Vaporization and Poor Wettability as the Main Challenges in Fabrication of TiB2-Cu Cermets Studied by SPS
Abstract
:1. Introduction
1.1. TiB2-Cu Cermets Produced by Liquid Phase Sintering Using Spark Plasma Sintering (SPS)
1.2. Wettability and Contact Angle
1.3. Capillary Forces
1.4. Effect of Contaminations on the Densification of TiB2
1.5. Chemical Stability and Vaporization of Copper
1.6. Goal of the Present Research
2. Results and Discussion
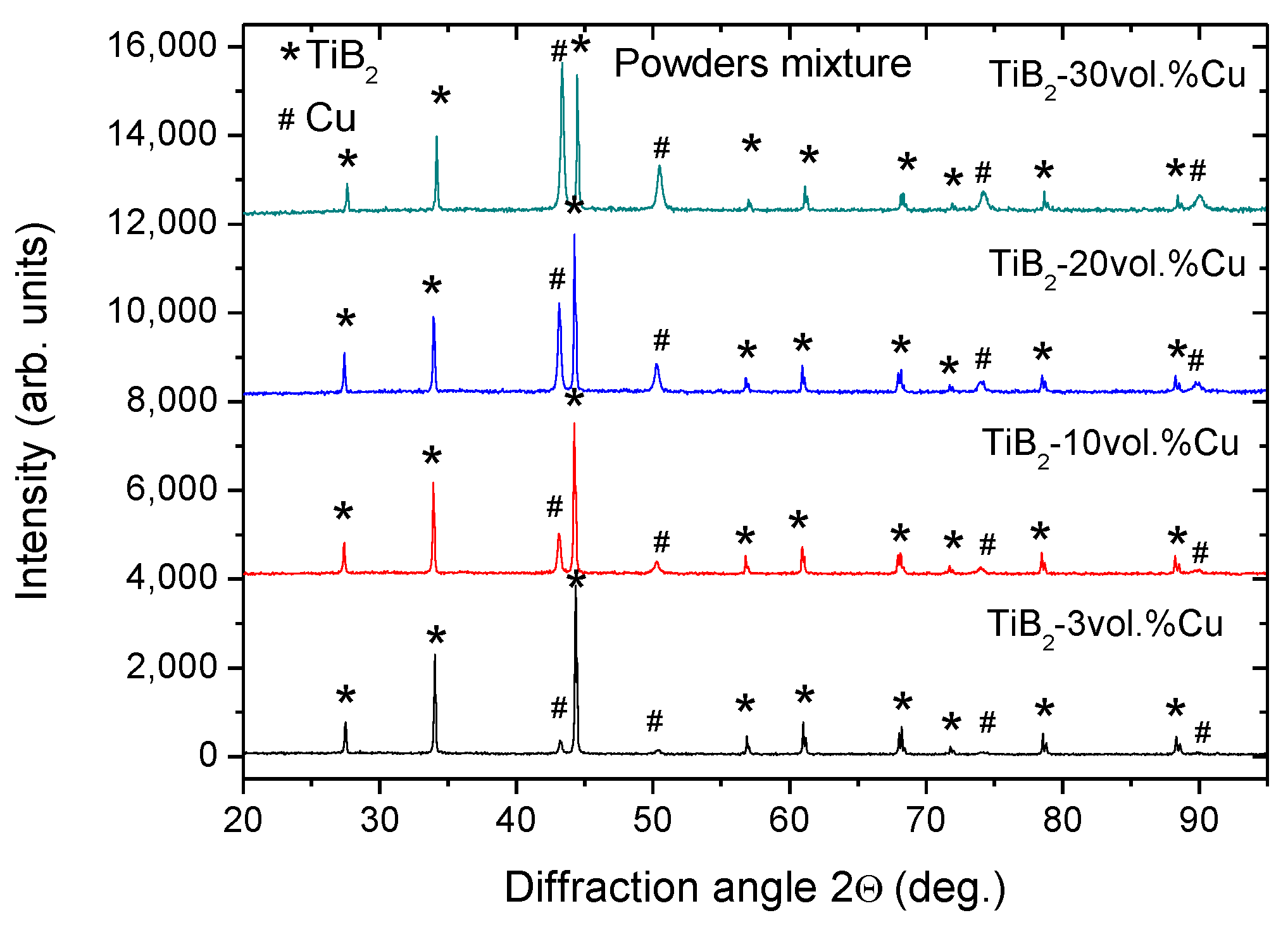
2.1. Effect of Sintering Temperature and Composition on Cu Vaporization

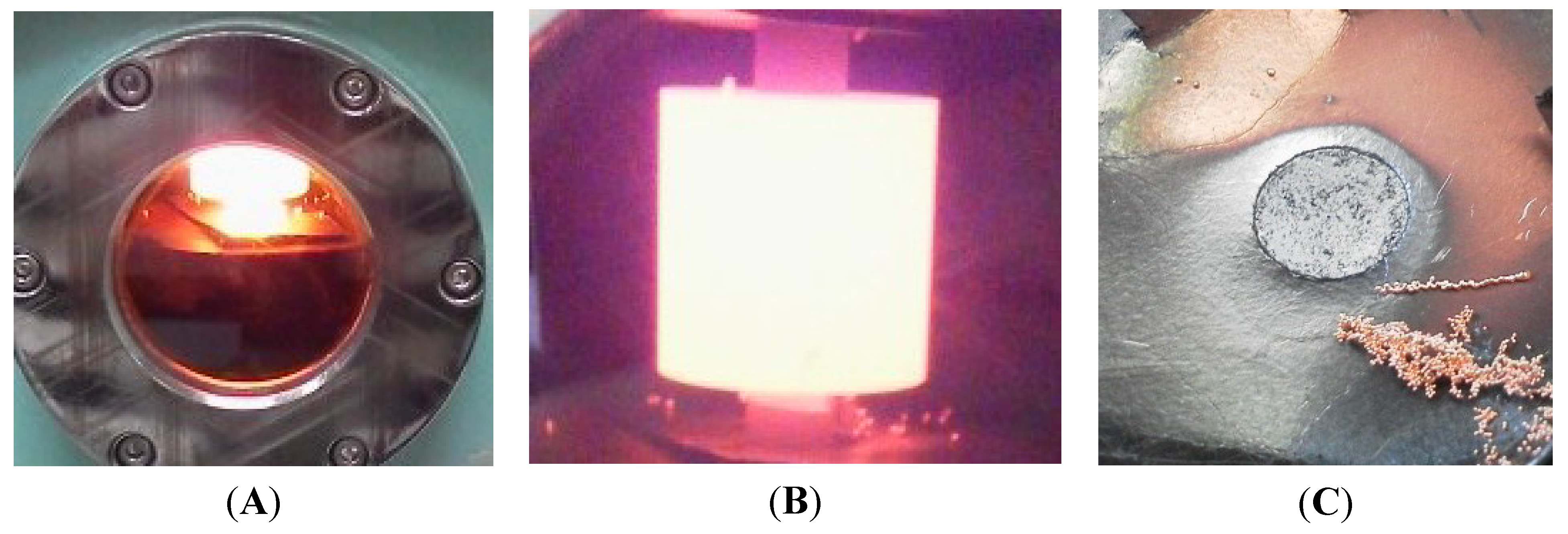

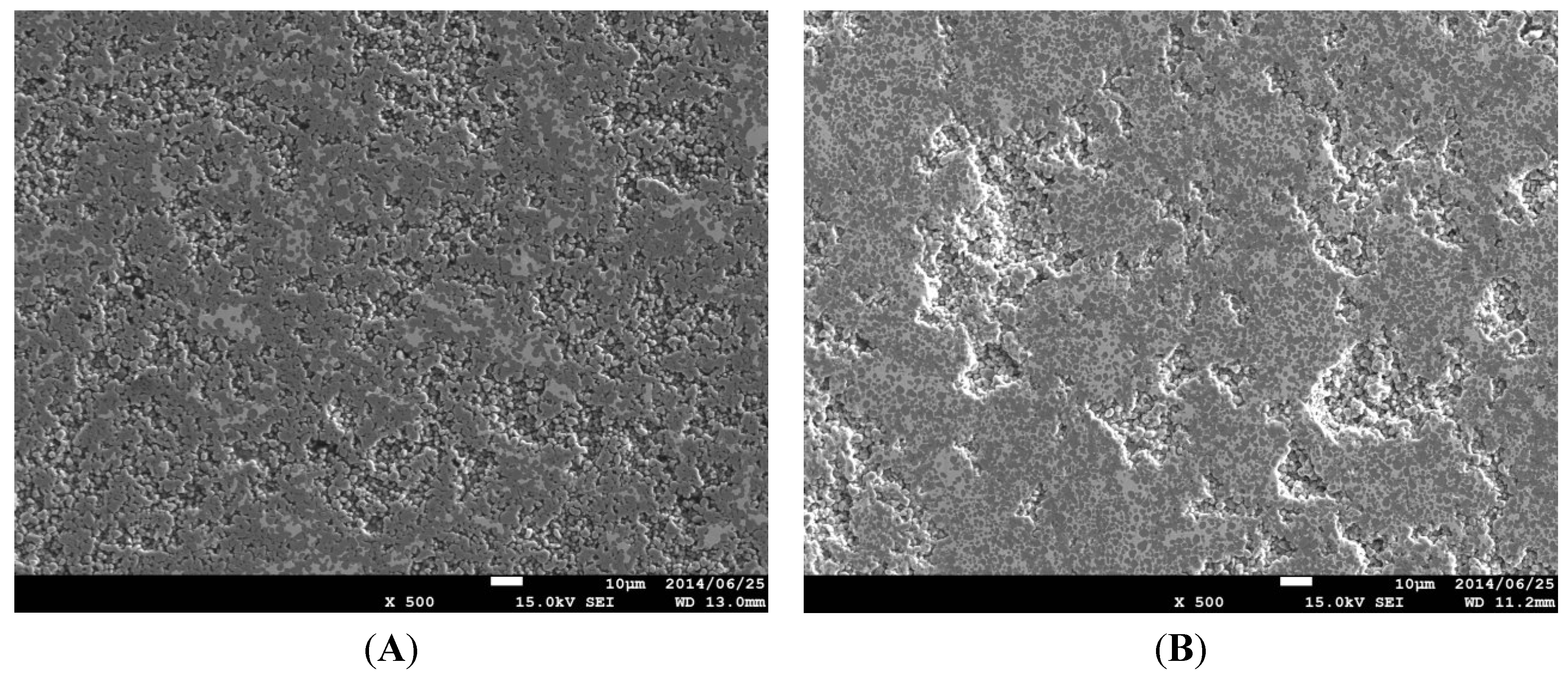
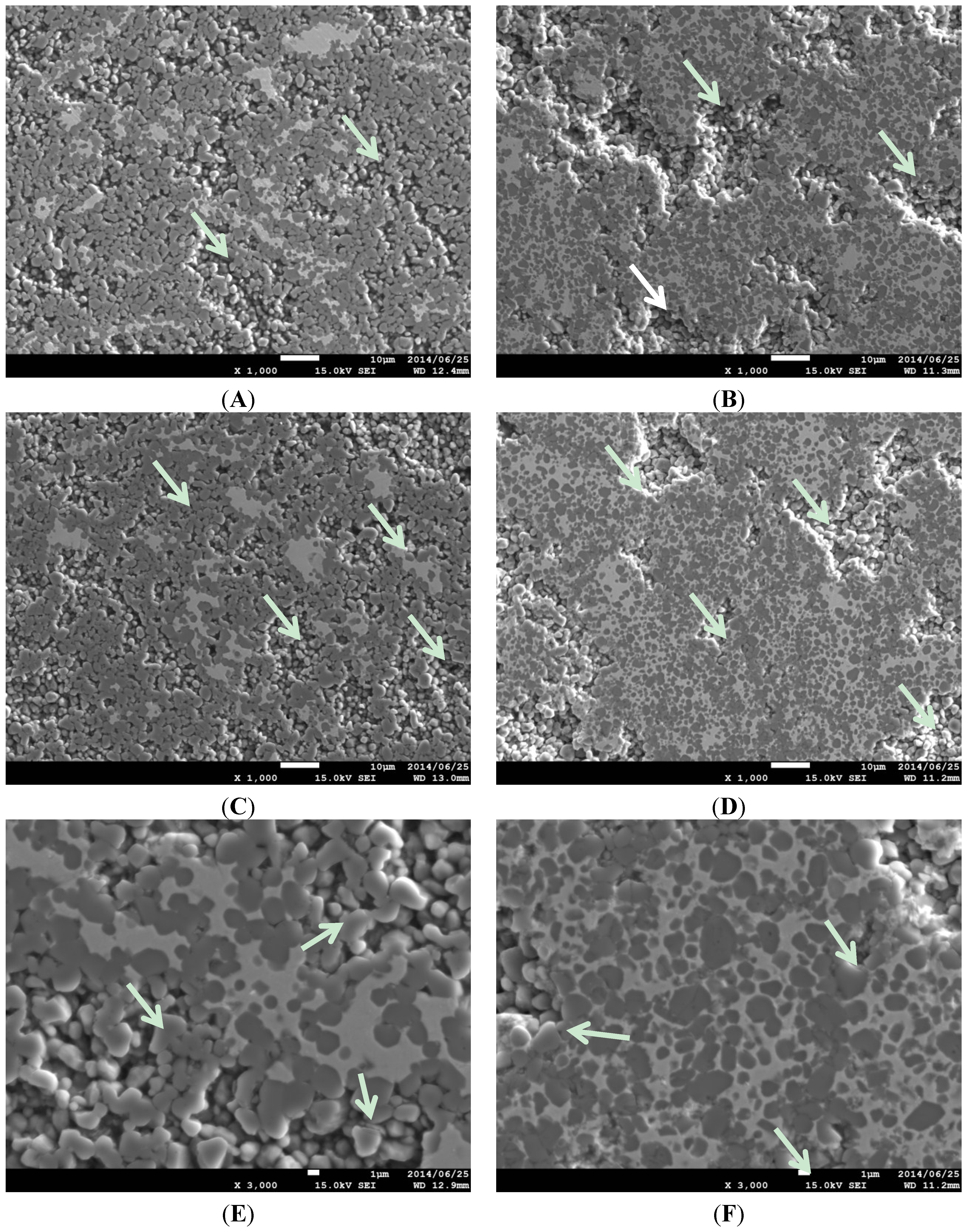
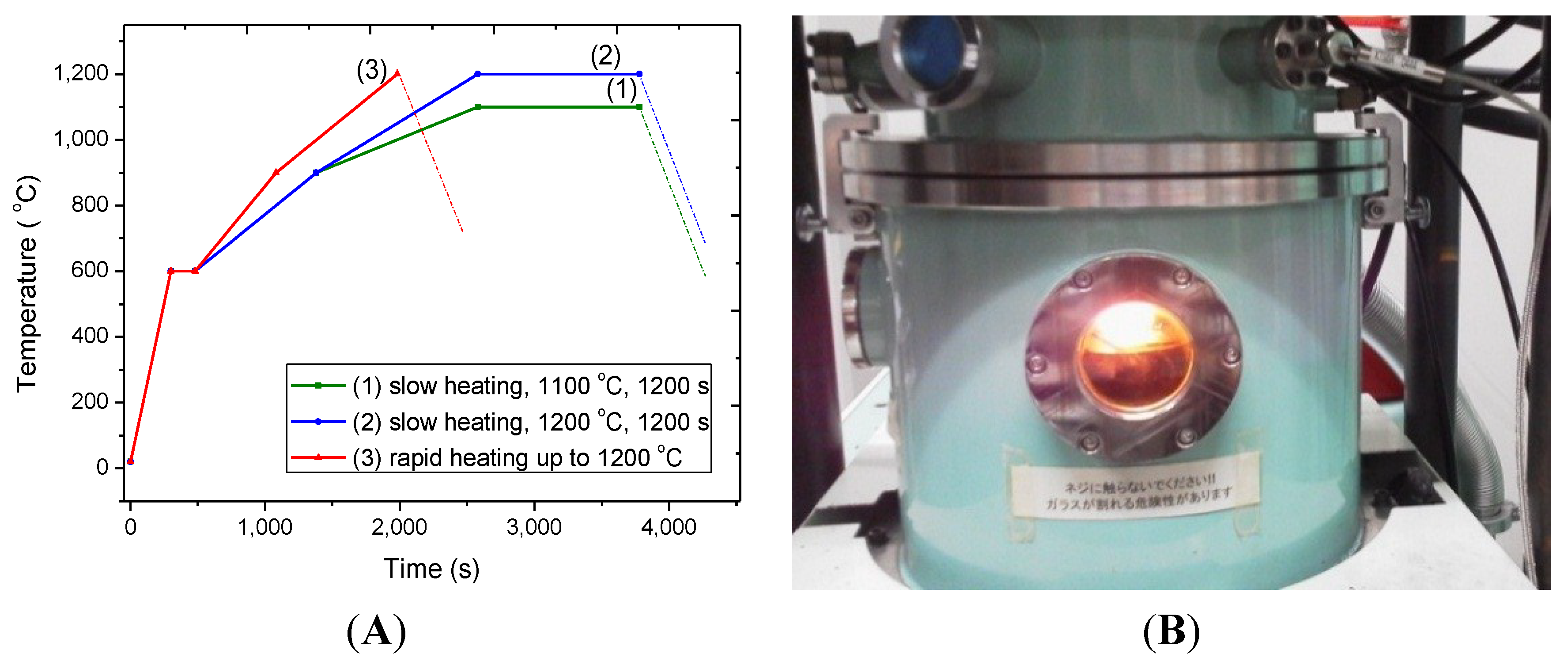
2.2. Effect of Heating Rate in SPS on the Cu evaporation
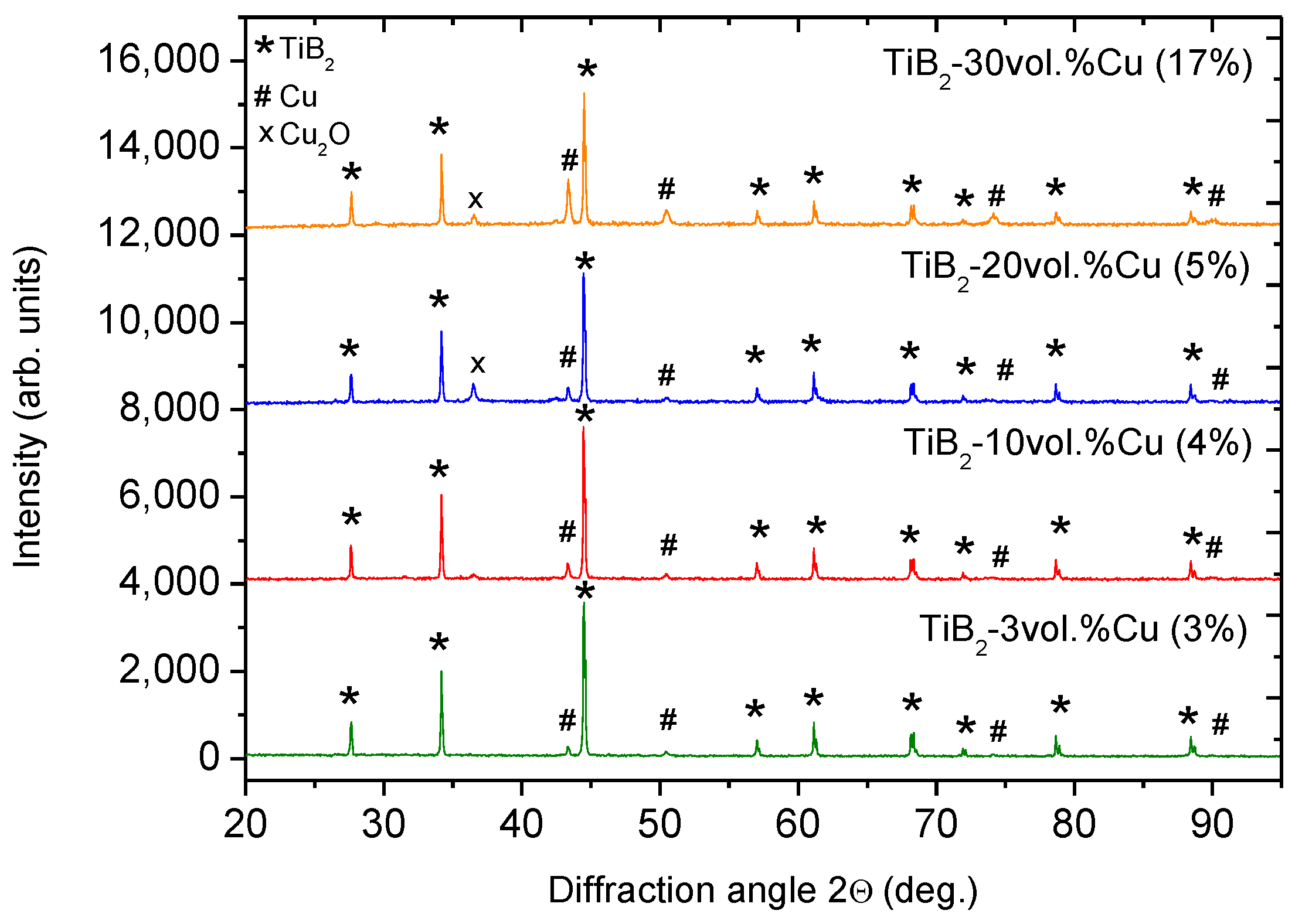
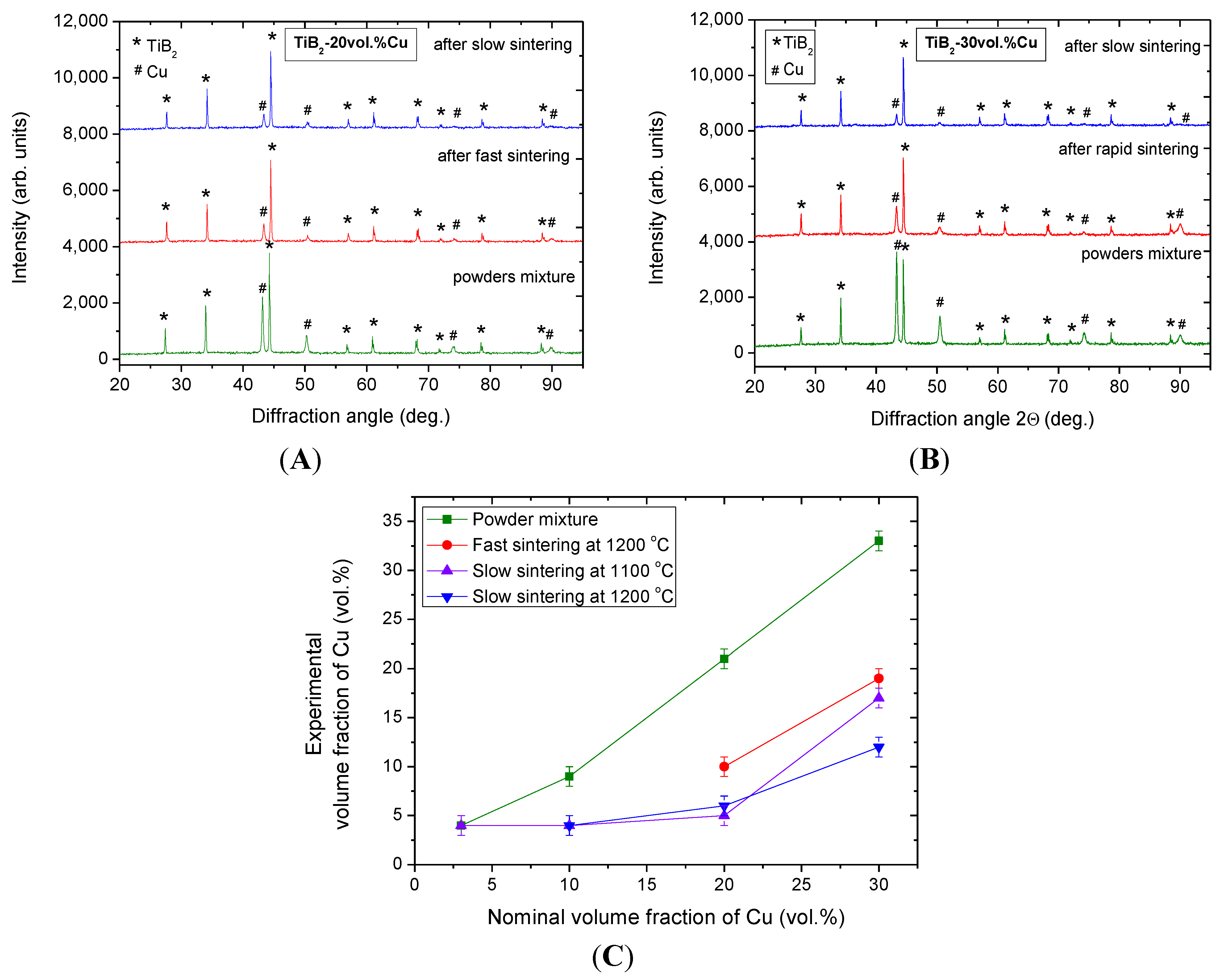
| Nominal (designed) composition of cermet (vol. % of Cu) | Cu vol. % in powder mixture before SPS | Cu vol. % after low hearing rate | Cu vol. % after rapid sintering | |
