Joining of Aluminium Alloy Sheets to Aluminium Alloy Foam Using Metal Glasses
Abstract
:1. Introduction
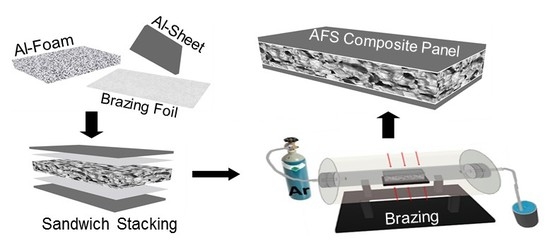
2. Materials and Methods
3. Results and Discussion
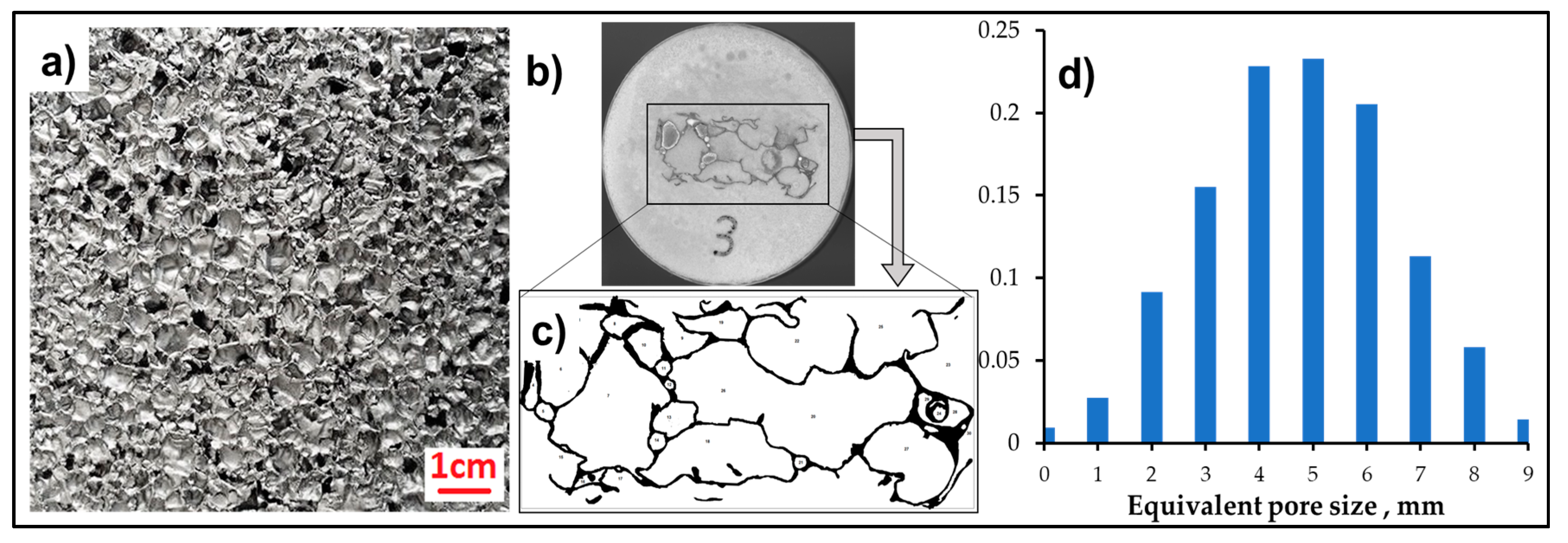
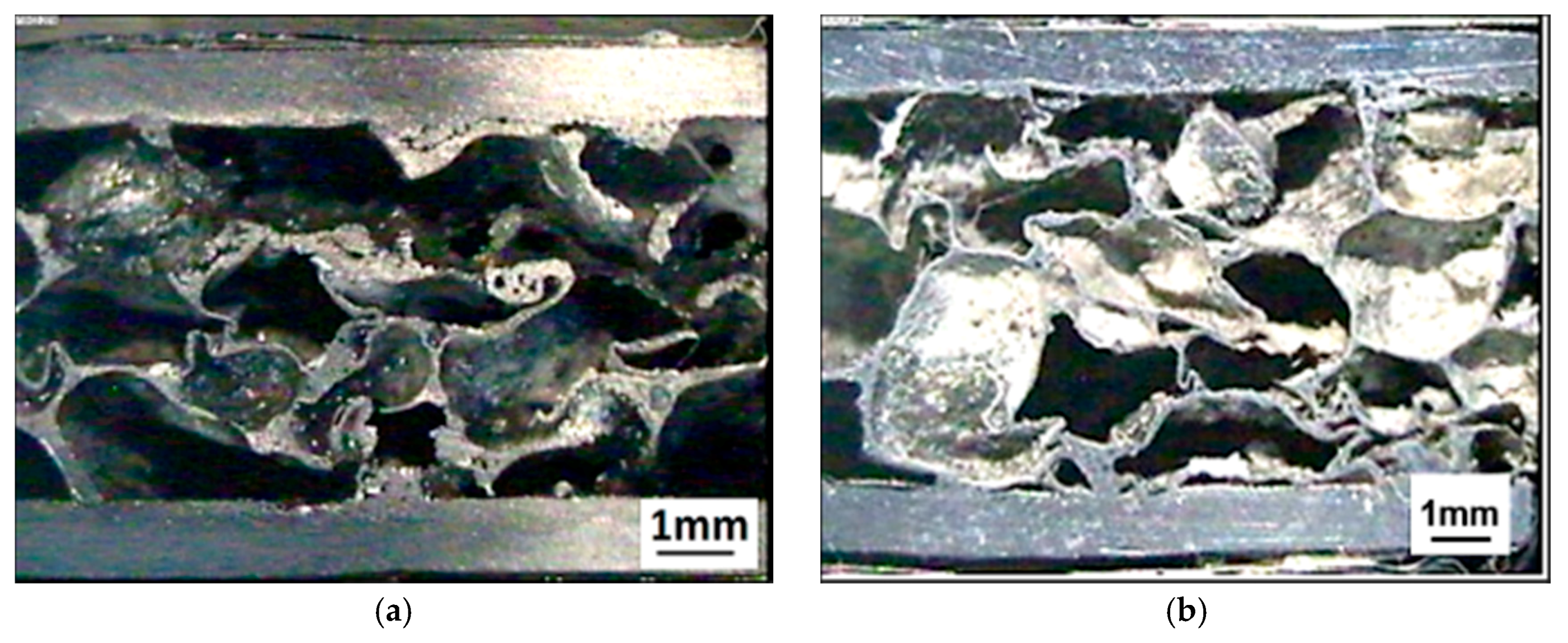
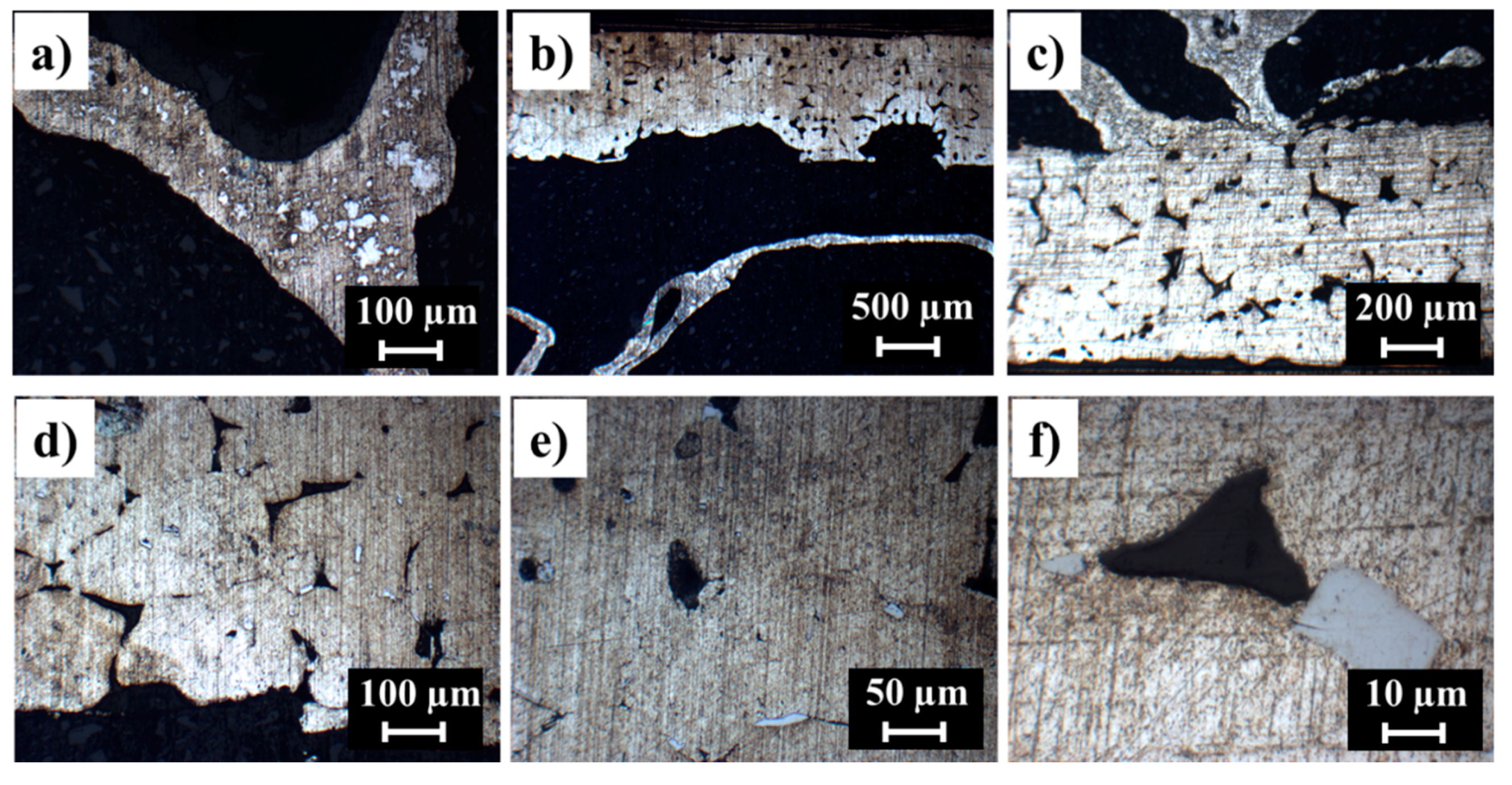
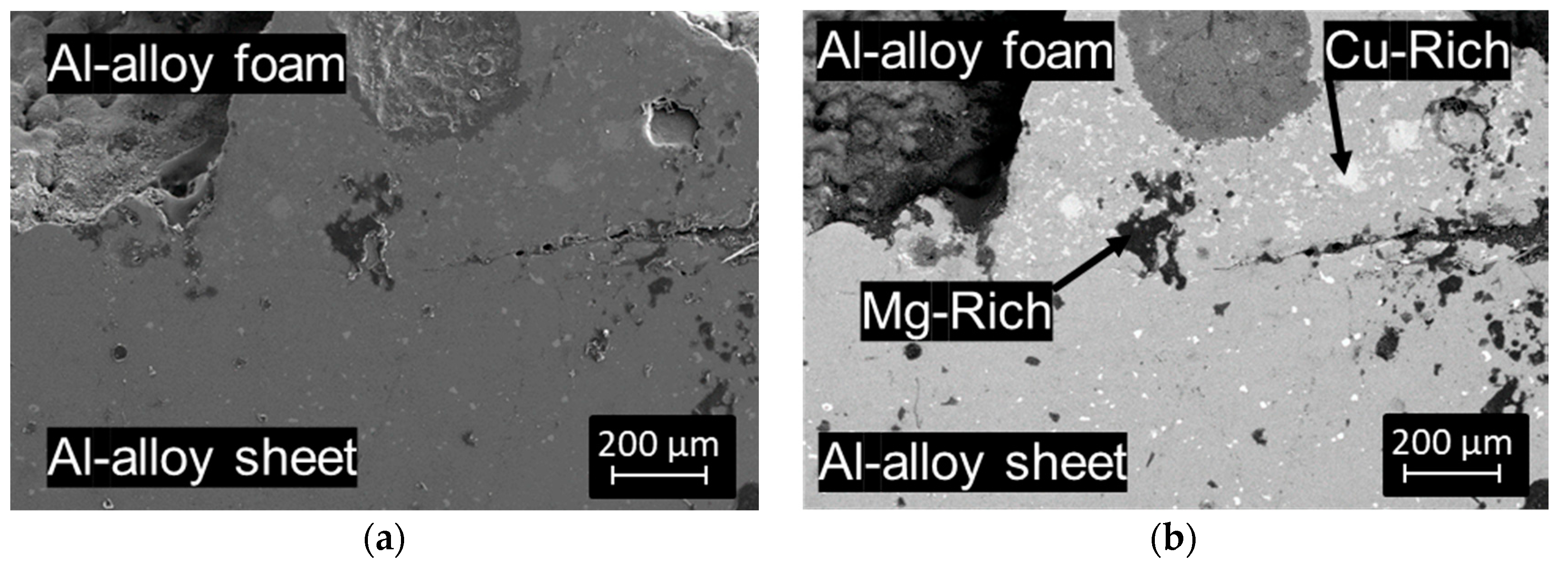
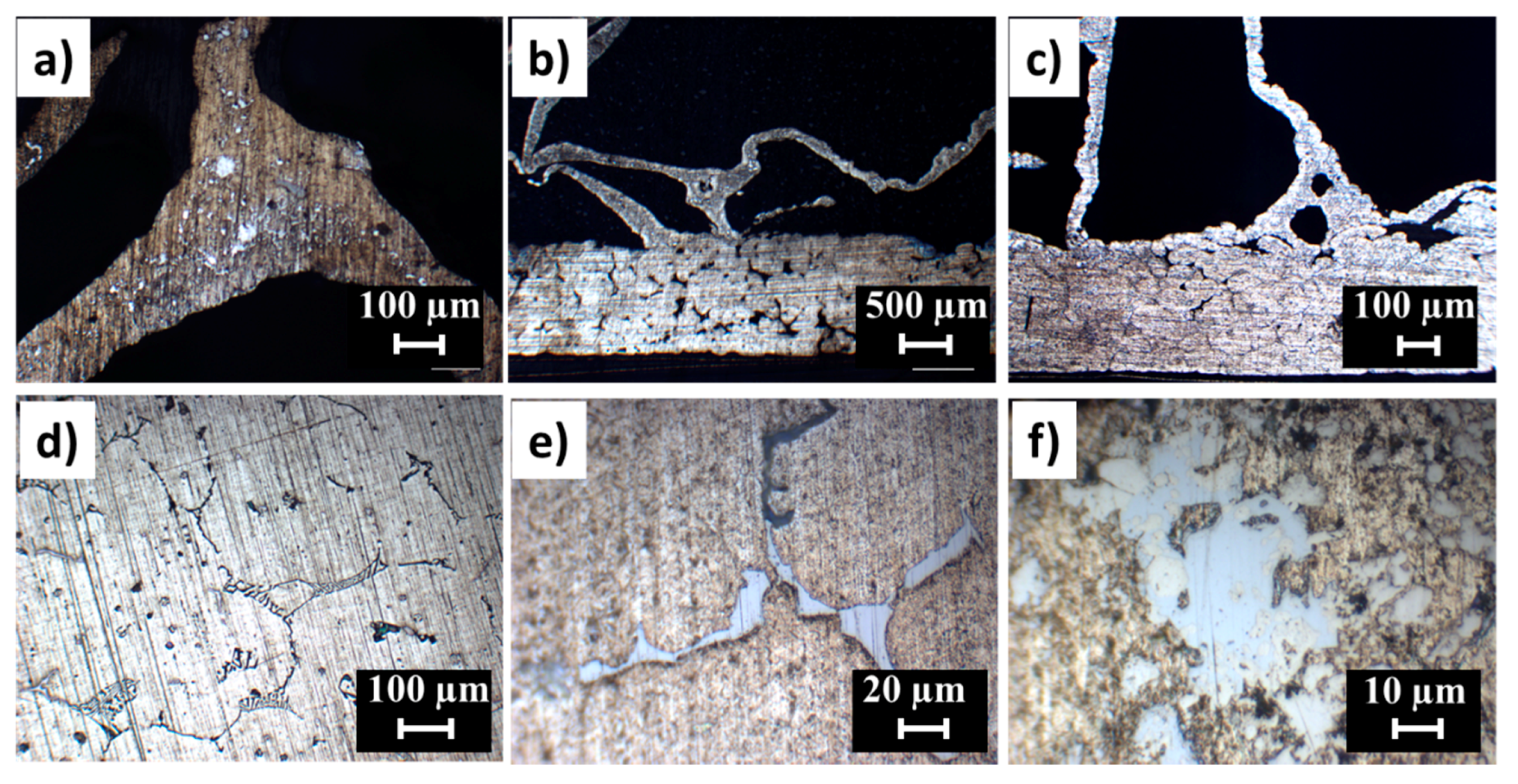
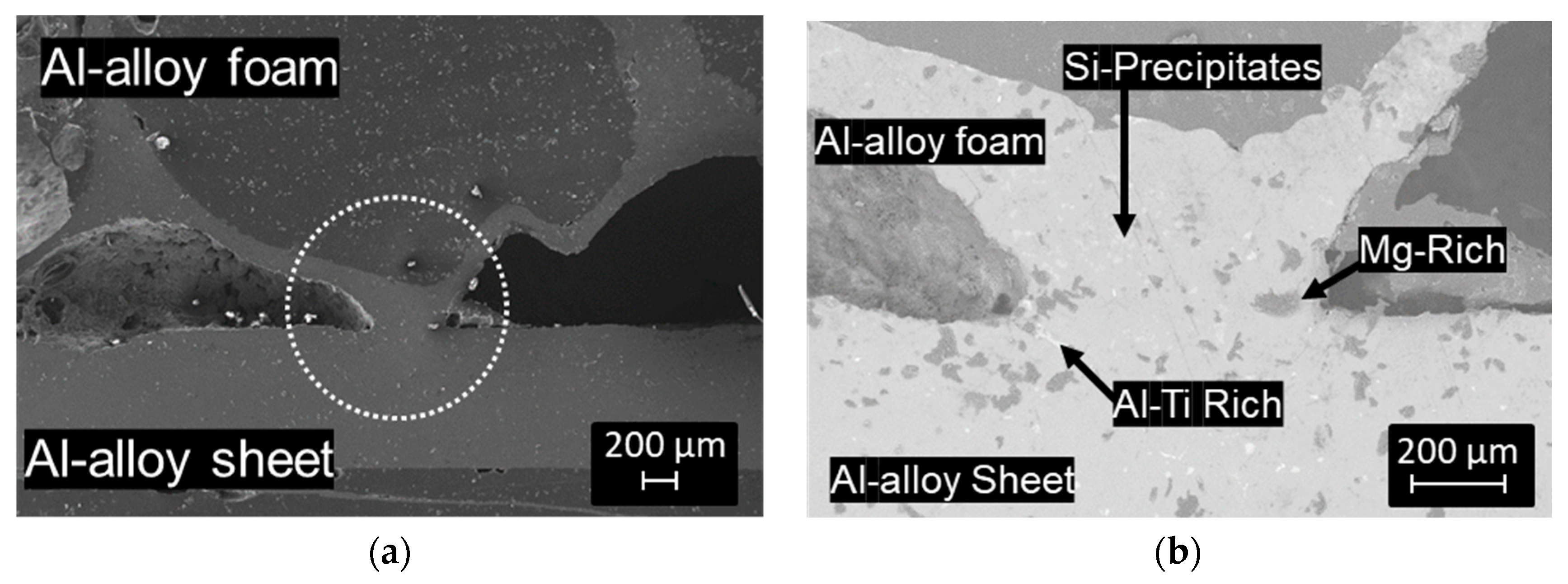
3.1. Al-Alloy Sheet/Al-Alloy Foam Joint Microstructure Analysis
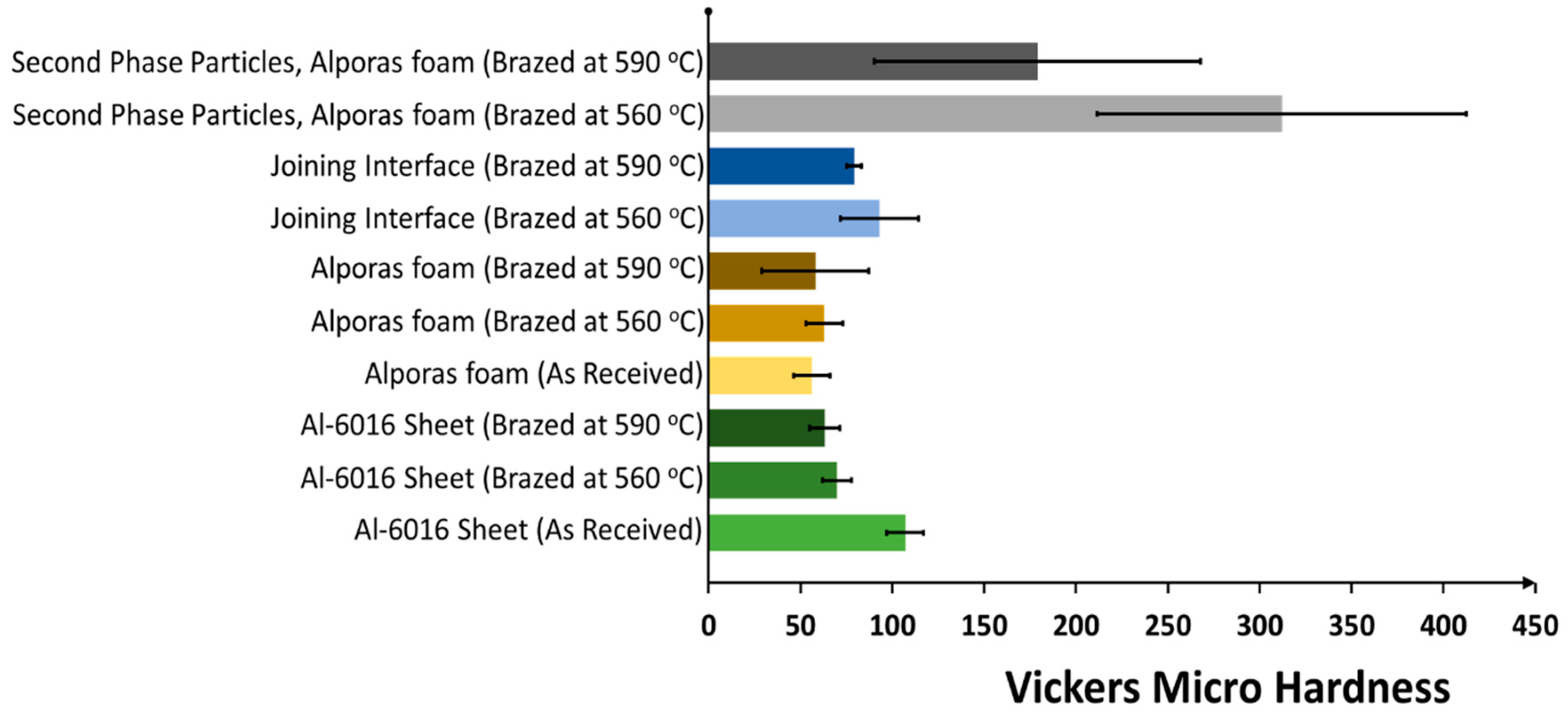
3.2. Microhardness Analysis
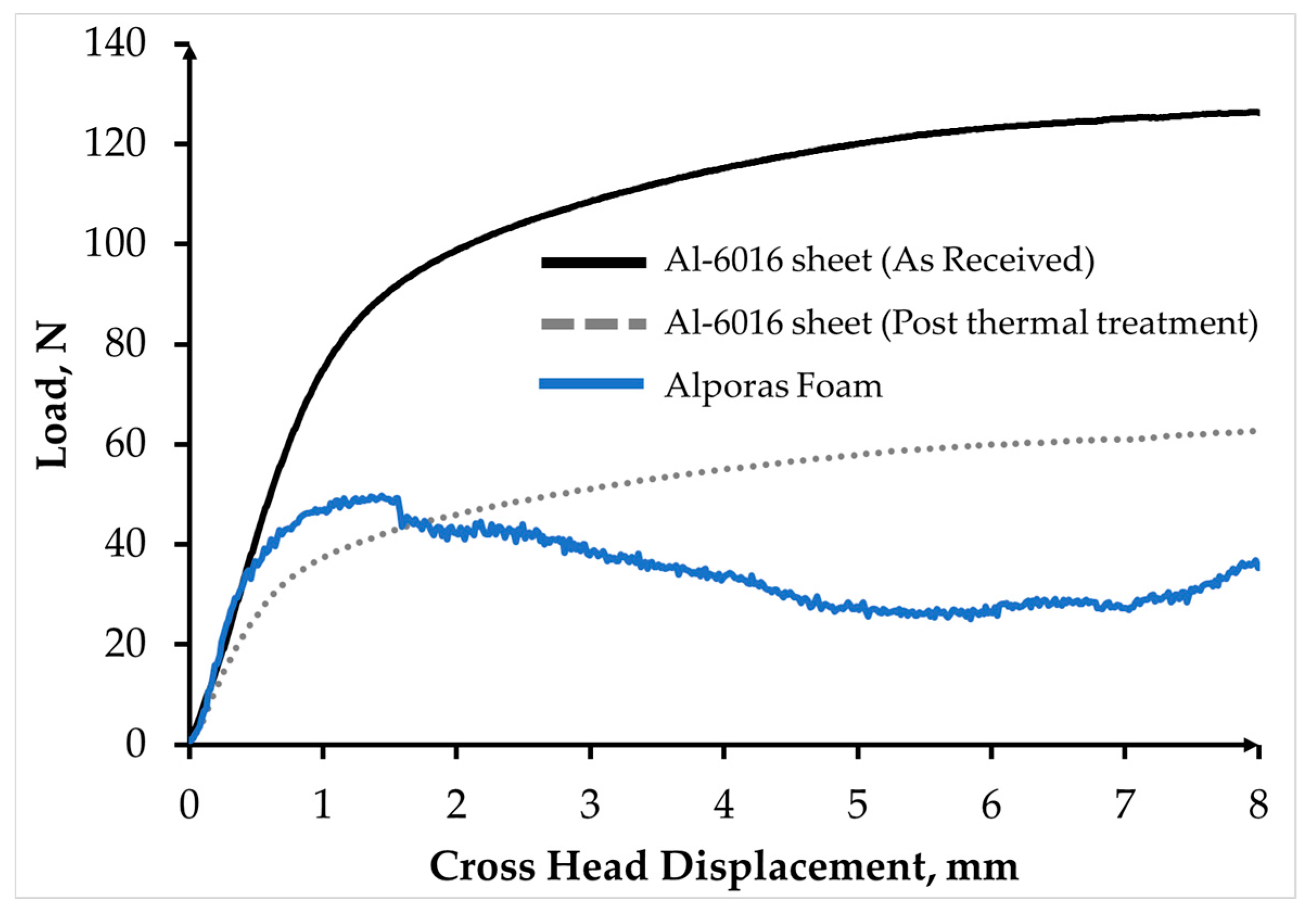
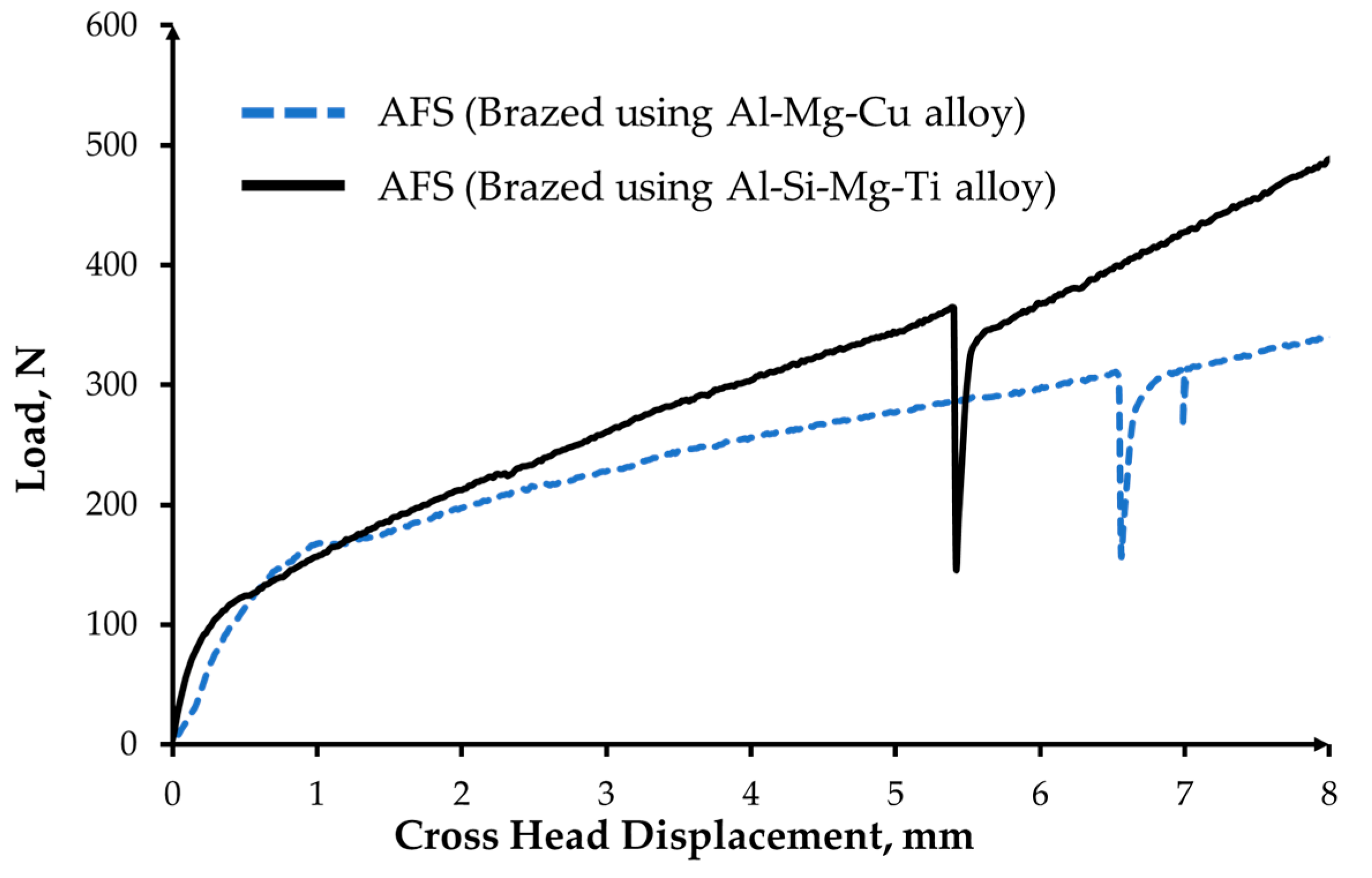
3.3. Mechanical Characterisation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crupi, V.; Epasto, G.; Guglielmino, E. Impact Response of Aluminum Foam Sandwiches for Light-Weight Ship Structures. Metals 2011, 1, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Banhart, J.; Seeliger, H.-W. Aluminium Foam Sandwich Panels: Manufacture, Metallurgy and Applications. Adv. Eng. Mater. 2008, 10, 793–802. [Google Scholar] [CrossRef]
- Güner, A.; Arıkan, M.; Nebioglu, M. New Approaches to Aluminum Integral Foam Production with Casting Methods. Metals 2015, 5, 1553–1565. [Google Scholar] [CrossRef] [Green Version]
