Innovative Integration of Citric Acid Leaching and Electrodialysis for Selective Lithium Recovery from NMC Cathode Material
Abstract
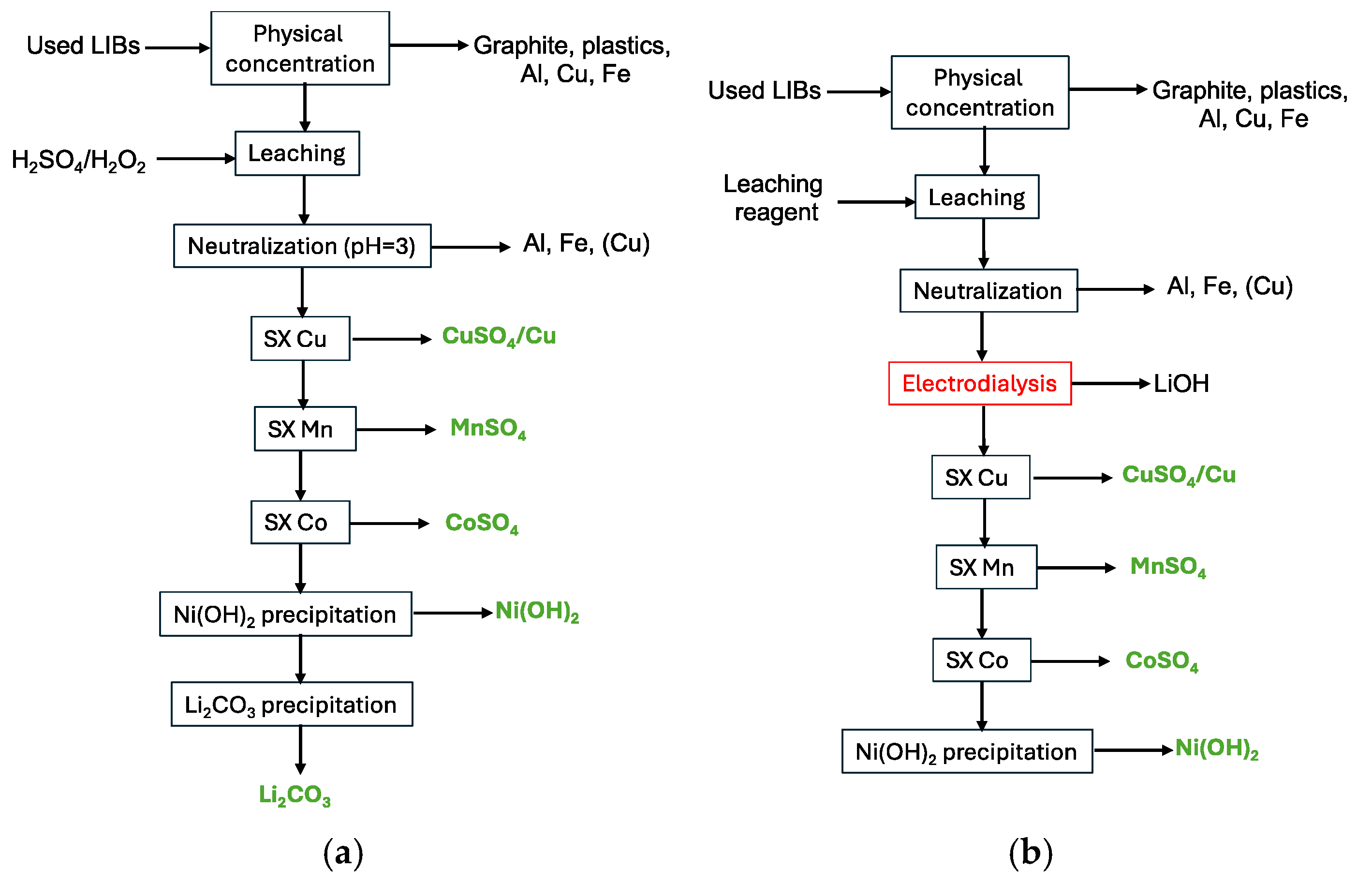
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Analyses
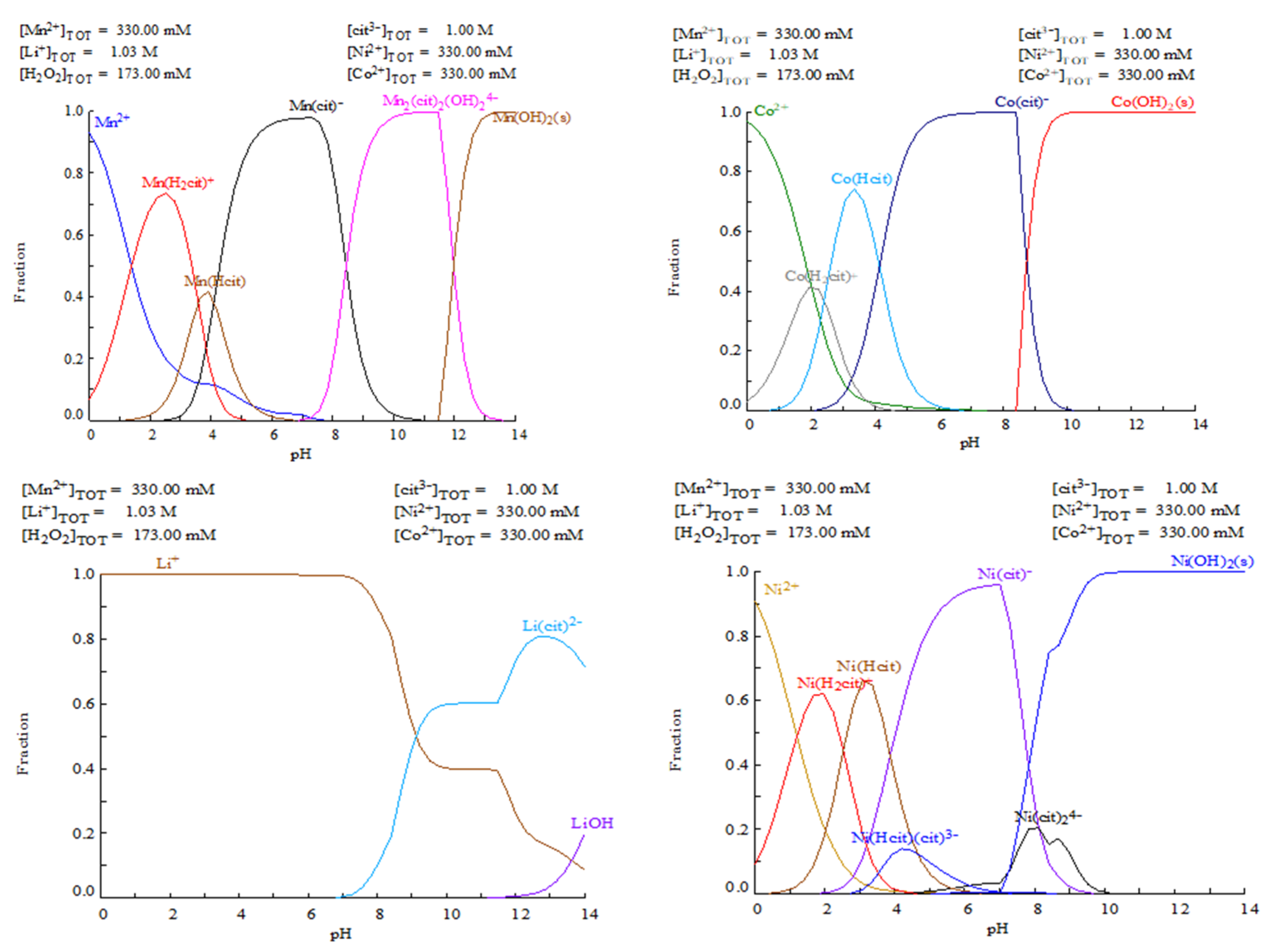
2.2.2. Speciation Calculations
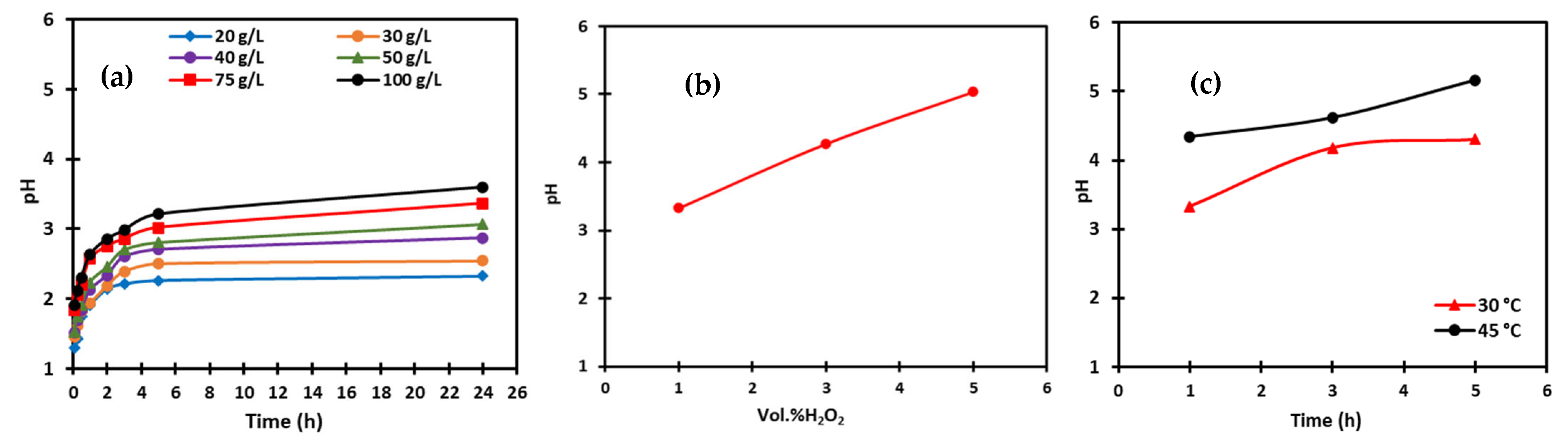
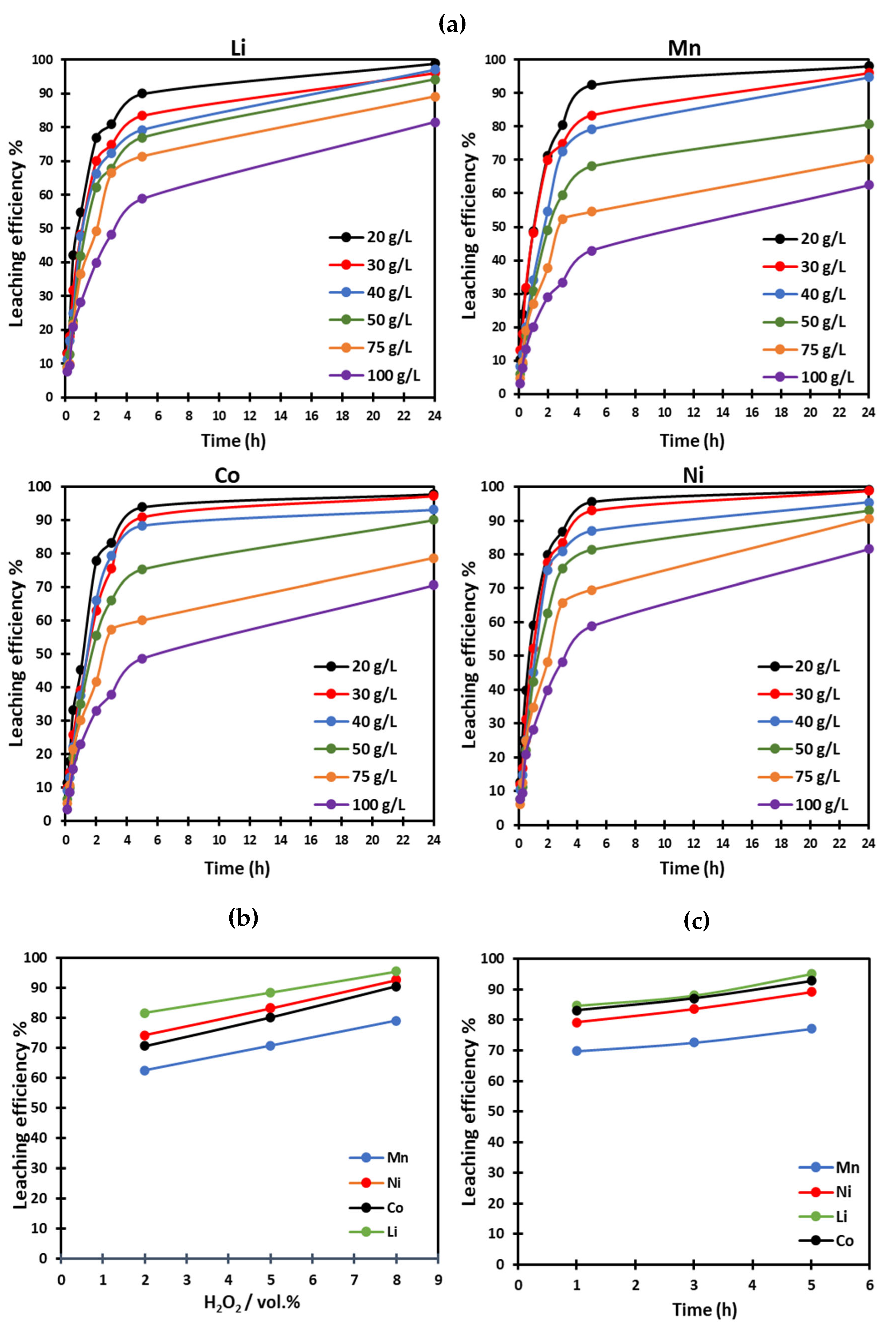
2.2.3. Leaching
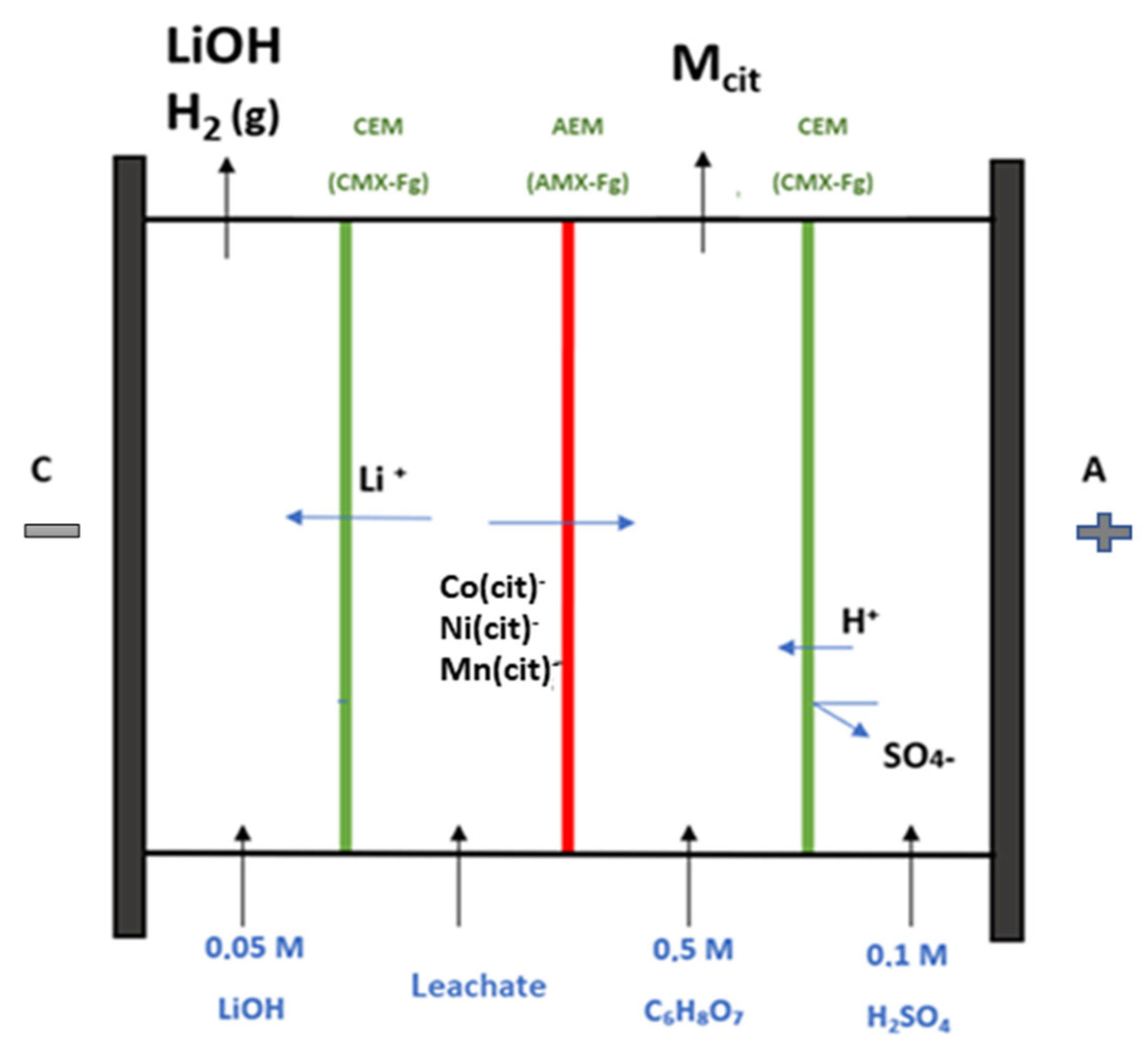
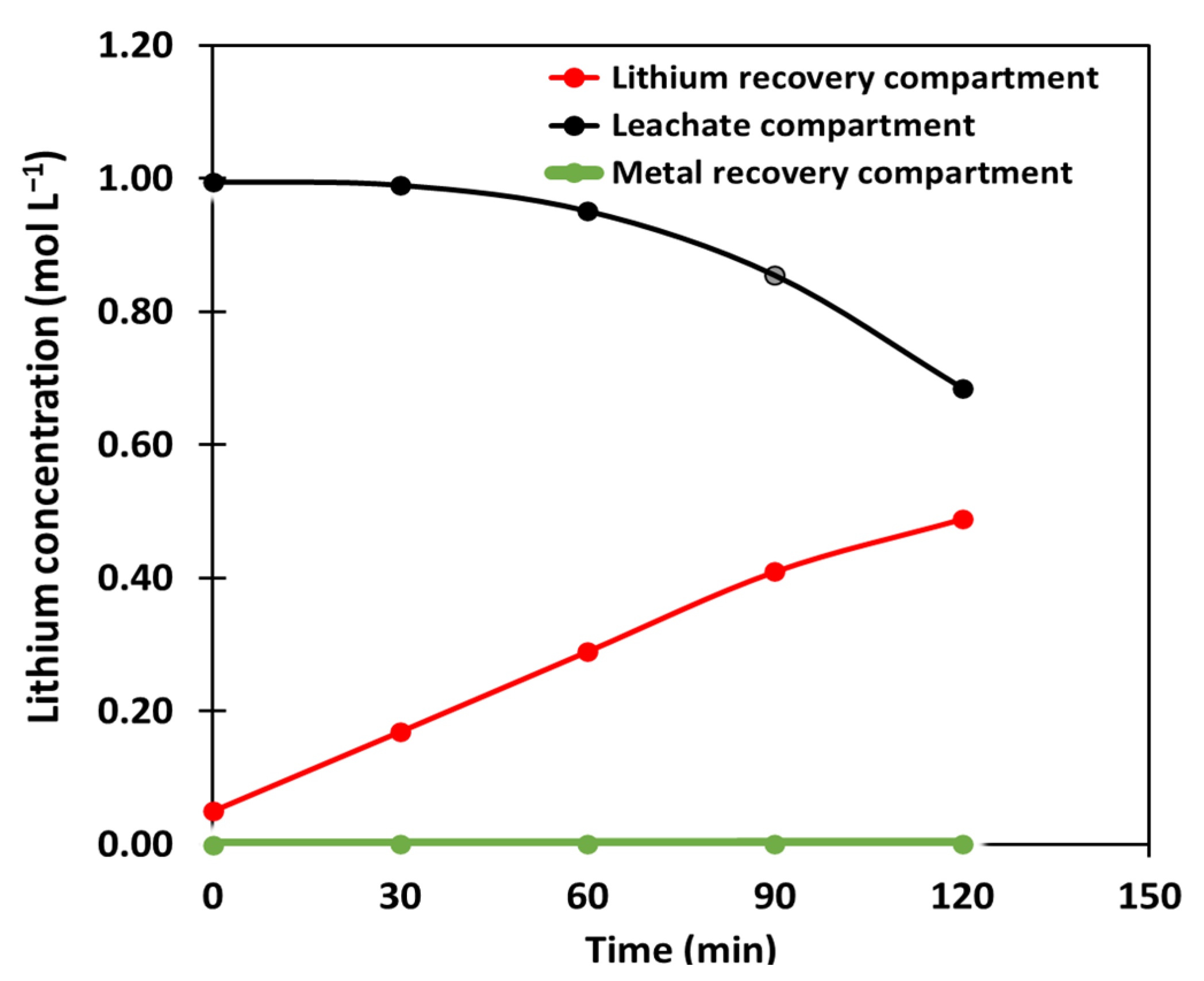
2.2.4. Electrodialysis
3. Results and Discussion
3.1. Speciation Calculations
+ 2 Mn (OA)2(aq)+ 2 Co(OA)2(aq) + 6 LiOA(aq) + 3 O2(g) + 12 H2O(l)
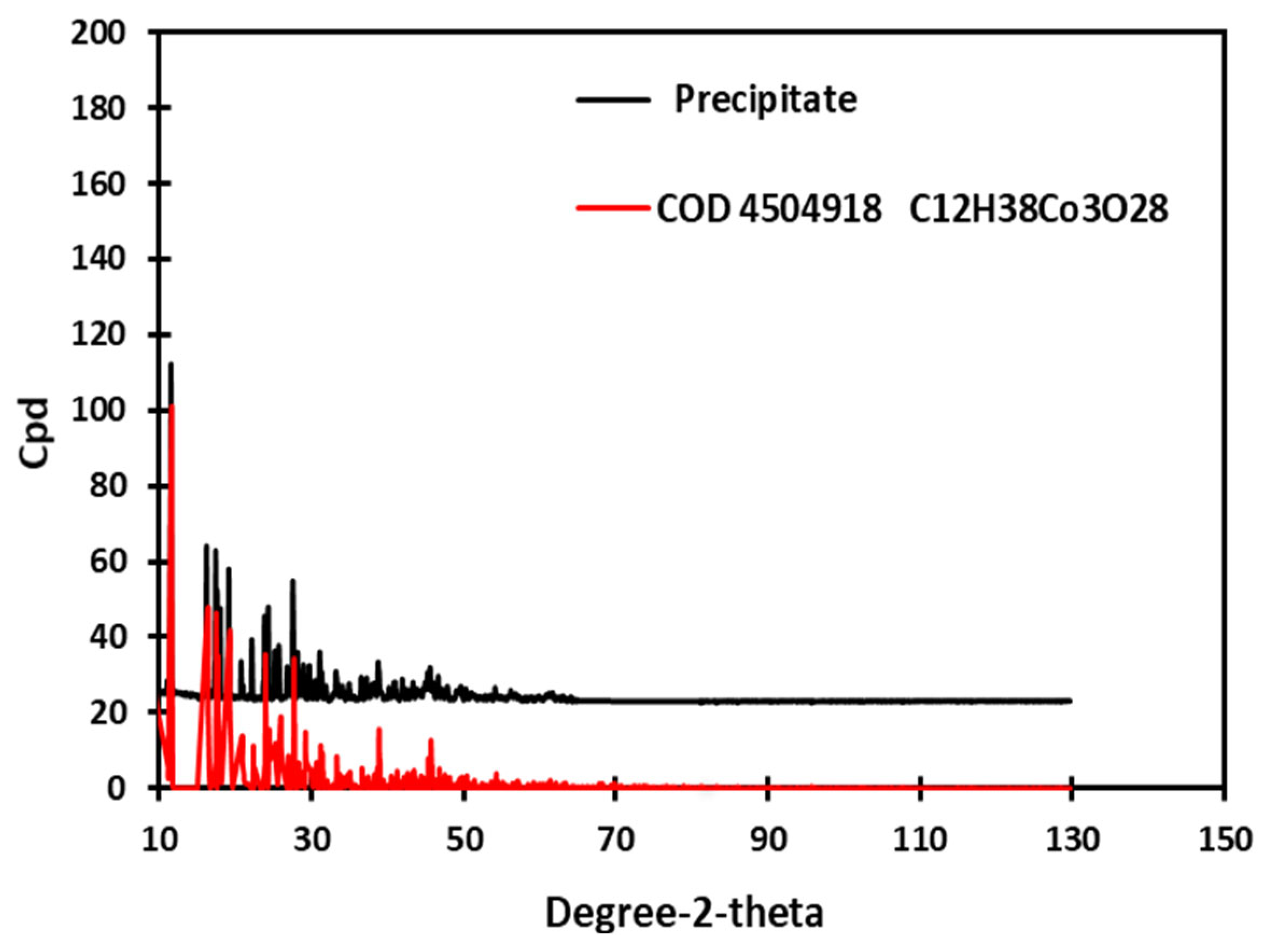
3.2. Crystallization After Leaching
3.3. Electrodialysis
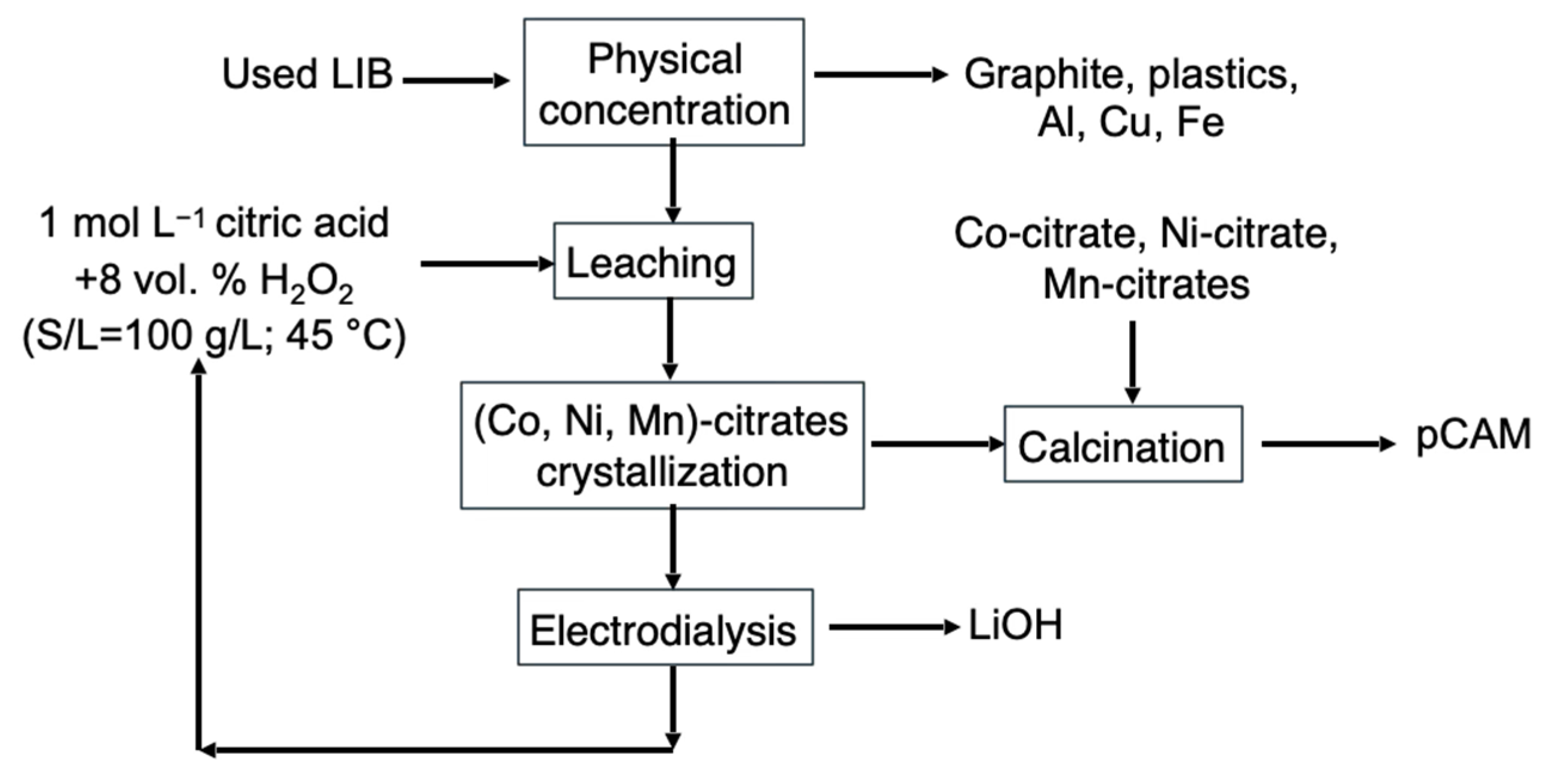
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Thomas, H.R.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Chagnes, A. Métallurgie Extractive-Hydrométallurgie; Techniques de l’ingénieur: Saint-Denis, France, 2022. (In French) [Google Scholar]
- Marcinov, V.; Klimko, J.; Takáčová, Z.; Pirošková, J.; Miškufová, A.; Sommerfeld, M.; Dertmann, C.; Friedrich, B.; Oráč, D. Lithium Production and Recovery Methods: Overview of Lithium Losses. Metals 2023, 13, 1213. [Google Scholar] [CrossRef]
