The Role of Anode Manufacturing Processes in Net Carbon Consumption †
Abstract
:1. Introduction
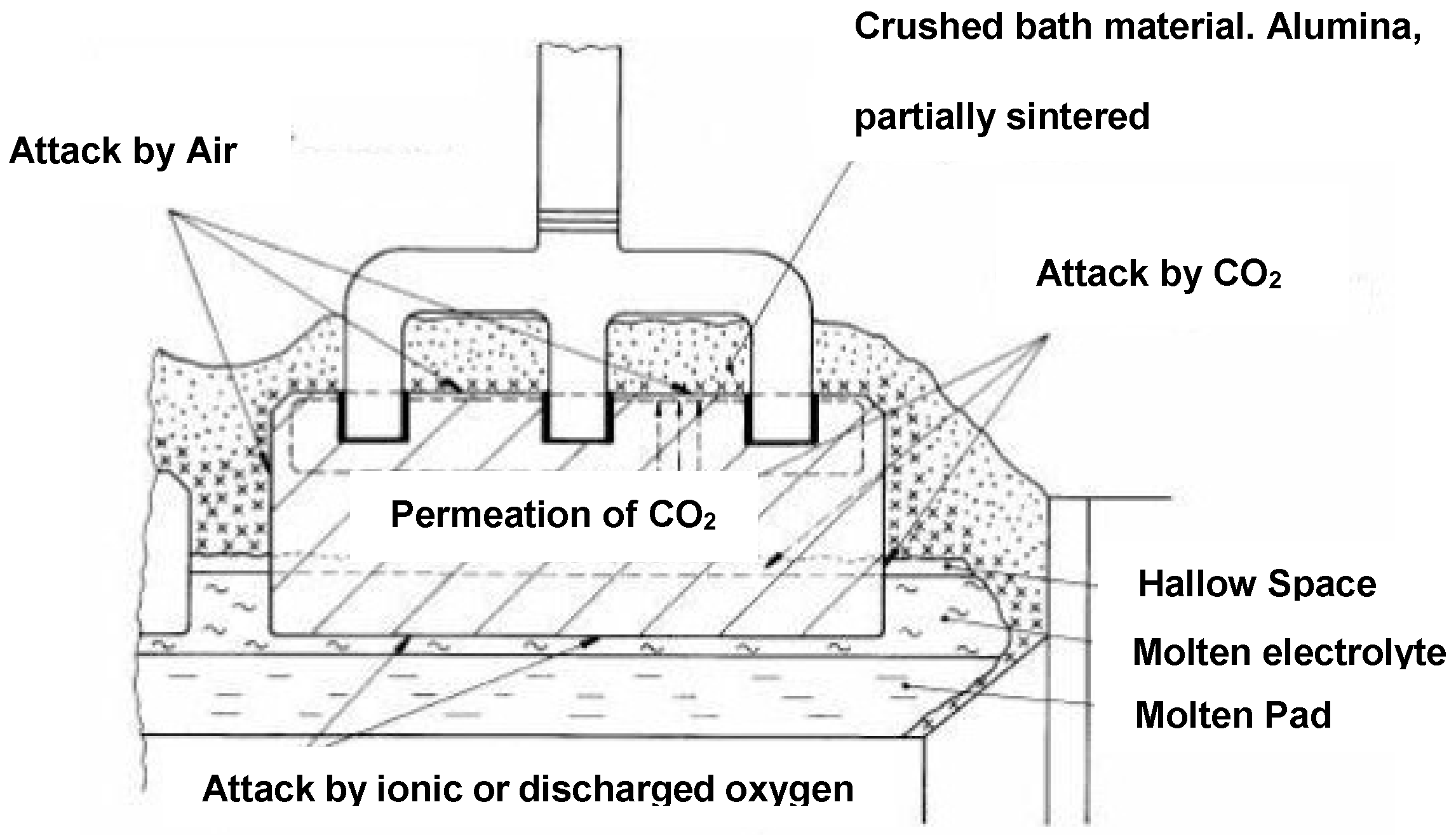
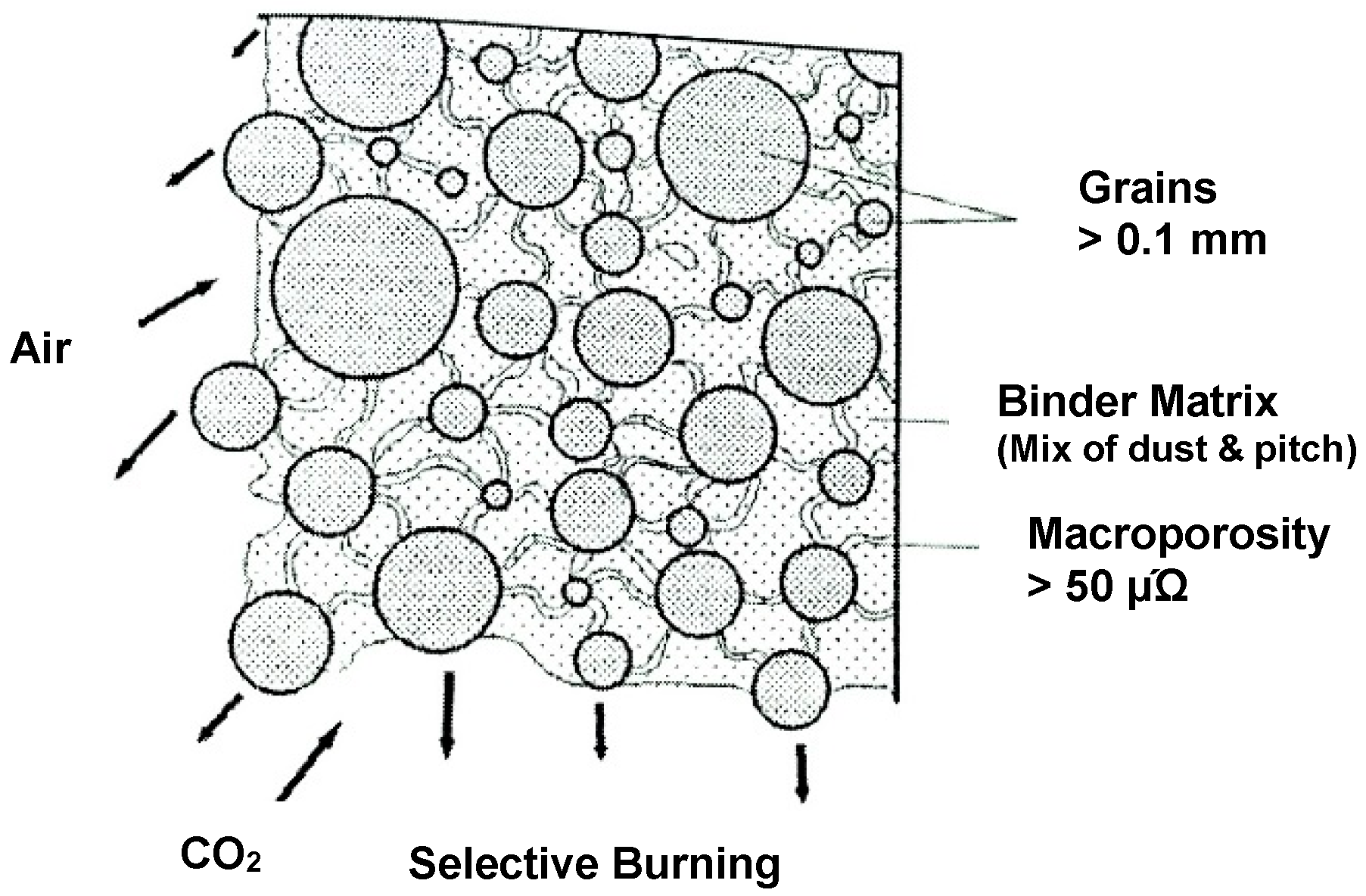
2. Anode Consumption
- electrochemical formation of carbon dioxide,
- electrochemical formation of carbon monoxide,
- carboxy (Boudouard) reaction,
- air burn, and
- dusting as a consequence of preferential oxidation.