|---|---|---|---|---|
| Sintering at 1100 °C | Sintering at 1200 °C | |||
| TiB2-3 vol. % Cu | 4 | 3 (75.0%) | - | - |
| TiB2-10 vol. % Cu | 9 | 4 (44.4%) | 4 (44.4%) | - |
| TiB2-20 vol. % Cu | 21 | 5 (23.8%) | 6 (28.6%) | 10 (47.6%) |
| TiB2-30 vol. % Cu | 33 | 17 (51.5%) | 12 (36.4%) | 19 (57.6%) |
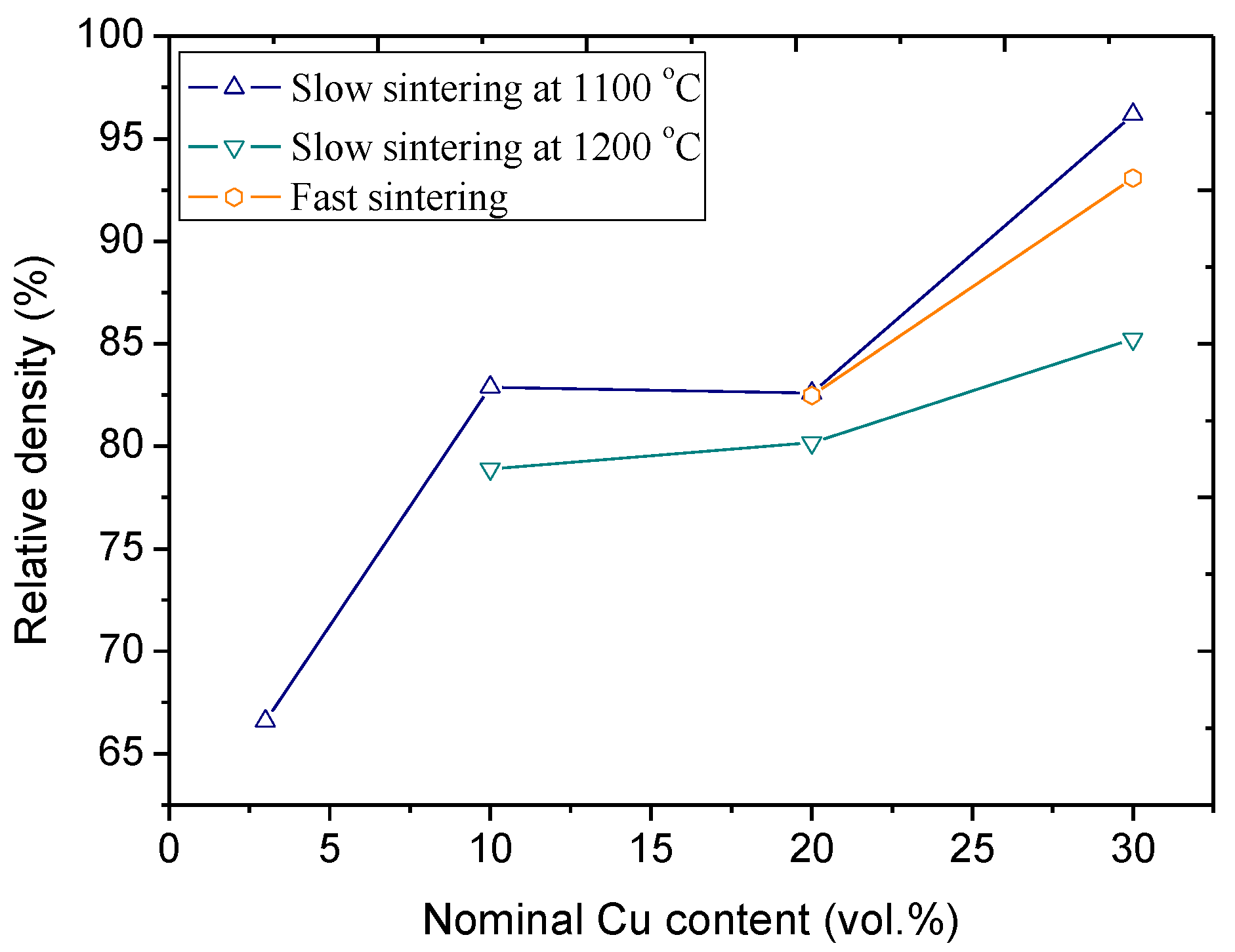
2.3. Discussion
3. Experimental Section
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dong, L.; Tao, X.; Hamdi, M.; Zhang, L.; Zhang, X.; Ferreira, A.; Nelson, B.J. Nanotube boiler: Attogram copper evaporation driven by electric current, Joule heating, charge and ionization. IEEE Trans. Nanotechnol. 2009, 8, 565–568. [Google Scholar] [CrossRef]
- Eymann, K.; Riedl, T.; Bram, A.; Ruhnow, M.; Kirchner, A.; Kieback, B. Consolidation of mechanically alloyed nanocrystalline Cu-Nb-ZrO2 powder by spark plasma sintering. J. Alloy. Compd. 2012, 535, 62–69. [Google Scholar] [CrossRef]
