D. As-fabricated and heat-treated microstructures of the Ti-6Al-4V alloy processed by selective laser melting. Metall. Mater. Trans. A 2011, 42, 3190–3199. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.-J. Laser in additive manufacturing: A review. Int. J. Precis. Eng. Manuf-Gr. Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Chen, S.Y.; Huang, J.C.; Pan, C.T.; Lin, C.H.; Yang, T.L.; Huang, Y.S.; Ou, C.H.; Chen, L.Y.; Lin, D.Y.; Lin, H.K.; et al. Microstructure and mechanical properties of open-cell porous Ti-6Al-4V fabricated by selective laser melting. J. Alloy. Compd. 2017, 713, 248–254. [Google Scholar] [CrossRef]
- Zhang, L.C.; Attar, H. Selective laser melting of titanium alloys and titanium matrix composites for biomedical applications: a review. Adv. Eng. Mater. 2016, 18, 463–475. [Google Scholar] [CrossRef]
- Attar, H.; Löber, L.; Funk, A.; Calin, M.; Zhang, L.C.; Prashanth, K.G.; Scudino, S.; Zhang, Y.S.; Eckert, J. Mechanical behavior of porous commercially pure Ti and Ti–TiB composite materials manufactured by selective laser melting. Mater. Sci. Eng. A 2015, 625, 350–356. [Google Scholar] [CrossRef]
- Attar, H.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Manufacture by selective laser melting and mechanical behavior of commercially pure titanium. Mater. Sci. Eng. A 2014, 593, 170–177. [Google Scholar] [CrossRef]
- Okulov, I.V.; Volegov, A.S.; Attar, H.; Bönisch, M.; Ehtemam-Haghighi, S.; Calin, M.; Eckert, J. Composition optimization of low modulus and high-strength TiNb-based alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Ehtemam-Haghighi, S.; Prashanth, K.G.; Attar, H.; Chaubey, A.K.; Cao, G.; Zhang, L. Evaluation of mechanical and wear properties of Ti-xNb-7Fe alloys designed for biomedical applications. Mater. Des. 2016, 111, 592–599. [Google Scholar] [CrossRef]
- Watanabe, H.; Mukai, T.; Mabuchi, M.; Higashi, K. High-strain-rate superplasticity at low temperature in a ZK61 magnesium alloy produced by powder metallurgy. Scr. Mater. 1999, 41, 209–213. [Google Scholar] [CrossRef]
- Qin, L.L.; Chen, C.J.; Zhang, M.; Yan, K.; Cheng, G.P.; Jing, H.M.; Wang, X.N.; Zou, T. Effect of Zr on microstructure and mechanical properties of magnesium alloy by laser additive manufacturing. Appl. Laser 2016, 36, 391–396. (In Chinese) [Google Scholar]
- Wei, K.; Wang, Z.; Zeng, X. Influence of element vaporization on formability, composition, microstructure, and mechanical performance of selective laser melted Mg-Zn-Zr components. Mater. Lett. 2015, 156, 187–190. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Zhou, Y.; Feng, P.; Liu, X.; Peng, S. Laser rapid solidification improves corrosion behavior of Mg-Zn-Zr alloy. J. Alloy. Compd. 2017, 691, 961–969. [Google Scholar] [CrossRef]
- Yan, Y.; Cao, H.; Kang, Y.; Yu, K.; Xiao, T.; Luo, J.; Deng, Y.; Fang, H.; Xiong, H.; Dai, Y. Effects of Zn concentration and heat treatment on the microstructure, mechanical properties and corrosion behavior of as-extruded Mg-Zn alloys produced by powder metallurgy. J. Alloy. Compd. 2017, 693, 1277–1289. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Chen, C. Effect of laser processing parameters on porosity, microstructure and mechanical properties of porous Mg-Ca alloys produced by laser additive manufacturing. Mater. Sci. Eng. A 2017, 703, 359–371. [Google Scholar] [CrossRef]