- Chan, K.H.; Malik, M.; Azimi, G. Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis. Resour. Conserv. Recycl. 2022, 178, 106076. [Google Scholar] [CrossRef]
- Gmar, S.; Chagnes, A.; Lutin, F.; Muhr, L. Application of Electrodialysis for the Selective Lithium Extraction Towards Cobalt, Nickel and Manganese from Leach Solutions Containing High Divalent Cations/Li Ratio. Recycling 2022, 7, 14. [Google Scholar] [CrossRef]
- Afifah, D.N.; Ariyanto, T.; Supranto, S.; Prasetyo, I. Separation of Lithium Ion from Lithium-Cobalt Mixture using Electrodialysis Monovalent Membrane. Eng. J. 2018, 22, 165–179. [Google Scholar] [CrossRef]
- Iizuka, A.; Yamashita, Y.; Nagasawa, H.; Yamasaki, A.; Yanagisawa, Y. Separation of lithium and cobalt from waste lithium-ion batteries via bipolar membrane electrodialysis coupled with chelation. Sep. Purif. Technol. 2013, 113, 33–41. [Google Scholar] [CrossRef]
- Siekierka, A.; Yalcinkaya, F. Selective cobalt-exchange membranes for electrodialysis dedicated for cobalt recovery from lithium, cobalt and nickel solutions. Sep. Purif. Technol. 2022, 299, 121695. [Google Scholar] [CrossRef]