- Sharma, A.S.; Mishra, N.; Biswas, K.; Basu, B. Fretting wear study of Cu-10 wt. % TiB2 and Cu-10 wt. % TiB2-10 wt. % Pb composites. Wear 2013, 306, 138–148. [Google Scholar] [CrossRef]
- Sobhani, M.; Mirhabibi, A.; Arabi, H.; Brydson, R.M.D. Effect of in situ formation of TiB2 particles on age hardening behavior of Cu-1 wt. % Ti-1 wt. % TiB2. Mater. Sci. Eng. A 2013, 577, 16–22. [Google Scholar] [CrossRef]
- Lopez, M.; Corredor, D.; Camurri, C.; Vergara, V.; Jimenez, J. Performance and characterization of dispersion strengthened Cu-TiB2 composite for electrical use. Mater. Charact. 2005, 55, 252–262. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, C.; Han, J.; Zhang, H. Microstructure and mechanical properties of TiB2/(Cu,Ni) interpenetrating phase composites. Scr. Mater. 2006, 55, 565–568. [Google Scholar] [CrossRef]
- Li, G.; Ziemnicka-Sylwester, M. The TiB2-based Fe-matrix composites fabricated using elemental powders in one step process by means of SHS combined with pseudo-HIP. Int. J. Refract. Metals Hard Mater. 2014, 45, 141–146. [Google Scholar] [CrossRef]