- Banhart, J.; Berlin, H.; Heim, K.; Seeliger, H.; Gmbh, P.M. Light-weighting in transportation and defence using aluminium foam sandwich structures. In Proceedings of the International Symposium on Light Weighting for Defence, Aerospace and Transportation, Goa, India, 11 November 2017; pp. 1–10. [Google Scholar]
- Chen, N.; Feng, Y.; Chen, J.; Li, B.; Chen, F.; Zhao, J. Vacuum Brazing Processes of Aluminum Foam. Rare Met. Mater. Eng. 2013, 42, 1118–1122. [Google Scholar] [CrossRef]
- Matsumoto, R.; Tsuruoka, H.; Otsu, M.; Utsunomiya, H. Fabrication of skin layer on aluminum foam surface by friction stir incremental forming and its mechanical properties. J. Mater. Process. Technol. 2015, 218, 23–31. [Google Scholar] [CrossRef]
- Banhart, J.; Seeliger, H.-W. Recent Trends in Aluminium Foam Sandwich Technology Industrial Implementation of AFS Technology. Adv. Eng. Mater. 2011, 14, 1082–1087. [Google Scholar] [CrossRef]
- Polmear, I. Light Alloys-From Traditional Alloys to Nanocrystals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Luo, J.-G.; Acoff, V.L. Interfacial Reactions of Titanium and Aluminum during Diffusion Welding. Weld J. 2000, 79, 239–243. [Google Scholar]
- Banhart, J.; Seeliger, H.-W. Recent Trends in Aluminum Foam Sandwich Technology. Adv. Eng. Mater. 2012, 14, 1082–1087. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, J.; Lv, S.; Leng, J.; Li, Y. Fluxless soldering with surface abrasion for joining metal foams. Mater. Sci. Eng. A 2012, 552, 283–287. [Google Scholar] [CrossRef]