- Hooyar, A.; Ehtemam-Haghighi, S.; Kent, D.; Wu, X.; Dargusch, M.S. Comparative study of commercially pure titanium produced by laser engineered net shaping, selective laser melting and casting processes. Mater. Sci. Eng. A 2017, 705, 385–393. [Google Scholar] [CrossRef]
- Hooyar, H.; Ehtemam-Haghighi, S.; Kent, D.; Dargusch, M.S. Recent developments and opportunities in additive manufacturing of titanium-based matrix composites: A review. Int. J. Mach. Tool. Manuf. 2018, 133, 85–102. [Google Scholar] [CrossRef]
- Wits, W.W.; Carmignato, S.; Zanini, F.; Vaneker, T.H.J. Porosity testing methods for the quality assessment of selective laser melted parts. CRIP Ann. 2016, 65, 201–204. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Zhang, D.; Shen, Z.; Liu, W. Balling phenomena in selective laser melted tungsten. J. Mater. Process. Technol. 2015, 222, 33–42. [Google Scholar] [CrossRef]
- Davis, J.R. Metals Handbook Desk Edition, 2nd ed.; ASM International: Cleveland, OH, USA, 1998. [Google Scholar]
- Akin, M.; Veli, I.; Erdur, E.A.; Aksakalli, S.; Uysal, T. Different pulse modes of Er:YAG laser irradiation: Effects on bond strength achieved with self-etching primers. J. Orofac. Orthop. 2016, 77, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Abrams, H. Grain size measurement by intercept method. Metallography 1971, 4, 59–78. [Google Scholar] [CrossRef]
- Shuai, S.; Guo, E.; Zheng, Q.; Wang, M.; Jing, T.; Fu, Y. Three-dimensional α-Mg dendritic morphology and branching structure transition in Mg-Zn alloys. Mater. Charact. 2016, 118, 304–308. [Google Scholar] [CrossRef]
- Shuai, S.; Guo, E.; Wang, J.; Phillion, A.B.; Tao, J.; Ren, Z.; Lee, P.D. Synchrotron tomographic quantification of the influence of Zn concentration on dendritic growth in Mg-Zn alloys. Acta Mater. 2018, 156, 287–296. [Google Scholar] [CrossRef]
- Ma, H.T.; Yuan, R.; Xie, Y.P.; Gao, H.; Hu, L.J.; Li, X.D.; Qian, Y.C.; Dai, Z.H. The role of Ag, Ca, Zr and Al in strengthening effects of ZK series alloys by altering G.P. zones stability. Acta Mater. 2018, 147, 42–50. [Google Scholar] [CrossRef]
- Kim, W.J.; Moon, I.K.; Han, S.H. Ultrafine-grained Mg-Zn-Zr alloy with high strength and high-strain-rate superplasticity. Mater. Sci. Eng. A 2012, 538, 374–385. [Google Scholar] [CrossRef]
- Shahzad, M.; Wagner, L. The role of Zr-rich cores in strength differential effect in an extruded Mg-Zn-Zr alloy. J. Alloy. Compd. 2009, 486, 103–108. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Mendis, C.L.; Sasaki, T.T.; Ohkubo, T.; Hono, K. Effect of Zr addition on the precipitation in Mg-Zn-based alloy. Scr. Mater. 2012, 67, 967–970. [Google Scholar] [CrossRef]
- Wagner, D.C.; Chai, X.; Tang, X.; Kou, S. Liquation cracking in arc and friction-stir welding of Mg-Zn alloys. Metall. Mater. Trans. A 2015, 46, 315–327. [Google Scholar] [CrossRef]
- Matthews, R.P.; Knusten, R.D.; Westraadt, J.E.; Couvant, T. Intergranular oxidation of 316L stainless steel in the PWR primary water environment. Corros. Sci. 2017, 125, 175–183. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Cavaleiro, A.J.; Schell, N.; Stark, A.; Miranda, R.M.; Ocana, J.L.; Fernandes, F.M.B. Effects of laser processing on the transformation characteristics of NiTi: A contribute to additive manufacturing. Scr. Mater. 2018, 152, 122–126. [Google Scholar] [CrossRef]