- Shi, M.; Diaz, L.A.; Klaehn, J.R.; Wilson, A.D.; Lister, T.E. Li2 CO3 Recovery through a Carbon-Negative Electrodialysis of Lithium-Ion Battery Leachates. ACS Sustain. Chem. Eng. 2022, 10, 11773–11781. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z. Recovery of lithium from spent lithium-ion batteries using precipitation and electrodialysis techniques. Sep. Purif. Technol. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Arhoun, B.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Electrodialytic processes in solid matrices. New insights into battery recycling. A review. J. Chem. Technol. Biotechnol. 2019, 94, 1727–1738. [Google Scholar] [CrossRef]
- Gerold, E.; Schinnerl, C.; Antrekowitsch, H. Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling 2022, 7, 4. [Google Scholar] [CrossRef]
- Punt, T.; Van Wyk, A.P.; Bradshaw, S.M.; Akdogan, G. Citric Acid Leaching Performance at High Solid-to-Liquid Ratios for Lithium-Ion Battery Recycling. Min. Metall. Explor. 2024, 41, 3463–3474. [Google Scholar] [CrossRef]
- Richard, M. Recyclage Assisté des Métaux Stratégiques de Batteries Lithium-Ion Par Chauffage Micro-Ondes; HAL Theses: Bengaluru, India, 2022. (In French) [Google Scholar]