- Wan, L.; Huang, Y.; Huang, T.; Lv, S.; Feng, J. Novel method of fluxless soldering with self-abrasion for fabricating aluminum foam sandwich. J. Alloys Compd. 2015, 640, 1–7. [Google Scholar] [CrossRef]
- Fleming, K.M.; Zhu, A.; Scully, J.R. Corrosion of AA6061 brazed with an Al-Si alloy: Effects of Si on metallurgical and corrosion behavior. Corrosion 2012, 68, 1126–1145. [Google Scholar] [CrossRef]
- Han, Y.; Ma, K.; Li, L.; Chen, W.; Nagaumi, H. Study on microstructure and mechanical properties of Al-Mg-Si-Cu alloy with high manganese content. Mater. Des. 2012, 39, 418–424. [Google Scholar] [CrossRef]
- Ubertalli, G.; Ferraris, M.; Bangash, M.K. Joining of AL-6016 to Al-foam using Zn-based joining materials. Compos. Part A Appl. Sci. Manuf. 2017, 96, 122–128. [Google Scholar] [CrossRef]
- Duarte, I.; Oliveira, M. Aluminium Alloy Foams: Production and Properties. In Powder Metallurgy; InTech: Rijeka, Croatia, 2012; pp. 47–72. [Google Scholar]
- Campana, G.; Ascari, A.; Fortunato, A. Laser foaming for joining aluminum foam cores inside a hollow profile. Opt. Laser Technol. 2013, 48, 331–336. [Google Scholar] [CrossRef]