3. Plant Parameters
4. Findings
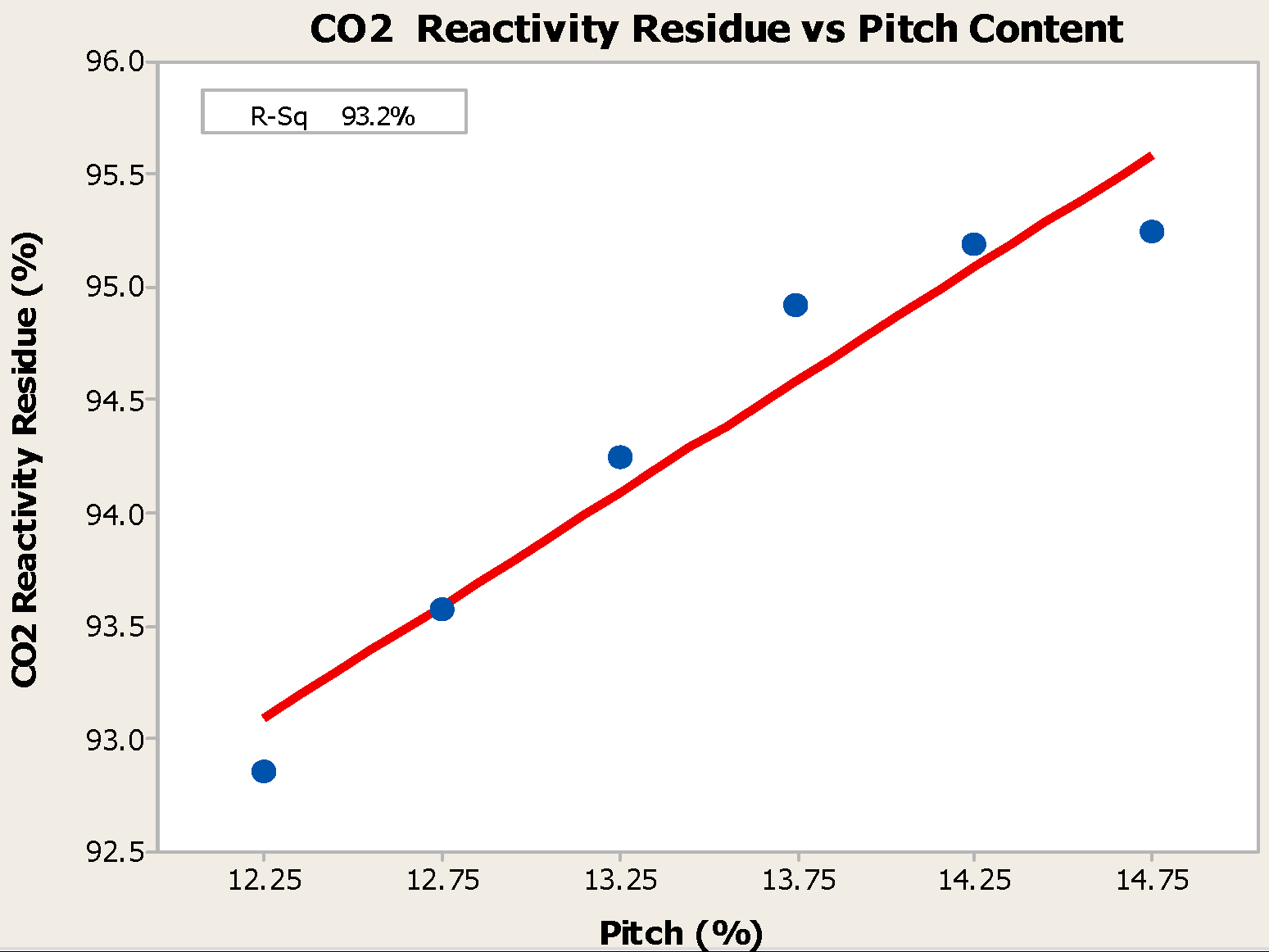
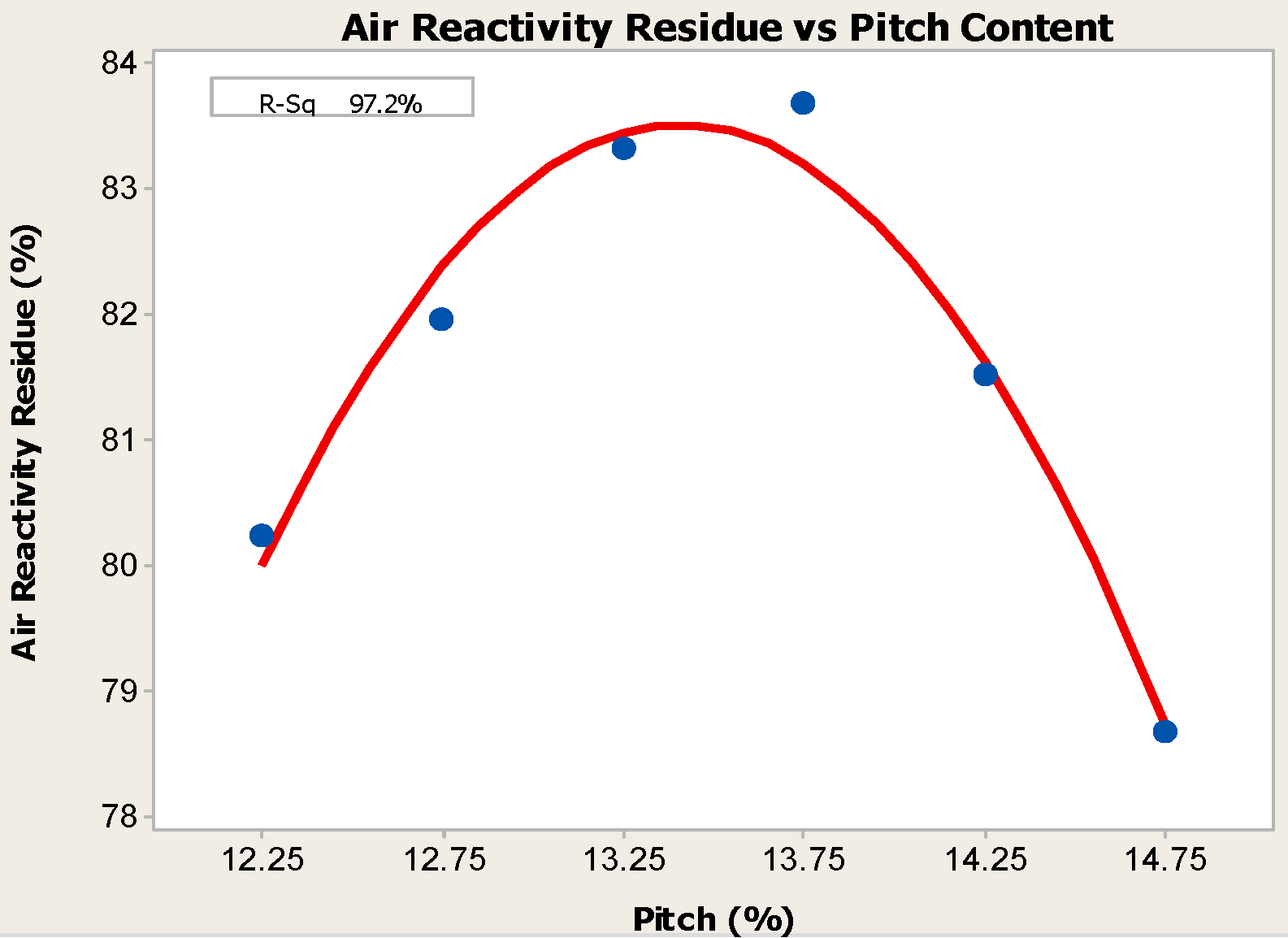
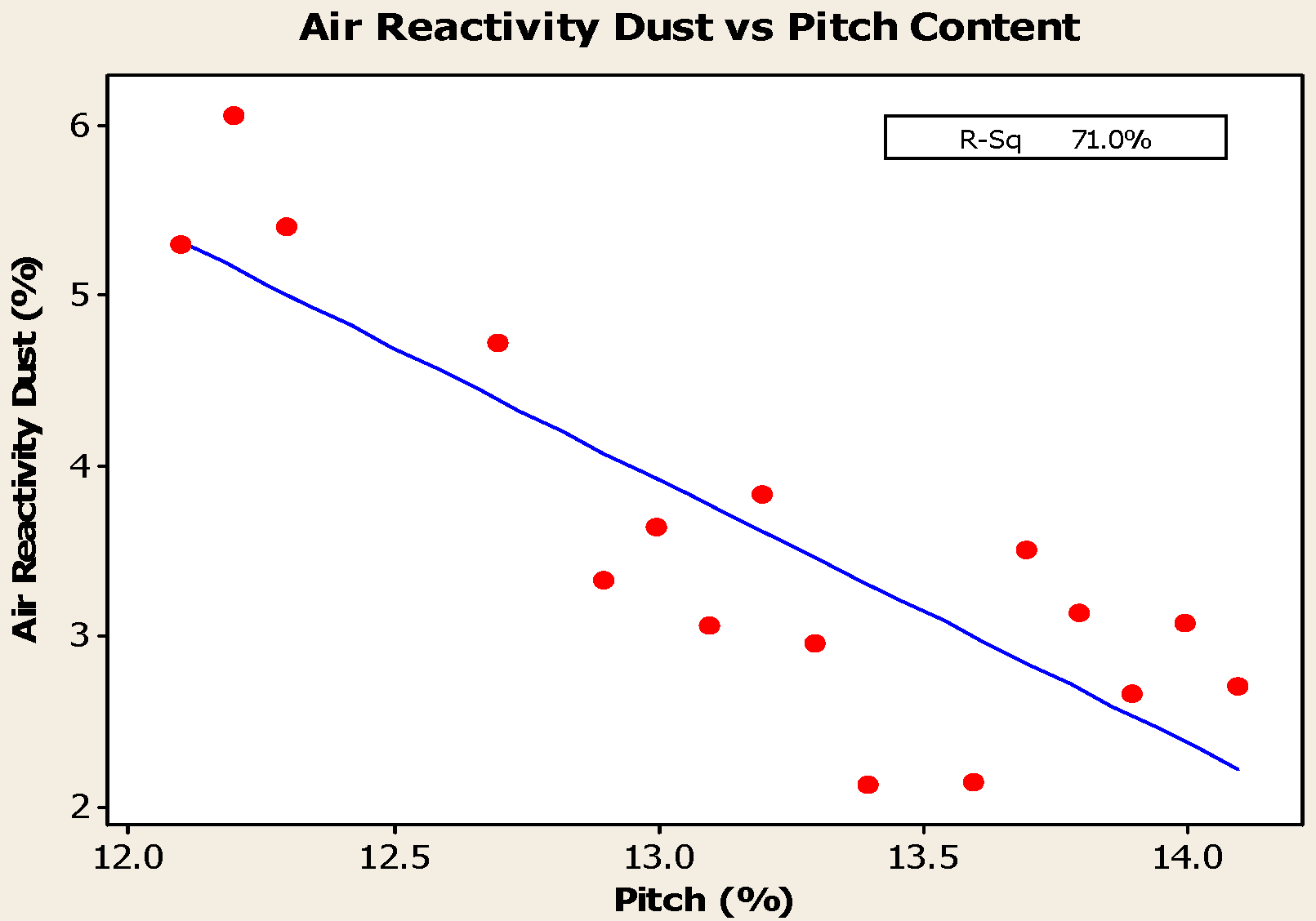
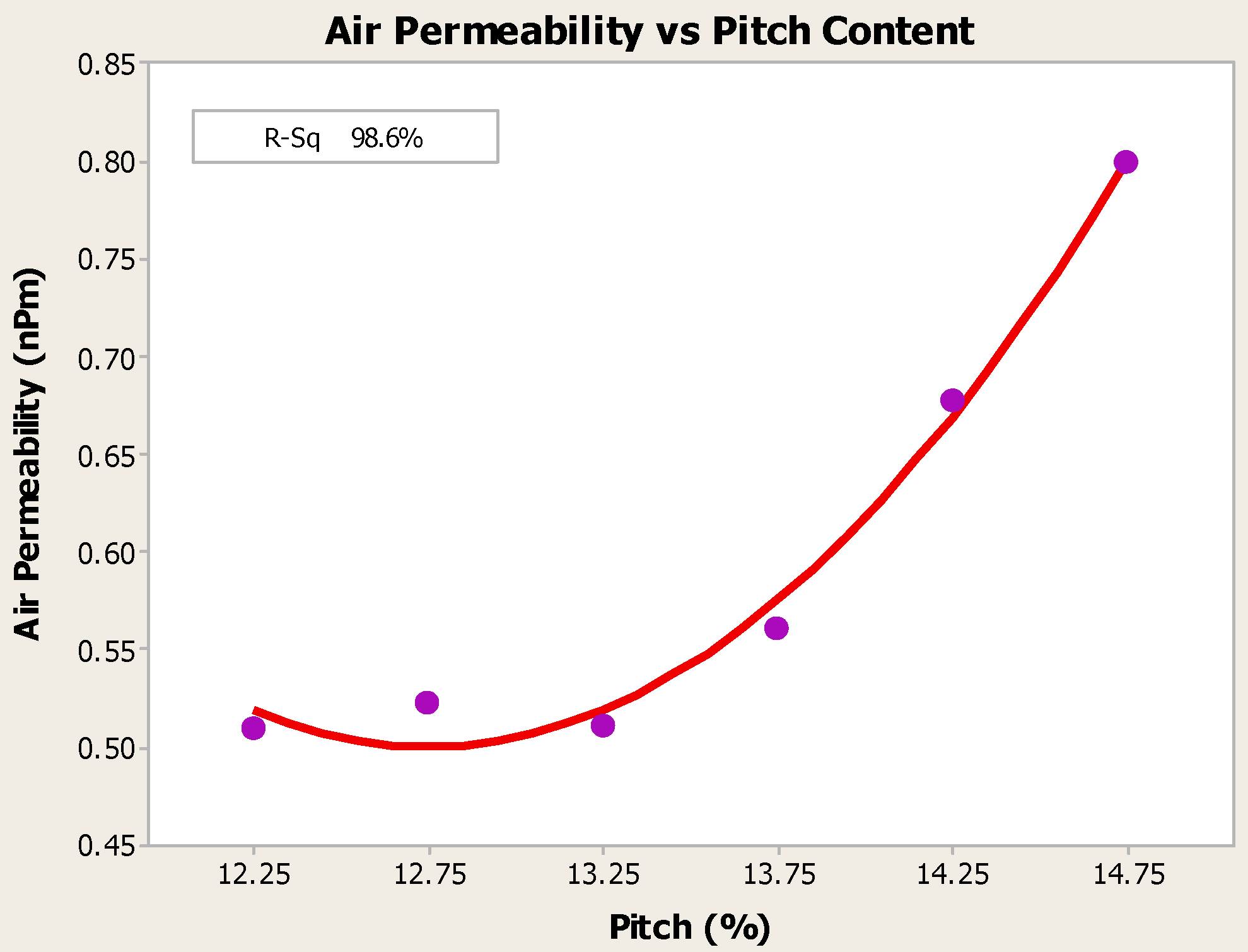