- Ziemnicka-Sylwester, M.; Matsuura, K.; Ohno, M. Phase evolution, microstructure and hardness of TiB2-based Co-containing composite by SHS under pseudo-isostatic pressure. ISIJ Int. 2012, 9, 1698–1704. [Google Scholar] [CrossRef]
- Ziemnicka-Sylwester, M. The Cu matrix cermets remarkably strengthened by TiB2 “in situ” synthesized via self-propagating high temperature synthesis. Mater. Des. 2014, 53, 758–765. [Google Scholar] [CrossRef]
- Venkateswaran, T.; Basu, B.; Raju, G.B.; Kim, D.Y. Densification and properties of transition metal borides-based cermets via spark plasma sintering. J. Eur. Ceram. Soc. 2006, 26, 2431–2440. [Google Scholar] [CrossRef]
- Sigl, L.S.; Schwetz, K.A.; Dworak, U. Continuous turning with TiB2 cermets: Preliminary cutting tests. Int. J. Refract. Metals Hard Mater. 1993, 12, 95–99. [Google Scholar] [CrossRef]
- Biswas, K.; Sharma, A.S.; Basu, B. On the densification mechanisms and properties of Cu–Pb and Cu–Pb–TiB2 nanocomposites densified using spark plasma sintering. Scr. Mater. 2013, 69, 122–126. [Google Scholar] [CrossRef]