- García-Moreno, F. Commercial applications of metal foams: Their properties and production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Banhart, J.; Vinod-Kumar, G.S.; Kamm, P.H.; Neu, T.R.; García-Moreno, F. Light-metal foams: Some recent developments. Ciência Tecnol. Dos Mater. 2016, 28, 1–4. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, D.; Tian, J.; Wang, D.; Wang, P.; Niu, J. Effects of Al–8.5Si–25Cu–xY filler metal foils on vacuum brazing of SiCp/Al composites. Mater. Sci. Technol. 2017, 0836, 1–11. [Google Scholar] [CrossRef]
- Qingxian, H.; Sawei, Q.; Yuebo, H. Development on Preparation Technology of Aluminum Foam Sandwich Panels. Rare Met. Mater. Eng. 2015, 44, 548–552. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Lehmhus, D.; Banhart, J. Properties of heat-treated aluminium foams. Mater. Sci. Eng. 2003, 349, 98–110. [Google Scholar] [CrossRef]
- Kailas, S.V. Chapter 6. Pase Diagram. In Material Science; Dept. of Mechanical Engineering, Indian Institute of Science: Bangalore, India, 2007; Available online: http://docplayer.net (accessed on 22 January 2018).
- Samson, S. The crsytal structure of the phase Mg2Al3. Acta Crystallogr. 1965, 19, 401–413. [Google Scholar] [CrossRef]
- Pearson Burton, W.; Villars, P.; Calvert, D.L. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases; American Society for Metals: Metals Park, OH, USA, 1987; Volume 1. [Google Scholar]
