Preparation of Bulk TiZrNbMoV and NbTiAlTaV High-Entropy Alloys by Powder Sintering
Abstract
:1. Introduction
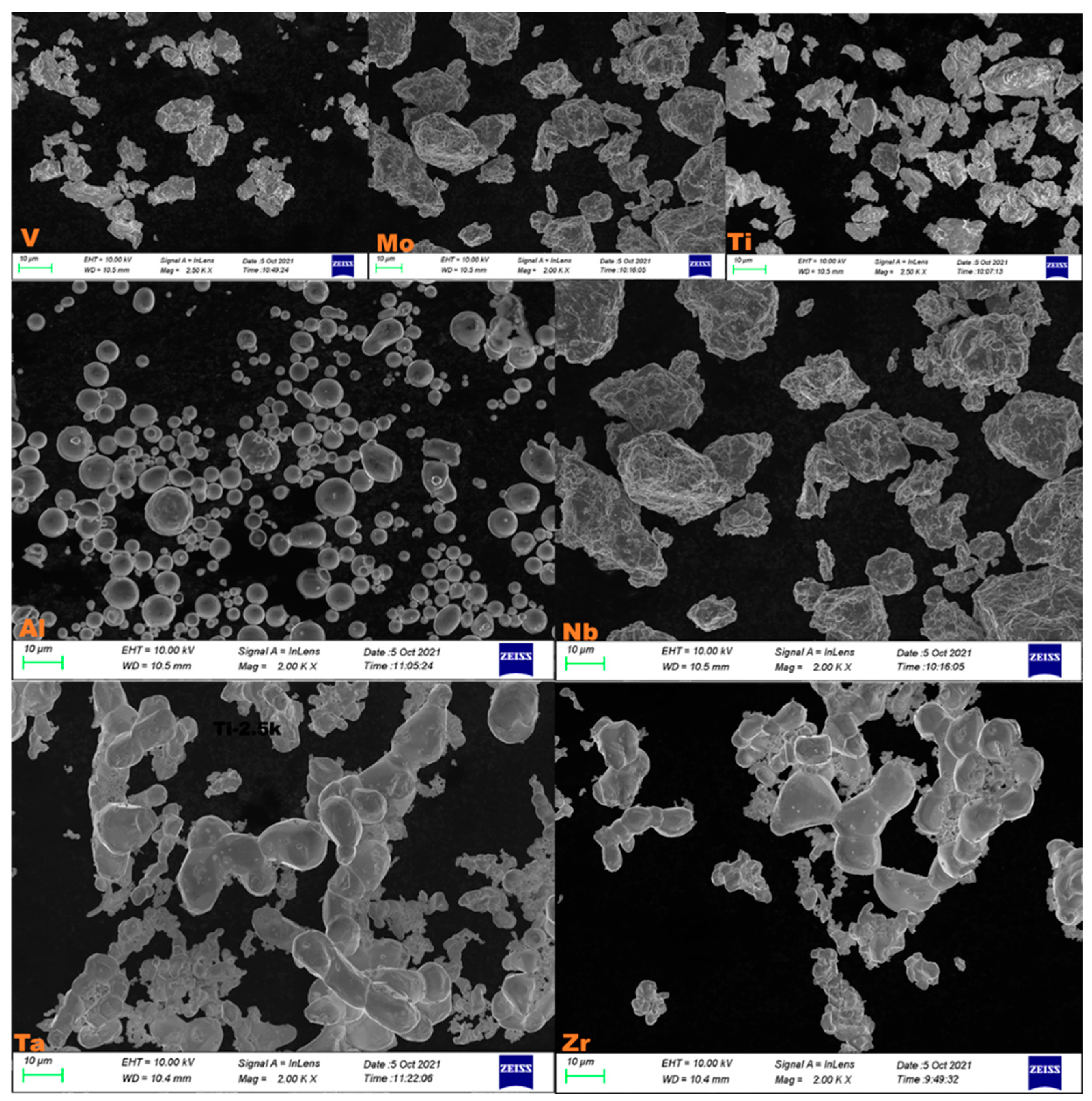
2. Experimental Procedures
3. Results
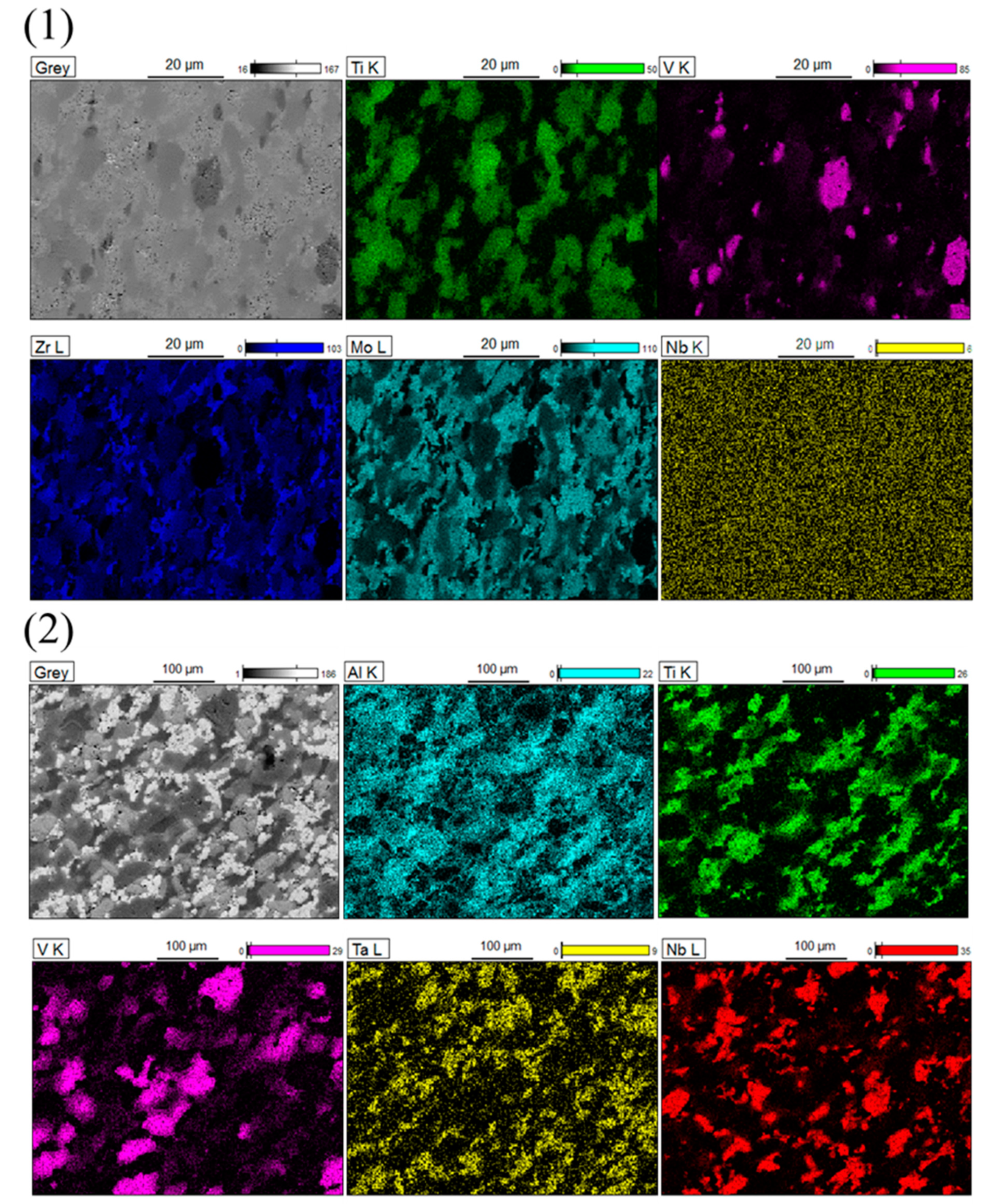
3.1. Crystal Structure and Microstructure
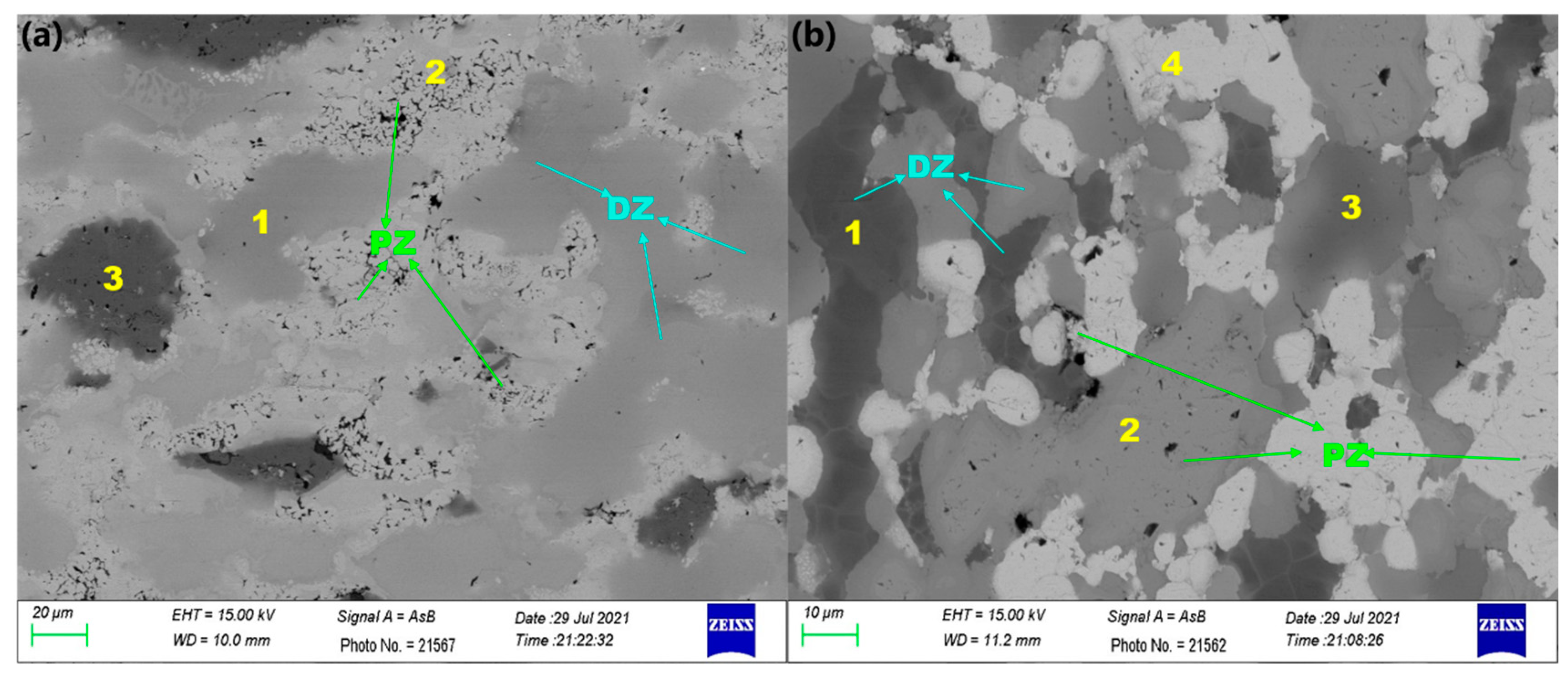
3.2. Determination of Porosity
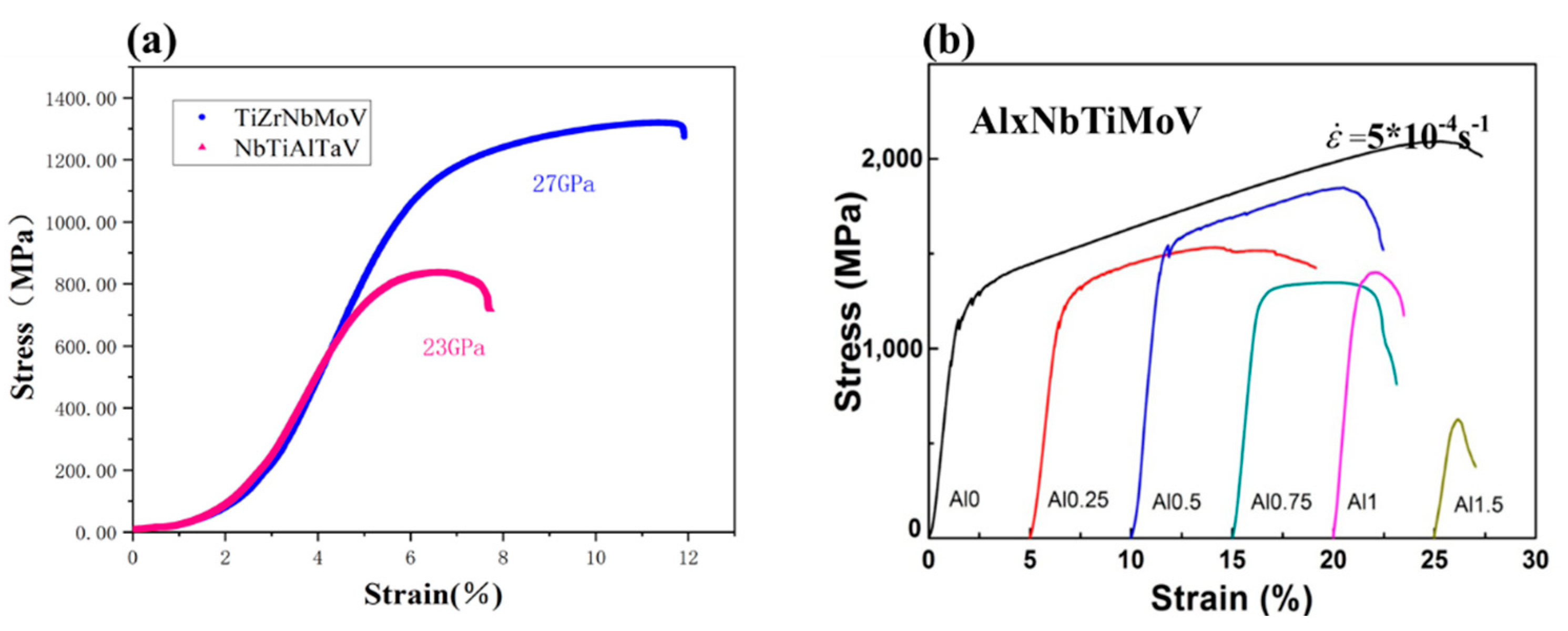
3.3. Compressive Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Qiu, S.; Miao, N.; Zhou, J.; Guo, Z.; Sun, Z. Strengthening mechanism of aluminum on elastic properties of NbVTiZr high-entropy alloys. Intermetallics 2018, 92, 7–14. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, C.; Liu, Y.; Liu, B.; Li, K. Microstructure and mechanical properties of Ti15MoxTiC composites fabricated by in-situ reactive sintering and hot swaging. J. Alloys Compd. 2018, 738, 188–196. [Google Scholar] [CrossRef]
- Coury, F.G.; Kaufman, M.; Clarke, A.J. Solid-solution strengthening in refractory high entropy alloys. Acta Mater. 2019, 175, 66–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R. Editorial for special issue on nanostructured high-entropy materials. Int. J. Miner. Metall. Mater. 2020, 27, 1309. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Shen, J.; Zeng, Z.; Park, J.M.; Choi, Y.T.; Schell, N.; Maawad, E.; Zhou, N.; Kim, H.S. Dissimilar laser welding of a CoCrFeMnNi high entropy alloy to 316 stainless steel. Scr. Mater. 2021, 206, 114–219. [Google Scholar] [CrossRef]
- Martin, A.C.; Oliveira, J.P.; Fink, C. Elemental Effects on Weld Cracking Susceptibility in AlxCoCrCuyFeNi High-Entropy Alloy. Metall. Mater. Trans. A 2019. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Curado, T.M.; Zeng, Z.; Lopes, J.G.; Rossinyol, E.; Park, J.M.; Schell, N.; Braz Fernandes, F.M.; Kim, H.S. Gas tungsten arc welding of as-rolled CrMnFeCoNi high entropy alloy. Mater. Des. 2020, 189, 108505. [Google Scholar] [CrossRef]
- Xiaoning, Y.; Weilin, D.; Xiaobo, H.; Linhai, T. Research on preparation methods of high-entropy alloy. Hot Work. Technol. 2014, 43, 30–33. [Google Scholar] [CrossRef]
- Zhou, S.; Liaw, P.K.; Xue, Y.; Zhang, Y. Temperature-dependent mechanical behavior of an Al0.5Cr0.9FeNi2.5V0.2 high-entropy alloy. Appl. Phys. Lett. 2021, 119, 121902. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. A Novel Low-Activation VCrFeTaxWx (x = 0.1, 0.2, 0.3, 0.4, and 1) High-Entropy Alloys with Excellent Heat-Softening Resistance. Entropy 2018, 20, 951. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Akhavan, B.; Zhou, H.; Chang, L.; Wang, Y.; Sun, L.; Bilek, M.M.; Liu, Z. High entropy alloy thin films of AlCoCrCu0.5FeNi with controlled microstructure. Appl. Surf. Sci. 2019, 495, 143560. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Ma, J.; Lu, Z.; Zhao, Y. Compositional gradient films constructed by sputtering in a multicomponent TiAl(Cr, Fe, Ni) system. J. Mater. Res. 2018, 33, 3330–3338. [Google Scholar] [CrossRef] [Green Version]
- Xing, Q.; Ma, J.; Zhang, Y. Phase thermal stability and mechanical properties analyses of (Cr,Fe,V)(Ta,W) multiple-based elemental system using a compositional gradient film. Int. J. Miner. Metall. Mater. 2020, 27, 1379–1387. [Google Scholar] [CrossRef]
- Firstov, S.A.; Gorban, V.F.; Danilenko, N.I.; Karpets, M.V.; Andreev, A.A.; Makarenko, E.S. Thermal stability of superhard nitride coatings from high entropy multicomponent TiVZrNbHf alloy. Powder Metall. Met. Ceram. 2014, 52, 560–566. [Google Scholar] [CrossRef]
- Lai, C.; Cheng, K.; Lin, S.; Yeh, J. Mechanical and tribological properties of multi-element (AlCrTaTiZr)N coatings. Surf. Coat. Technol. 2008, 202, 3732–3738. [Google Scholar] [CrossRef]