4.1. Pitch Addition
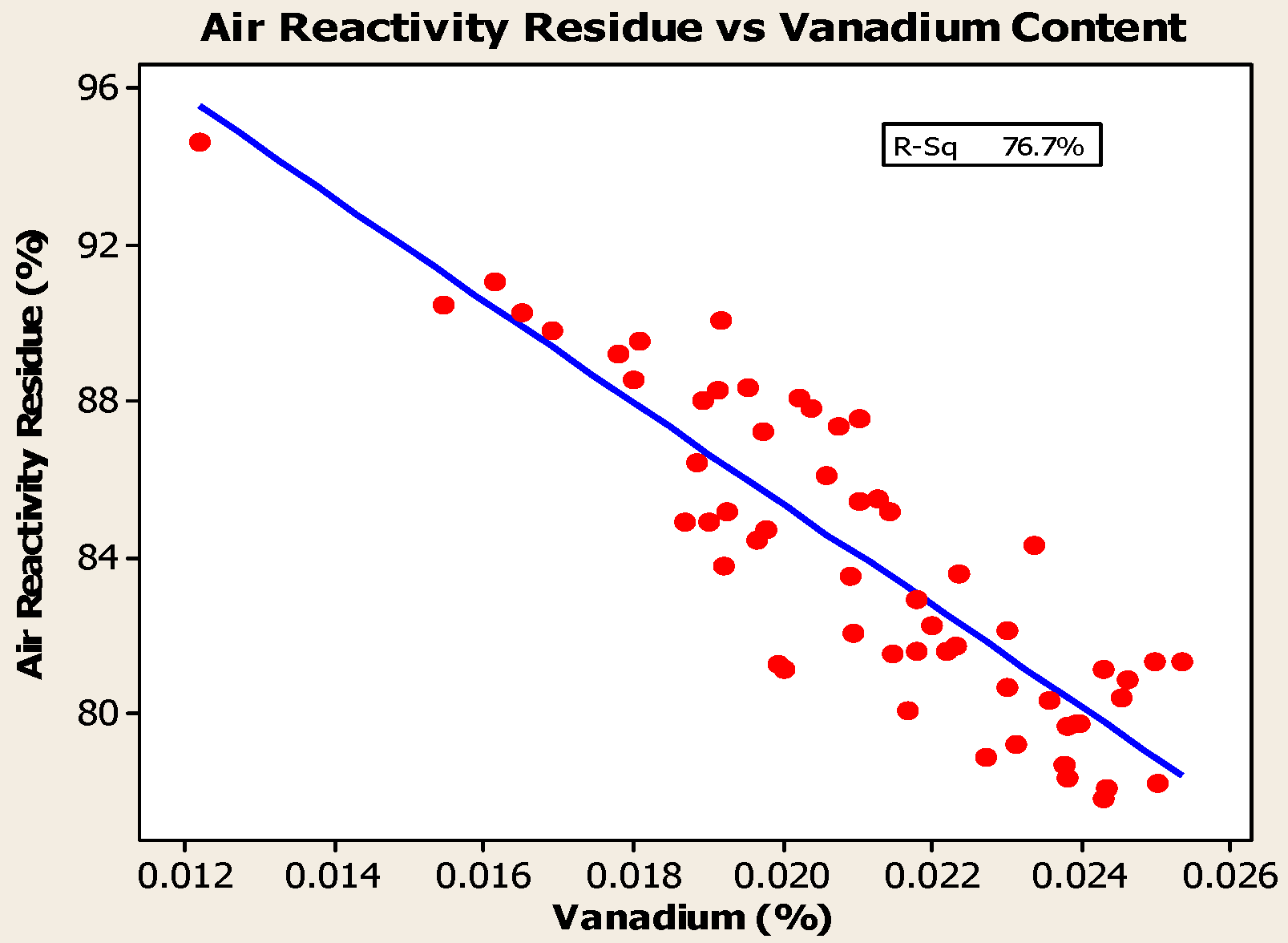
4.2. Impurities
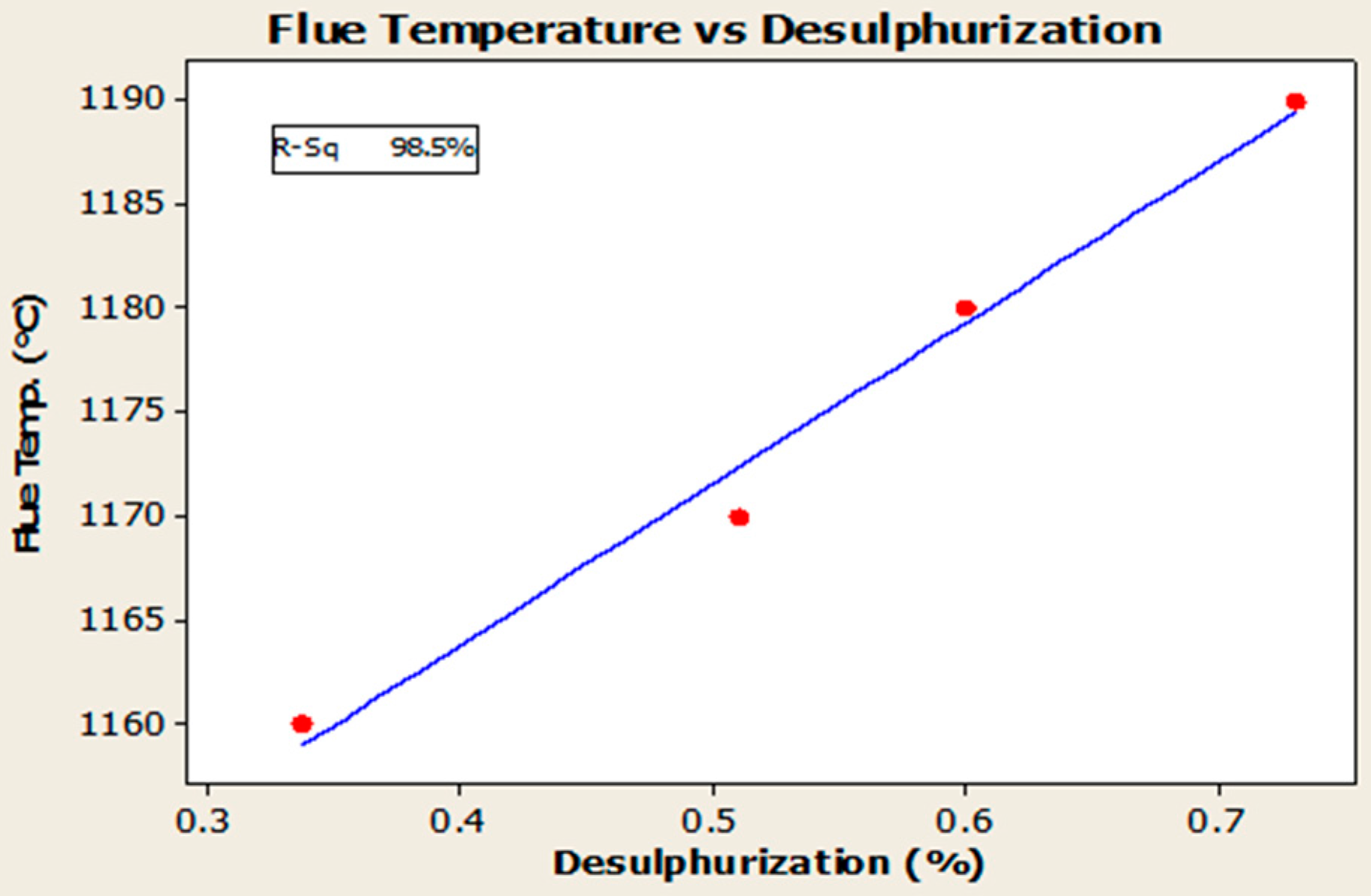
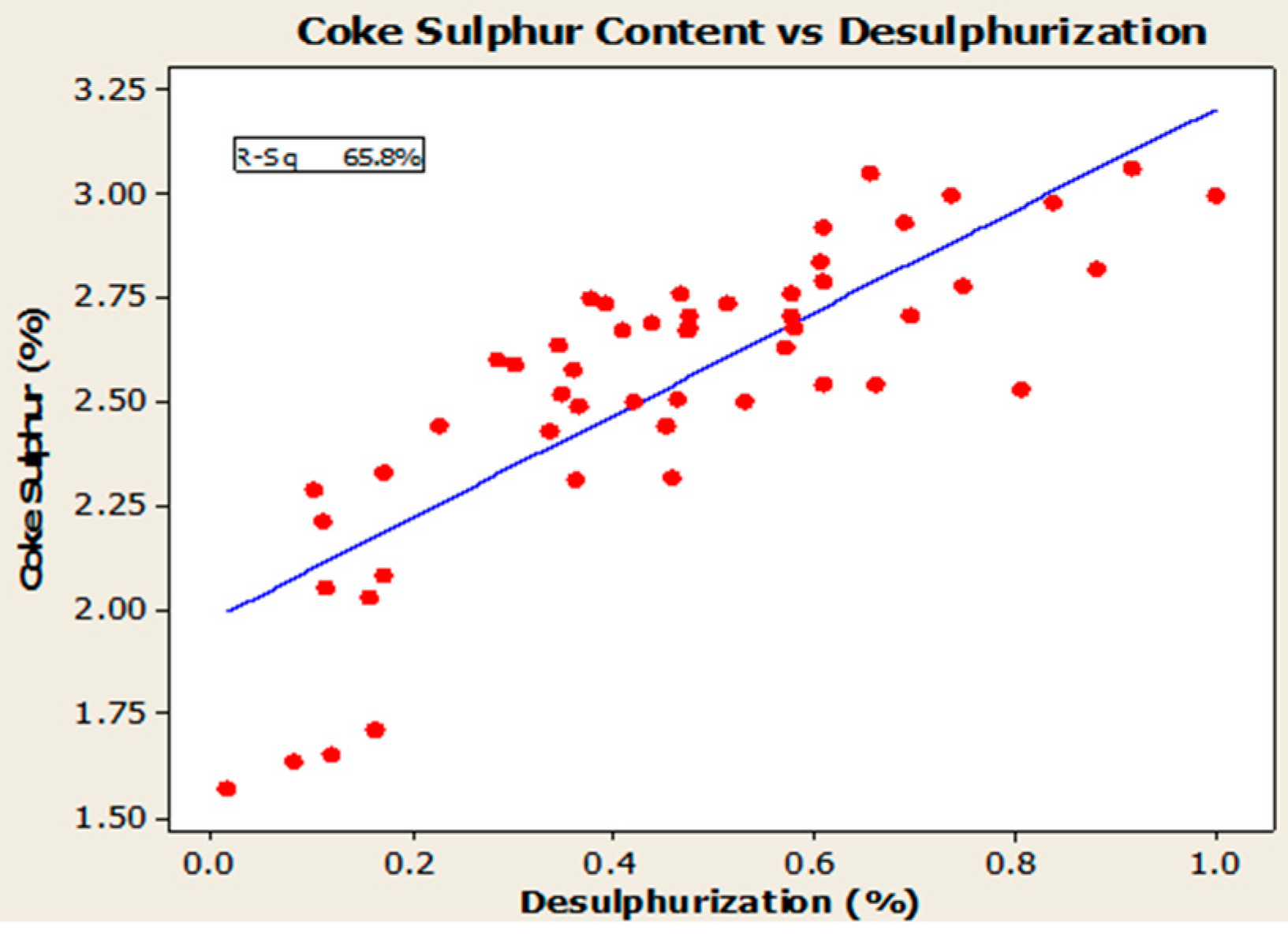
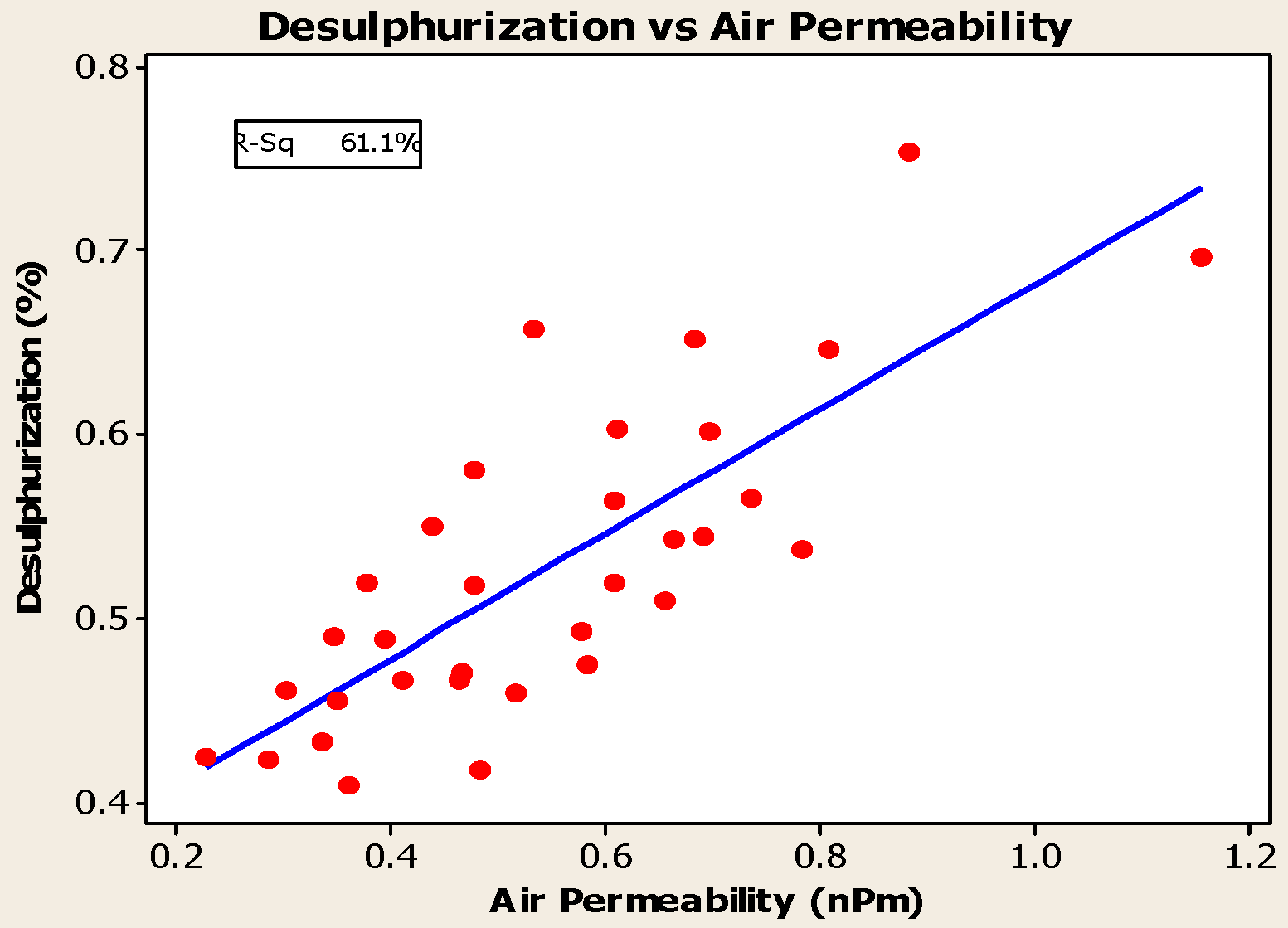