- Vušanovic, I.; Voronjec, D.; Krane, M.J.M. Microsegregation Phenomena in Al-Cu-Mg Alloy with Considering of Diffusion Phenomena in Primary Phase. Facta Univ. Ser. Mech. Eng. 2001, 1, 965–980. [Google Scholar]
- Bolingbroke, R.K.; Gray, A.; Lauzon, D. Optimisation of Nocolok(TM) Brazing Conditions for Higher Strength Brazing Sheet; SAE Technical Paper: Warrendale, PA, USA, 1997. [Google Scholar] [CrossRef]
- Orman, L.; Swidersky, H.W.; Lauzon, D. Brazing of Aluminum alloys with higher magnesium content using non-corrosive fluxes. Keikinzoku Yosetsu/J. Light Met. Weld Constr. 2014, 52, 24–29. [Google Scholar]
- Moller, C.; Grann, J. Aluminum Brazing—What Matters Most: Fundamentals and Case Studies. In Proceedings of the 5th International Brazing Soldering Conference, Las Vegas, NV, USA, 22–25 April 2012; pp. 1–9. [Google Scholar]
- Makhlouf, M.M.; Guthy, H.V. The aluminum-silicon eutectic reaction: Mechanisms and crystallography. J. Light Met. 2002, 1, 199–218. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Jansen, J.; Zandbergen, H.W. The influence of temperature and storage time at RT on nucleation of the β′ phase in a 6082 Al-Mg-Si alloy. Acta Mater. 2003, 51, 789–796. [Google Scholar] [CrossRef]