- Xuan, W. Développement d’un Procédé Hydrométallurgique Pour le Recyclage des Électrodes Positives de Type NMC Contenues dans les Batteries Lithium-Ion Usagées; HAL Theses: Bengaluru, India, 2022. (In French) [Google Scholar]
- Chen, X.; Zhou, T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 1083–1093. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Puigdomenech, I. MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms); School of Chemical Science and Engineering, Royal Institute of Technology: Stockholm, Sweden, 2013; Available online: https://www.kth.se/che/medusa/downloads-1.386254 (accessed on 24 May 2025).
- Loewenstein, A.; Roberts, J.D. The Ionization of Citric Acid Studied by the Nuclear Magnetic Resonance Technique. J. Am. Chem. Soc. 1960, 82, 2705–2710. [Google Scholar] [CrossRef]
- Gergoric, M.; Ravaux, C.; Steenari, B.-M.; Espegren, F.; Retegan, T. Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids. Metals 2018, 8, 721. [Google Scholar] [CrossRef]
- Francis, A.; Dodge, C. Bio/PhotoChemical Separation and Recovery of Uranium; Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2008. [Google Scholar]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Xuan, W.; Chagnes, A.; Xiao, X.; Olsson, R.T.; Forsberg, K. Antisolvent Precipitation for Metal Recovery from Citric Acid Solution in Recycling of NMC Cathode Materials. Metals 2022, 12, 607. [Google Scholar] [CrossRef]
- Yao, L.; Feng, Y.; Xi, G. A new method for the synthesis of LiNi1/3 Co1/3 Mn1/3 O2 from waste lithium ion batteries. RSC Adv. 2015, 5, 44107–44114. [Google Scholar] [CrossRef]
- Chagnes, A.; Forsberg, K. Raw Material Supply for Lithium-Ion Batteries in the Circular Economy. Metals 2023, 13, 1590. [Google Scholar] [CrossRef]
| Methods | Cathode Materials + Complexing Agent | Membrane Type | U and/or j | Flowrate | Complementary Information | Ref. |
|---|---|---|---|---|---|---|
| Conventional electrodialysis and selective electrodialysis | Leach solution of NMC111 cathodic materials: 0.07 g L−1 Li+, 0.2 g L−1 Co2+, 0.2 g L−1 Ni2+, 0.18 g L−1 Mn2+, and SO42− + EDTA | Neosepta® CMS membrane, Neosepta®AMX, and Neosepta® CMX | 18 V (Stage 1) 18 V (Stage 2) 3 V (Stage 3) | 0.75 L min− 1 | jLi+ = 0.165 mol/(m2/h) SLi+/Mn+ = 92% | [5] |
| Conventional electrodialysis | Leached solution produced from spent LiCoO2 [Li+] = [Co2+] = 0.02 mol L−1 + EDTA | 1 AEM (Selemion AMV), 1 CEM (Selemion CMV), and two bipolar membranes (Neosepta® BP-1E) | 20 V | 0.375 L min−1 | R (Li+) = R (Co2+) = 99% | [8] |