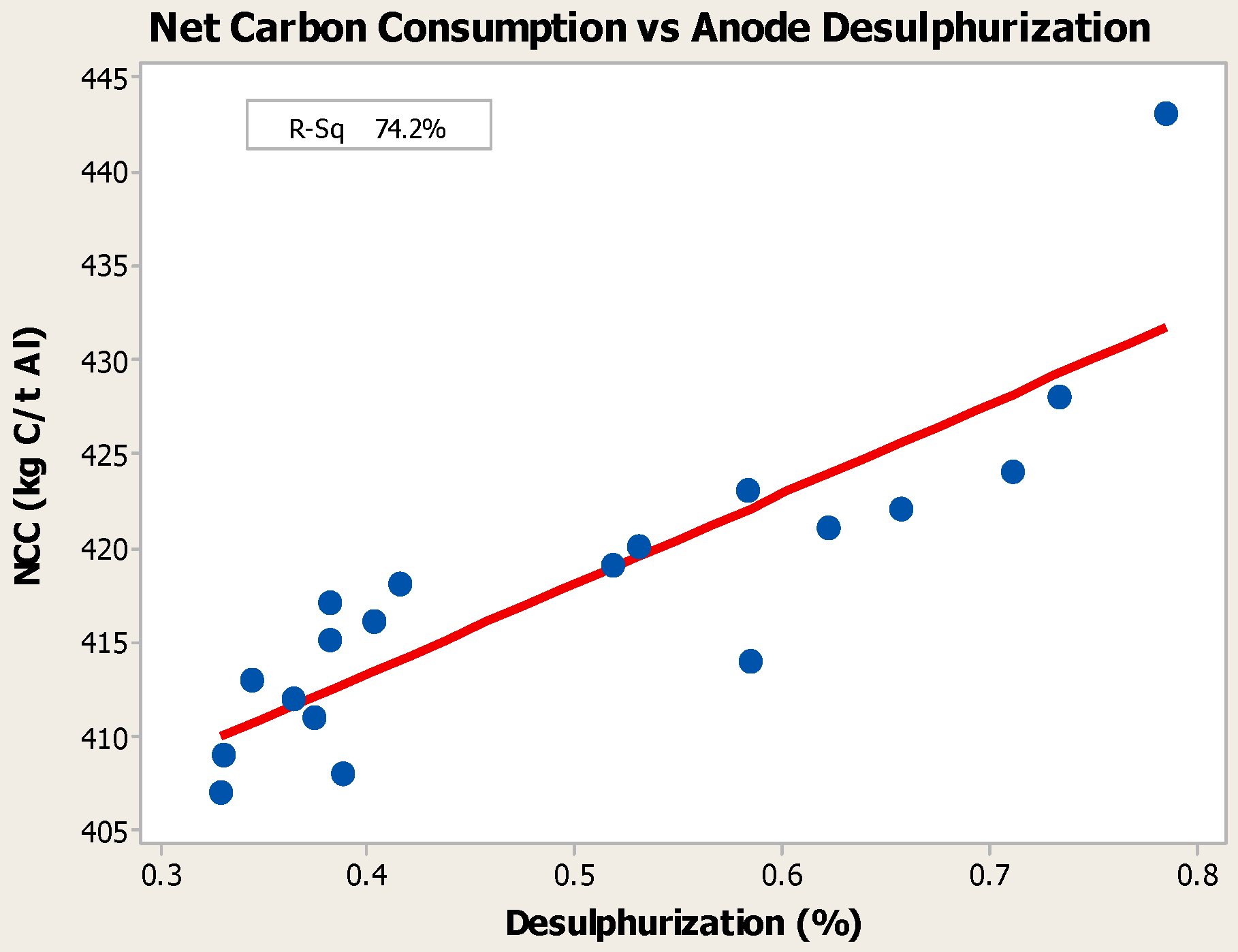
4.3. Desulfurization of Anodes
5. Discussion
6. Conclusions
- Anodes with higher pitch content have higher CO2 reactivity residue and lower CO2 reactivity dust, both of