- Kabir, K.; Vodenitcharova, T.; Hoffman, M. Response of aluminium foam-cored sandwich panels to bending load. Compos. Part B Eng. 2014, 64, 24–32. [Google Scholar] [CrossRef]
- Shabestari, S.G.; Wanderka, N.; Seeliger, W.; Banhart, J. Optimisation of the Strength of Aluminium Foam Sandwich (AF) Panels by Different Heat Treatments. Mater. Sci. Forum 2006, 521, 1221–1226. [Google Scholar] [CrossRef]
- Wan, L.; Huang, Y.; Lv, S.; Feng, J. Fabrication and interfacial characterization of aluminum foam sandwich via fluxless soldering with surface abrasion. Compos. Struct. 2015, 123, 366–373. [Google Scholar] [CrossRef]
- Crupi, V.; Montanini, R. Aluminium foam sandwiches collapse modes under static and dynamic three-point bending. Int. J. Impact Eng. 2007, 34, 509–521. [Google Scholar] [CrossRef]
- Duarte, I.; Vesenjak, M.; Krstulović-Opara, L.; Anžel, I.; Ferreira, J.M. Manufacturing and bending behaviour of in situ foam-filled aluminium alloy tubes. Mater. Des. 2015, 66, 532–544. [Google Scholar] [CrossRef]
- Bastawros, A. Experimental analysis of deformation mechanisms in a closed-cell aluminum alloy foam. J. Mech. Phys. Solids 2000, 48, 301–322. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.; Yu, J.; Qian, C.; Lu, F. Deformation and failure mechanisms of sandwich beams under three-point bending at elevated temperatures. Compos. Struct. 2014, 111, 285–290. [Google Scholar] [CrossRef]