| Selective electrodialysis | Typical mixing solution for used NMC [Li] = 2.6 g L−1; [Co] = 7.88 g L−1 [Mn] = 8.01 g L−1; [Ni] = 4.4 g L−1 + Sulfuric acid H2SO4 | 2 MEA 1 MEC monovalent (Neosepta®) | 12.5 mA cm−2 | 100 mL min−1 | Rf(Li) = 67.1% P(Li/Co) = 5.6 P(Li/Ni) = 6.1 P(Li/Mn) = 5.4 | [6] |
| Selective electrodialysis | Soluble nitrate salts (LiNO3 + Co (NO3) 2·6H2O) [Li] = 0.1 g L−1; [Co] = 0.3 g L−1 | 5 MEC (PC-MVK) 5 MEA (PC-MVA) | 15 V 15 A/m2 | 15 L h −1 | S (%) = 99.4% Lithium purity = 95.73% | [7] |
| Selective electrodialysis | [Li] = 0.027 g L−1; [Co] = 0.108 g L−1; [Ni]= 0.049 g L−1 | PAN-5C8Q membrane | 5 V | 2.2 L.h−1 | jLi+ =0.047 mol/(m2/h) | [9] |
| Selective electrodialysis | Leached solution produced from spent lithium ion [Li] = 1.616 g L−1; [Co] = 0.059 g L−1; [Mn] = 1.149 g L−1 + Sulfuric acid H2SO4 | 1 MEC monovalent (Selemion CSO membrane) | 5 V | 17 cm3/min | Li2CO3 purity = 99.6% SEC(Li) = 0.5 kW h/g Rf(Li) = 48.4% | [10] |