which are favorable in reducing net carbon consumption.
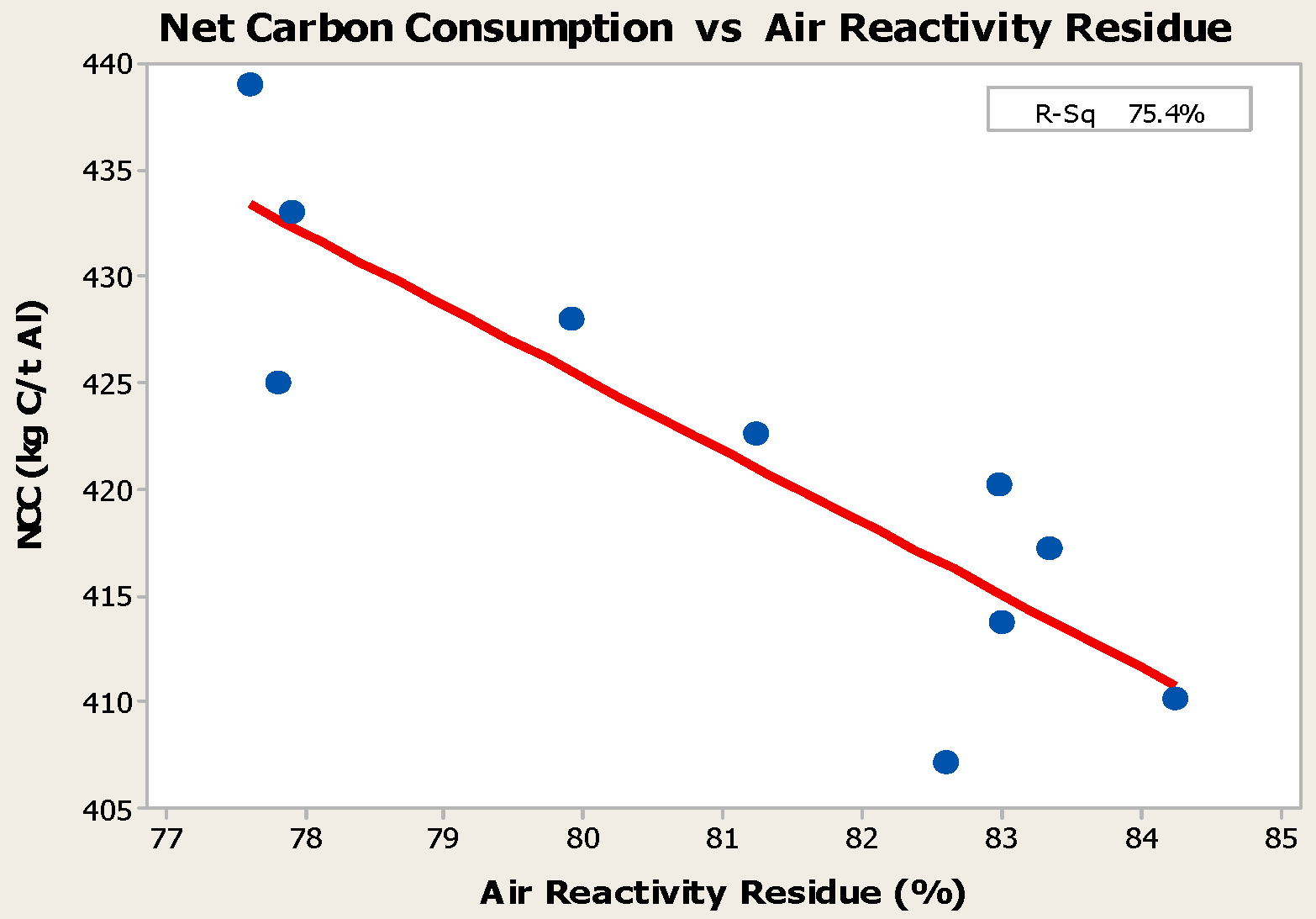
- Anodes with higher metallic impurity content have lower CO2 reactivity residue and higher CO2 reactivity dust, which increase net carbon consumption.
- Higher baking temperatures favor desulfurization of anodes. Desulfurization of anodes lowers CO2 reactivity residue while increasing air permeability, thereby resulting in increased net carbon consumption.
- Pitch addition, impurity content, and desulfurization need to be optimized by adjusting anode manufacturing parameters to reduce net carbon consumption.