- Ritasalo, R.; Cura, M.E.; Liu, X.W.; Soderberg, O.; Ritvonen, T.; Hannula, S.-P. Spark plasma sintering of submicron-sized Cu powder—Influence of processing parameters and powder oxidization on microstructure and mechanical properties. Mater. Sci. Eng. A 2010, 527, 2733–2737. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, F.C.; Lee, S.K.; Liu, Y.; Cheng, J.W.; Liang, Y. Microstructure characteristic, mechanical properties and sintering mechanism of nanocrystalline copper obtained by SPS process. Mater. Sci. Eng. A 2009, 523, 134–138. [Google Scholar] [CrossRef]
- Brown, C.W. The wettability of TiB2-based cathodes in low-temperature slurry-electrolyte reduction cells. Alum. Innov. Part. III JOM 1998, 50, 38–40. [Google Scholar]
- Zhang, X.H.; Yan, C.; Yu, Z.Z. In-situ combustion synthesis of ultrafine TiB2 particles reinforced Cu matrix composites. J. Mater. Sci. 2004, 39, 4683–4685. [Google Scholar] [CrossRef]
- Nicholas, M.G.; Mortimer, D.A.; Jones, L.M.; Crispin, R.M. Some observations on the wetting and bonding of nitride ceramics. J. Mater. Sci. 1990, 25, 2679–2689. [Google Scholar] [CrossRef]
- Mattia, D.; Desmaison-Brut, M.; Tetard, D.; Desmaison, J. Wetting of HIP AlN-TiB2 ceramic composites by liquid metals and alloys. J. Eur. Ceram. Soc. 2005, 25, 1797–1803. [Google Scholar] [CrossRef]
- Ziemnicka-Sylwester, M. Superhard TiB2-based composites with different matrix fabricated from elemental powders by SHS-p-HIP. Adv. Sci. Technol. 2012, 77, 146–152. [Google Scholar] [CrossRef]
- Ahangarkani, M.; Borgi, S.; Abbaszadeh, H.; Rahmani, A.A.; Zangeneh-Madar, K. The effect of additive and sintering mechanism on the microstructural characteristics of W-40Cu composites. Int. J. Refract. Metals Hard Mater. 2012, 32, 39–44. [Google Scholar] [CrossRef]
- Aizenshtein, M.; Frage, N.; Froumin, N.; Shipiro-Tsoref, E.; Dariel, M.P. Interface interaction in the (B4C + TiB2)/Cu system. J. Mater. Sci. 2006, 41, 5185–5189. [Google Scholar] [CrossRef]