- Murr, L.E.; Martinez, E.; Amato, K.N.; Gaytan, S.M.; Hernandez, J.; Ramirez, D.A.; Shindo, P.W.; Medina, F.; Wicker, R.B. Fabrication of metal and alloy components by additive manufacturing: Examples of 3D materials science. J. Mater. Res. Technol. 2012, 1, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Niu, P.; Yuan, T.; Cao, P.; Chen, C.; Zhou, K. Selective laser melting of an equiatomic CoCrFeMnNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical property. J. Alloys Compd. 2018, 746, 125–134. [Google Scholar] [CrossRef]
- Brif, Y.; Thomas, M.; Todd, I. The use of high-entropy alloys in additive manufacturing. Scr. Mater. 2015, 99, 93–96. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Feng, T.; Zhang, Y.; Sha, G.; Lewandowski, J.J.; Liaw, P.K.; Zhang, Y. High-entropy Al0.3CoCrFeNi alloy fibers with high tensile strength and ductility at ambient and cryogenic temperatures. Acta Mater. 2017, 123, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Lukac, F.; Dudr, M.; Musalek, R.; Klecka, J.; Cinert, J.; Cizek, J.; Chraska, T.; Cizek, J.; Melikhova, O.; Kuriplach, J.; et al. Spark plasma sintering of gas atomized high-entropy alloy HfNbTaTiZr. J. Mater. Res. 2018, 33, 1–11. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Liu, B.; Zhang, W. Precipitation behavior during hot deformation of powder metallurgy Ti-Nb-Ta-Zr-Al high entropy alloys. Intermetallics 2018, 100, 95–103. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.-k.; Wu, W.-q.; Song, M.; Zhang, W.; Liu, B. Progress of powder metallurgical high entropy alloys. Chin. J. Nonferrous Met. 2019, 9, 2155–2184. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Liu, Y.; Fang, Q.; Chen, S.; Liu, C.T. Microstructure and mechanical properties of equimolar FeCoCrNi high entropy alloy prepared via powder extrusion. Intermetallics 2016, 75, 25–30. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Li, D.; Zuo, T.; Zhou, K.; Gao, M.; Sun, B.; Shen, T. Metals Compositional Design of Soft Magnetic High Entropy Alloys by Minimizing Magnetostriction Coefficient in (Fe0.3Co0.5Ni0.2)100-x(Al 1/3Si2/3)x System. Metals 2019, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Y. Tensile Properties and Impact Toughness of AlCoxCrFeNi3.1–x(x=0.4,1) High-Entropy Alloys. Front. Mater. 2020, 7, 92. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y. Utrastrong and ductile BCC high-entropy alloys with low-density via dislocation regulation and nanoprecipitates. JMST. submitted.