| Conventional electrodialysis | Typical mixing solution for used NMC [Li] = 3.27 g L−1; [Co] = 0.25 g L−1; [Mn] = 0.28 g L−1; [Ni] = 0.25 g L−1 + Sodium phosphate Na3PO4 | 1 MEC (Nafion 117) | 3.5 V | - | SC(P/Li) =3; Li2CO3 purity = 99.3%; SEC(Li) = 0.027 kWh/g; Current efficiency η(Li) = 50% | [11] |
| Cathode Materials | Citric Acid Concentration (mol L−1) | Temperature (°C) | Leach Duration (min) | H2O2 Content (% in vol.) | S/L (g/L) | Leaching Yields (%) | pH of Leachate Solution | Ref. |
|---|---|---|---|---|---|---|---|---|
| LiNi0.45Mn0.4Co0.15O2 + LiCoO2 | 1.5 | 95 | 30 | 2% | 20 | Li = 95.3%, Co = 89.8%, Ni = 93.6%, Mn = 94.4% | 2.5 | [14] |
| LiCoO2 | 1 M | 80 °C | 60 | 4% | 20 | Li = 99%, Co = 99.21% | 1.29 | [15] |
| LiNi0.33Mn0.33Co0.33O2 (NMC111) | 1.5 M | 50 °C | 120 | 0% | 20 | Li = 82%, Co =63%, Ni = 74%, Mn = 95% | 2 | [16] |
| LiNi0.33Mn0.33Co0.33O2 (NMC111) | 2 M | 80 °C | 90 | 2% | 30 | Ni = 97% Co = 95%, Mn = 94% Li = 99% | 2.31 | [17] |
| LiCoO2 (LCO) | 0.5 M | 60 °C | 300 | 6% | 25 | Li = 100%, Co = 96% | 2.5 | [18] |
| ED Compartments | ||||||
|---|---|---|---|---|---|---|
| Li+ Recovery Compartment | Leachate Compartment | Metal Recovery Compartment | ||||
| Time (min) | pH | κ (mS cm−1) | pH | κ (mS cm−1) | pH | κ (mS cm−1) |
| 0 | 12.75 | 9.26 | 4.8 | 23.88 | 1.60 | 5.42 |