- Dubé, D.; Fiset, M.; Couture, A.; Nakatsugawa, I. Characterization and performance of laser melted AZ91D and AM60B. Mater. Sci. Eng. A 2001, 299, 38–45. [Google Scholar] [CrossRef]
- Furukawa, M.; Iwahashi, Y.; Horita, Z.; Nemoto, M.; Tsenev, N.K.; Valiev, R.Z.; Langdon, T.G. Structural evolution and the hall-petch relationship in an Al-Mg-Li-Zr alloy with ultra-fine grain size. Acta Mater. 1997, 45, 4751–4757. [Google Scholar] [CrossRef]
- Varvenne, C.; Leyson, G.P.M.; Ghazisaeidi, M.; Curtin, W.A. Solute strengthening in random alloys. Acta Mater. 2017, 124, 660–683. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.M.; Ma, Z.Y.; Geng, L. Effect of microstructural evolution on mechanical properties of friction stir welded ZK60 alloy. Mater. Sci. Eng. A 2008, 486, 49–55. [Google Scholar] [CrossRef]
- Galindo-Nava, E.I.; Rae, C.M.F. Microstructure-sensitive modelling of dislocation creep in polycrystalline FCC alloys: Orowan theory revisited. Mater. Sci. Eng. A 2016, 651, 116–126. [Google Scholar] [CrossRef]
- Hahn, S.-I.; Hwang, S.J. Estimate of the Hall-Petch and Orowan effects in the nanocrystalline Cu with Al2O3 dispersoid. J. Alloy. Compd. 2009, 483, 207–208. [Google Scholar] [CrossRef]
- Stamboulis, A.G.; Boeeaeeini, A.R.; Hench, L.L. Novel biodegradable polymeb/bioactive glass composites for tissue engineering application. Adv. Eng. Mater. 2002, 4, 105–109. [Google Scholar] [CrossRef]




| Abbreviation | Powder Component (wt.%) | Zn | Zr | Mg |
|---|---|---|---|---|
| E1 | Mg-5.2%Zn-0.3%Zr | 5.2 | 0.30 | Bal. |
| E2 | Mg-15%Zn-0.3%Zr | 15.0 | 0.27 | Bal. |
| E3 | Mg-30%Zn-0.3%Zr | 30.0 | 0.22 | Bal. |
| Processing Parameters | Value |
|---|---|
| Laser power P, (W) | 60, 70, 80, 90, 100, 110 |
| Pulse width W, (ms) | 2.5 |
| Frequency f, (HZ) | 30 |
| Hatch spacing d, (mm) | 1.5 |
| Scanning velocity V, (mm/s) | 10 |
| Laser spot size D, (mm) | 0.1 |
| Defocusing amount J, (mm) | 0 |
| Layer thickness T, (mm) | 0.04 |
| Samples | Grain Size (μm) | Grain Szie (μm) |
|---|---|---|
| Cross Section | Longitudinal Section | |
| E1 | 6.1 | 5.2 |
| E2 | 2.3 | 2.9 |
| E3 | 1.1 | 1.6 |
| Position | Mg (wt.%) | Zn (wt.%) | O (wt.%) | Zr (wt.%) |
|---|---|---|---|---|
| A | 88.81 | 6.30 | 5.09 | 0.80 |
| B | 75.70 | 12.65 | 9.80 | 1.85 |
| C | 71.53 | 17.55 | 8.75 | 2.28 |
| D | 80.53 | 12.05 | 6.62 | 0.80 |
| E | 67.51 | 16.68 | 15.07 | 1.85 |
| F | 67.77 | 21.20 | 9.38 | 1.55 |
| G | 75.69 | 20.33 | 5.10 | 0.88 |
| H | 61.69 | 30.52 | 6.21 | 1.58 |
| Samples | UCS (MPA) | Elastic Modulus (GPA) | Microhardness (HV) |
|---|---|---|---|
| E1 (HC) | 16.03 | 0.529 | 57.67 |
| E2 (HC) | 50.95 | 0.870 | 75.51 |
| E3 (HC) | 23.96 | 0.910 | 106.75 |
| E1 (LC) | 28.36 | 0.667 | 58.28 |
| E2 (LC) | 73.07 | 1.785 | 80.23 |
| E3 (LC) | 53.11 | 1.333 | 109.36 |
| Compact bone | 100–230 | 3–20 | |
| Cancellous bone | 0.2–80 | 0.01–2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Chen, C.; Liu, C.; Wang, S. Study on Porous Mg-Zn-Zr ZK61 Alloys Produced by Laser Additive Manufacturing. Metals 2018, 8, 635. https://doi.org/10.3390/met8080635
Zhang M, Chen C, Liu C, Wang S. Study on Porous Mg-Zn-Zr ZK61 Alloys Produced by Laser Additive Manufacturing. Metals. 2018; 8(8):635. https://doi.org/10.3390/met8080635
Chicago/Turabian StyleZhang, Min, Changjun Chen, Chang Liu, and Shunquan Wang. 2018. "Study on Porous Mg-Zn-Zr ZK61 Alloys Produced by Laser Additive Manufacturing" Metals 8, no. 8: 635. https://doi.org/10.3390/met8080635