- Duarte, I.; Teixeira-Dias, F.; Graça, A.; Ferreira, A.J.M. Failure Modes and Influence of the Quasi-static Deformation Rate on the Mechanical Behavior of Sandwich Panels with Aluminum Foam Cores. Mech. Adv. Mater. Struct. 2010, 17, 335–342. [Google Scholar] [CrossRef]
- Swidersky, H.W. Aluminium Brazing with Non-corrosive Fluxes State of the Art and Trends in NOCOLOK® Flux Technology. In Proceedings of the 6th International Conference Brazing, High Temperature Brazing and Diffusion Bonding, Aachen, Germany, 8–10 May 2001. [Google Scholar]


| Joint | Joining Parameters | Joining Material | Specimen Dimensions, (mm) | Span Length, (mm) | Approx. Service Temperature, (°C) | Bending Load at Failure, (N) | Reference |
|---|---|---|---|---|---|---|---|
| Al-6016/Al-alloy foam | 10 min at 560 °C | Al-Cu-Mg amorphous alloy | 1 l = 60 | 47 | 520 | 341 ± 30 | Current work |
| 2 b = 20 | |||||||
| 3 c = 9 | |||||||
| 4 t = 1.2 | |||||||
| Al-6016/Al-alloy foam | 10 min at 590 °C | Al-Si-Mg-Ti amorphous alloy | l = 60 | 47 | 520 | 284 ± 20 | Current work |
| b = 20 | |||||||
| c = 9 | |||||||
| t = 1.2 | |||||||
| Al-6016/Al-alloy foam | 1 min at 420 °C | Pure Zn foils | l = 60 | 47 | 380 | 785 ± 50 | [15] |
| b = 20 | |||||||
| c = 9 | |||||||
| t = 1.2 | |||||||