- Hwang, K.S.; German, R.M.; Lenel, F.V. Capillary forces between spheres during agglomeration and liquid phase sintering. Metall. Trans. A 1987, 18A, 11–17. [Google Scholar] [CrossRef]
- Gessinger, G.H.; Fischmeister, H.F.; Lukas, H.L. A model for second-stage liquid-phase sintering with a partially wetting liquid. Acta Metall. 1973, 21, 715–724. [Google Scholar] [CrossRef]
- Xu, K.; Mehrabadi, M.M. A micromechanical model for the initial rearrangement stage of liquid phase sintering. Mech. Mater. 1997, 25, 137–157. [Google Scholar] [CrossRef]
- Huppmann, W.J.; Riegger, H. Modeling of rearrangement process in liquid phase sintering. Acta Metall. 1975, 23, 965–971. [Google Scholar] [CrossRef]
- Baik, S.; Becher, P.F. Effect of oxygen contamination on densification of TiB2. J. Am. Ceram. Soc. 1987, 70, 527–530. [Google Scholar] [CrossRef]
- Lee, B.S.; Barnes, M.C.; Kim, D.Y.; Hwang, N.M. Spontaneous generation of charged clusters of a few nanometers during thermal evaporation of copper. J. Cryst. Growth 2002, 234, 599–602. [Google Scholar] [CrossRef]