Author Contributions
Conflicts of Interest
References
- Rhedey, P. A Review of Factors Affecting Carbon Anode Consumption in the Electrolytic Production of Aluminum. In Light Metals; TMS: Warrendale, PA, USA, 1971; pp. 385–408. [Google Scholar]
- Fisher, W.K.; Keller, F.; Perruchoud, R.C. Interdependence between Anode Net Consumption and Pot Design, Pot Operating Parameters and Anode Properties. In Light Metals; TMS: Warrendale, PA, USA, 1991; pp. 681–686. [Google Scholar]
- Sadler, B.A.; Algie, S.H. A Porosimetric Study of Sub-Surface Carboxy Oxidation in Anodes. In Light Metals; TMS: Warrendale, PA, USA, 1991. [Google Scholar]
- Jones, S.S.; Hildebrandt, R.D. Anode Carbon Reactivity. In Light Metals; TMS: Warrendale, PA, USA, 1974; pp. 901–932. [Google Scholar]
- Jones, S.S.; Hildebrand, R.D.; Hedlund, M.C. Influence of High-Sulphur Cokes on Anode Performance. In Light Metals; TMS: Warrendale, PA, USA, 1979; pp. 553–574. [Google Scholar]
- Belitskus, D.; Danka, D. A Comprehensive Determination of Effects of Calcined Petroleum Coke Properties. In Light Metals; TMS: Warrendale, PA, USA, 1989; pp. 429–439. [Google Scholar]
- Coste, B.; Schneider, J.P. Influence of Coke Real Density on Anode Reactivity Consequence on anode Baking. In Light Metals; TMS: Warrendale, PA, USA, 1994; pp. 583–591. [Google Scholar]
- Dreyer, C. Anode Reactivity: Influence of the Baking Process. In Light Metals; TMS: Warrendale, PA, USA, 1989; pp. 595–602. [Google Scholar]
- Rolle, J.G.; Hoang, Y.K. Studies of the Impact of Vanadium and Sodium on the Air Reactivity of Coke and Anodes. In Light Metals; TMS: Warrendale, PA, USA, 1995; pp. 741–745. [Google Scholar]
- Ken Ries, F.V.; Smith, M. Anode Desulfurization in Baking. In Light Metals; TMS: Warrendale, PA, USA, 1995; pp. 691–700. [Google Scholar]
- Dreyer, C.; Samanos, B.; Vogt, F. Coke Calcination Levels and Aluminum Anode Quality. In Light Metals; TMS: Warrendale, PA, USA, 1996; pp. 535–542. [Google Scholar]
- Edwards, L.C.; Neyrey, K.J.; Lossius, L. Coke and Anode Desulfurization. In Light Metals; TMS: Warrendale, PA, USA, 2007; pp. 895–900. [Google Scholar]
- Abbas, H.; Khaji, K.; Sulaiman, D. Desulphurization Control during Baking: Its Impact on Anode Performance and Operational Cost—ALBA’s Experience. In Light Metals; TMS: Warrendale, PA, USA, 2010; pp. 1011–1014. [Google Scholar]
- Khaji, K.; Qassemi, M.A. Factors Influencing Baked Anode Properties. In Light Metals; TMS: Warrendale, PA, USA, 2015; pp. 1135–1141. [Google Scholar]
- Samanos, B.; Dreyer, C. Impact of Coke Calcination Level and Baking Temperature on Anode Properties. In Light Metals; TMS: Warrendale, PA, USA, 2001; pp. 681–688. [Google Scholar]
- Lhuissier, J.; Bezamanifary, L.; Gendre, M.; Chollier, M. Use of Under Calcined Coke for the Production of Low Reactivity Anodes. In Light Metals; TMS: Warrendale, PA, USA, 2009. [Google Scholar]
- Sulaiman, D.; Garg, R. Use of Under Calcined Coke to Produce Baked Anodes for Aluminium Reduction Lines. In Light Metals; TMS: Warrendale, PA, USA, 2012; pp. 1147–1151. [Google Scholar]






| Mechanism | Anode Consumption, Mass % Prebaked Cells |
|---|---|
| Basic reaction: 2Al2O3 + 3C = 4Al + 3CO2 | 66–76 |
| Excess consumption: C + O2 = CO2 and 2C + O2 = 2CO | 8–15 |
| Boudouard reaction: CO2 + C = 2CO | 5–6 |
| Unreacted dust | 0.3 |
| Re-oxidation of metal | 7–8 |
| Pyrolysis and vaporisation | 0.2 |
| Sulphur, metal impurities and carbon loss by butts return | 3.5–4.5 |
| Net carbon consumption [kg C/t Al] | 400–450 |
| Analysis | Unit | Coke A | Coke B | Coke C |
|---|---|---|---|---|
| Average | Average | Average | ||
| Fe | % | 0.014 | 0.007 | 0.002 |
| Si | % | 0.014 | 0.001 | 0.003 |
| S | % | 2.57 | 1.05 | 2.83 |
| V | % | 0.024 | 0.005 | 0.019 |
| Ni | % | 0.022 | 0.008 | 0.009 |
| Ca | % | 0.013 | 0.001 | 0.001 |
| Na | % | 0.006 | 0.004 | 0.002 |
| Ash | % | 0.19 | 0.05 | 0.08 |
| Moisture | % | 0.06 | 0.08 | 0.08 |
| Real density | g/cm³ | 2.081 | 2.072 | 2.079 |
| Vibrated bulk density (VBD) | g/cm³ | 0.938 | 0.925 | 0.864 |
| Volatile matter (VM) | % | 0.40 | 0.44 | 0.42 |
| Hard grove index (HGI) | no | 31.5 | 36.0 | 31.8 |
| +4 Mesh | % | 36.3 | 27.4 | 35.3 |
| −20 Mesh | % | 24.6 | 26.1 | 22.9 |
| CO2 reactivity | % | 12.2 | 7.0 | 2.7 |
| Air reactivity | %/min | 0.29 | 0.07 | 0.07 |
| Electrical resistivity | μΩ·m | 464.4 | 441.5 | 492.8 |
| Lc | nm | 3.09 | 2.88 | 3.05 |
| Calciner type | Shaft | Rotary | Rotary |
| Parameter | Unit | Value |
|---|---|---|
| Dry aggregate GSR | No. | 3.5 to 4.5 |
| Pitch content | % | 12.5 to 14.5 |
| Mixer energy | kWh/t paste | 7.5 |
| Vibro-compaction time | s | 45 to 55 |
| Top tool pressure | kPa | 250 to 450 |
| Vacuum | kPa | 2 to 3 |
| Baking temperature | °C | 1160 to1190 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaji, K.; Al Qassemi, M. The Role of Anode Manufacturing Processes in Net Carbon Consumption. Metals 2016, 6, 128. https://doi.org/10.3390/met6060128
Khaji K, Al Qassemi M. The Role of Anode Manufacturing Processes in Net Carbon Consumption. Metals. 2016; 6(6):128. https://doi.org/10.3390/met6060128
Chicago/Turabian StyleKhaji, Khalil, and Mohammed Al Qassemi. 2016. "The Role of Anode Manufacturing Processes in Net Carbon Consumption" Metals 6, no. 6: 128. https://doi.org/10.3390/met6060128