| Al-6016/Al-alloy foam | 5 min at 430 °C | Zn-2Al alloy strips | l = 60 | 47 | 380 | 904 ± 60 | [15] |
| b = 20 | |||||||
| c = 9 | |||||||
| t = 1.2 | |||||||
| Al 1100-0/Al-alloy foam | Room temperature | Epoxy | l = 150 | 50 | 25 | 310 | [34] |
| b = 35 | |||||||
| c = 12 | |||||||
| t = 0.34 | |||||||
| Al 3104-H19/Al-alloy foam | Room temperature | Epoxy | l = 150 | 50 | 25 | 400 | [34] |
| b = 35 | |||||||
| c = 12 | |||||||
| t = 0.34 | |||||||
| Al-5056/Al-alloy foam | 10min at 420 °C (Without vibration) | Zn6.2Al4.3Cu1.2Mg0.8Mn0.5Ag alloy | l = 60 | 40 | 380 | 1000 | [36] |
| b = 15 | |||||||
| c = 15 | |||||||
| t = 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bangash, M.K.; Ubertalli, G.; Di Saverio, D.; Ferraris, M.; Jitai, N. Joining of Aluminium Alloy Sheets to Aluminium Alloy Foam Using Metal Glasses. Metals 2018, 8, 614. https://doi.org/10.3390/met8080614
Bangash MK, Ubertalli G, Di Saverio D, Ferraris M, Jitai N. Joining of Aluminium Alloy Sheets to Aluminium Alloy Foam Using Metal Glasses. Metals. 2018; 8(8):614. https://doi.org/10.3390/met8080614
Chicago/Turabian StyleBangash, Muhammad Kashif, Graziano Ubertalli, Davide Di Saverio, Monica Ferraris, and Niu Jitai. 2018. "Joining of Aluminium Alloy Sheets to Aluminium Alloy Foam Using Metal Glasses" Metals 8, no. 8: 614. https://doi.org/10.3390/met8080614