- Yoon, S.; Dornseiffer, J.; Xiong, Y.; Gruner, D.; Shen, Z.; Iwaya, S.; Pithan, C.; Waser, R. Synthesis, spark plasma sintering and electrical conduction mechanism in BaTiO3-Cu composites. J. Eur. Ceram. Soc. 2011, 31, 773–782. [Google Scholar] [CrossRef]
- Liu, H.; Kuo, C. Quantitative multiphase determination using the Rietveld method with high accuracy. Mater. Lett. 1996, 26, 171–175. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziemnicka-Sylwester, M. Vaporization and Poor Wettability as the Main Challenges in Fabrication of TiB2-Cu Cermets Studied by SPS. Metals 2014, 4, 623-638. https://doi.org/10.3390/met4040623
Ziemnicka-Sylwester M. Vaporization and Poor Wettability as the Main Challenges in Fabrication of TiB2-Cu Cermets Studied by SPS. Metals. 2014; 4(4):623-638. https://doi.org/10.3390/met4040623
Chicago/Turabian StyleZiemnicka-Sylwester, Marta. 2014. "Vaporization and Poor Wettability as the Main Challenges in Fabrication of TiB2-Cu Cermets Studied by SPS" Metals 4, no. 4: 623-638. https://doi.org/10.3390/met4040623