| 30 | 13.47 | 31.64 | 4.85 | 23.53 | 1.66 | 5.48 |
| 60 | 13.55 | 51.60 | 4.85 | 23.74 | 1.98 | 5.55 |
| 90 | 13.59 | 70.70 | 4.75 | 21.62 | 2.04 | 5.62 |
| 120 | 13.67 | 91.00 | 4.56 | 18.90 | 2.08 | 5.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badre-Eddine, S.; Muhr, L.; Chagnes, A. Innovative Integration of Citric Acid Leaching and Electrodialysis for Selective Lithium Recovery from NMC Cathode Material. Metals 2025, 15, 598. https://doi.org/10.3390/met15060598
Badre-Eddine S, Muhr L, Chagnes A. Innovative Integration of Citric Acid Leaching and Electrodialysis for Selective Lithium Recovery from NMC Cathode Material. Metals. 2025; 15(6):598. https://doi.org/10.3390/met15060598
Chicago/Turabian StyleBadre-Eddine, Soukayna, Laurence Muhr, and Alexandre Chagnes. 2025. "Innovative Integration of Citric Acid Leaching and Electrodialysis for Selective Lithium Recovery from NMC Cathode Material" Metals 15, no. 6: 598. https://doi.org/10.3390/met15060598
APA StyleBadre-Eddine, S., Muhr, L., & Chagnes, A. (2025). Innovative Integration of Citric Acid Leaching and Electrodialysis for Selective Lithium Recovery from NMC Cathode Material. Metals, 15(6), 598. https://doi.org/10.3390/met15060598