- Chen, S.Y.; Yang, X.; Dahmen, K.A.; Liaw, P.K.; Zhang, Y. Microstructures and Crackling Noise of AlxNbTiMoV High Entropy Alloys. Entropy 2014, 16, 870–884. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy Design and Properties Optimization of High-Entropy Alloys. JOM 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y. A body-centered cubic Zr50Ti35Nb15 medium-entropy alloy with unique properties. Scr. Mater. 2020, 178, 329–333. [Google Scholar] [CrossRef]
- Porous Materials for Powder Metallurgy; Metal Research Institute (Ed.) Metallurgical Industry Press: Beijing, China, 1979. [Google Scholar] [CrossRef]



| Number | Alloy Composition | References | Phase |
|---|---|---|---|
| GS101 | Al0.3CoCrFeNi | [21] | FCC |
| GS102 | Fe28.5Co47.5Ni19Al1.6Si3.4 | [26] | FCC |
| GS201 | AlCoCrFeNiTi0.2 | [27] | BCC + B2 |
| GS202 | W0.2Ta0.2FeCrV | [12] | BCC |
| GS203 | Zr45Ti31.5Nb13.5Al10 | [28] | BCC |
| GS301 | AlCo0.4CrFeNi2.7 | [27] | FCC + B2 |
| Elements-(a) | Ti-K | V-K | Zr-K | Nb-L | Mo-L |
|---|---|---|---|---|---|
| at.% | |||||
| Point 1 | 62.26 | 10.11 | 19.80 | 7.84 | |
| Point 2 | 1.81 | 1.95 | 96.10 | ||
| Point 3 | 0.50 | 97.75 | 1.26 | 0.49 | |
| Elements-(b) | Al-K | Ti-K | V-K | Nb-L | Ta-L |
| at.% | |||||
| Point-1 | 31.35 | 66.74 | 1.15 | 0.76 | |
| Point-2 | 0.57 | 98.16 | |||
| Point-3 | 0.50 | 97.83 | |||
| Point-4 | 17.01 | 5.06 | 72.80 | 3.10 | 2.04 |
| Samples | m/(g) | ma/(g) | ρ/(g/cm3) | η(%) | ||
|---|---|---|---|---|---|---|
| TiZrNbMoV | 6.5711 6.5714 6.5712 | 6.5712 | 5.8141 5.8140 5.8140 | 5.8140 | 6.8476 | 4.44 |
| NbTiAlTaV | 7.1121 7.1119 7.1115 | 7.1118 | 6.3582 6.3583 6.3582 | 6.3582 | 7.4459 | 5.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Liaw, P.K.; Zhang, Y. Preparation of Bulk TiZrNbMoV and NbTiAlTaV High-Entropy Alloys by Powder Sintering. Metals 2021, 11, 1748. https://doi.org/10.3390/met11111748
Wu Y, Liaw PK, Zhang Y. Preparation of Bulk TiZrNbMoV and NbTiAlTaV High-Entropy Alloys by Powder Sintering. Metals. 2021; 11(11):1748. https://doi.org/10.3390/met11111748
Chicago/Turabian StyleWu, Yaqi, Peter K. Liaw, and Yong Zhang. 2021. "Preparation of Bulk TiZrNbMoV and NbTiAlTaV High-Entropy Alloys by Powder Sintering" Metals 11, no. 11: 1748. https://doi.org/10.3390/met11111748








