A Study on the Effect of Ultrafine SiC Additions on Corrosion and Wear Performance of Alumina-Silicon Carbide Composite Material Produced by SPS Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Production
2.2. Powder Characterisation
2.3. Spark Plasma Sintering (SPS)
2.4. Sintered Samples Characterisation
3. Results and Discussion
3.1. Powder Characteristics
3.2. Composite Response to SPS
3.3. Characteristics of the Sintered Composites
4. Conclusions
- Ultrafine particles were produced after milling, evident from the peak broadening observed during XRD analyses. The morphology of the mixed powders revealed Al2O3 matrix with small SiC particles evenly dispersed amongst much larger and agglomerated particles. A shift of the Al2O3 peaks during XRD showed that the presence of SiC particles altered the Al2O3 matrix after mechanical alloying.
- The addition of SiC increased temperature from the onset of densification. Furthermore, the addition of SiC delayed densification. In addition, the degree of agglomeration observed in the initial milling stage of the powders might have prolonged densification.
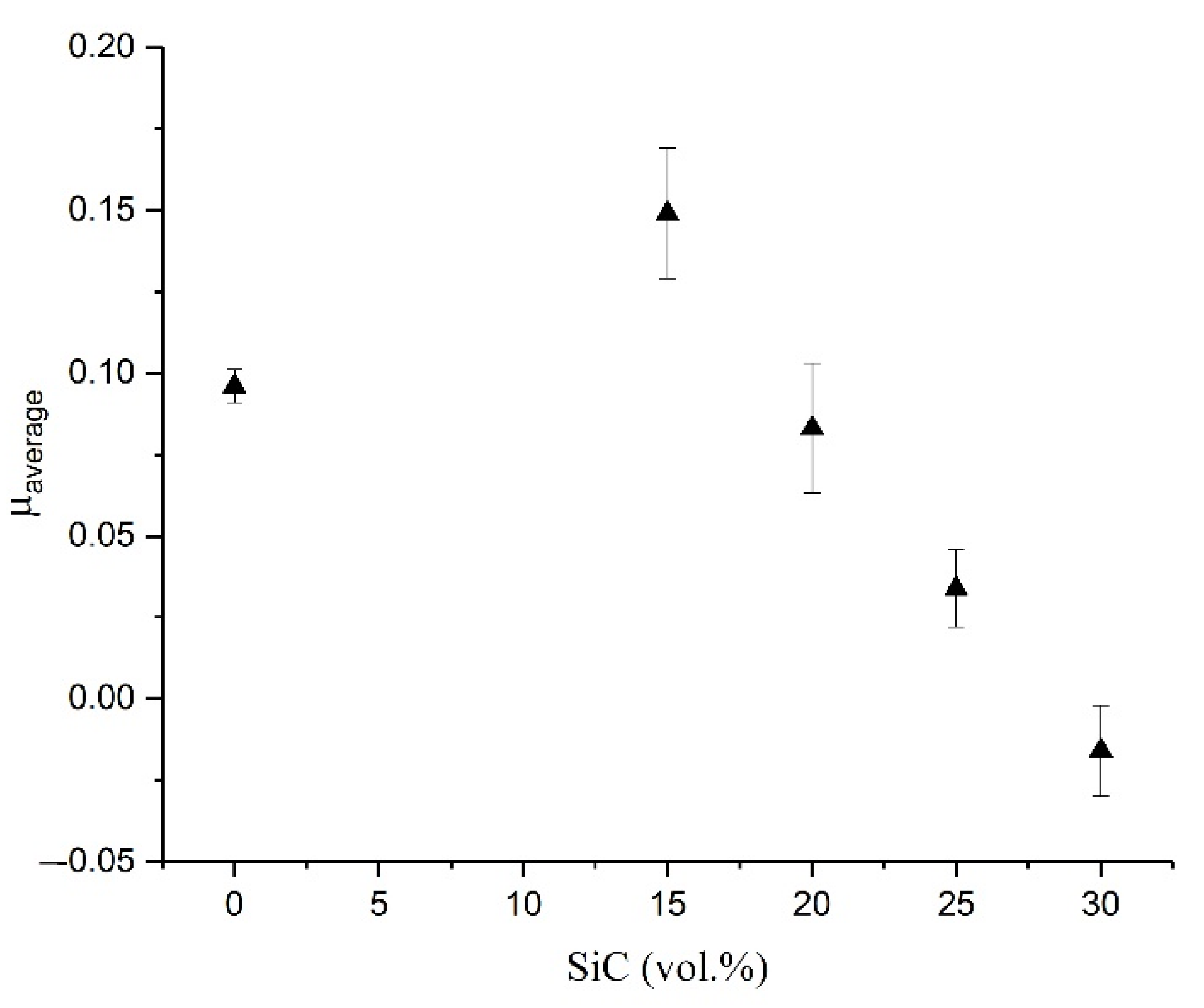
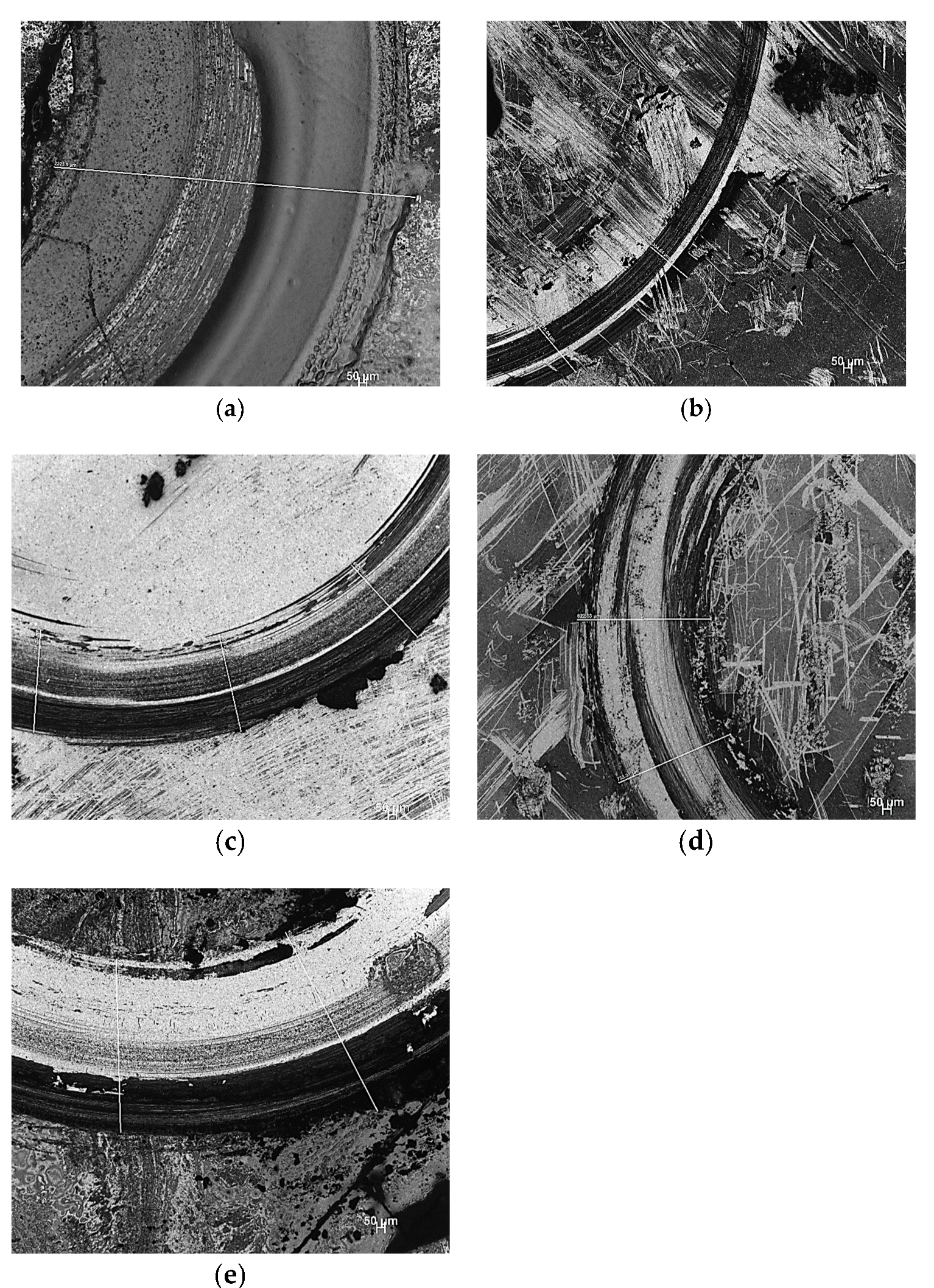
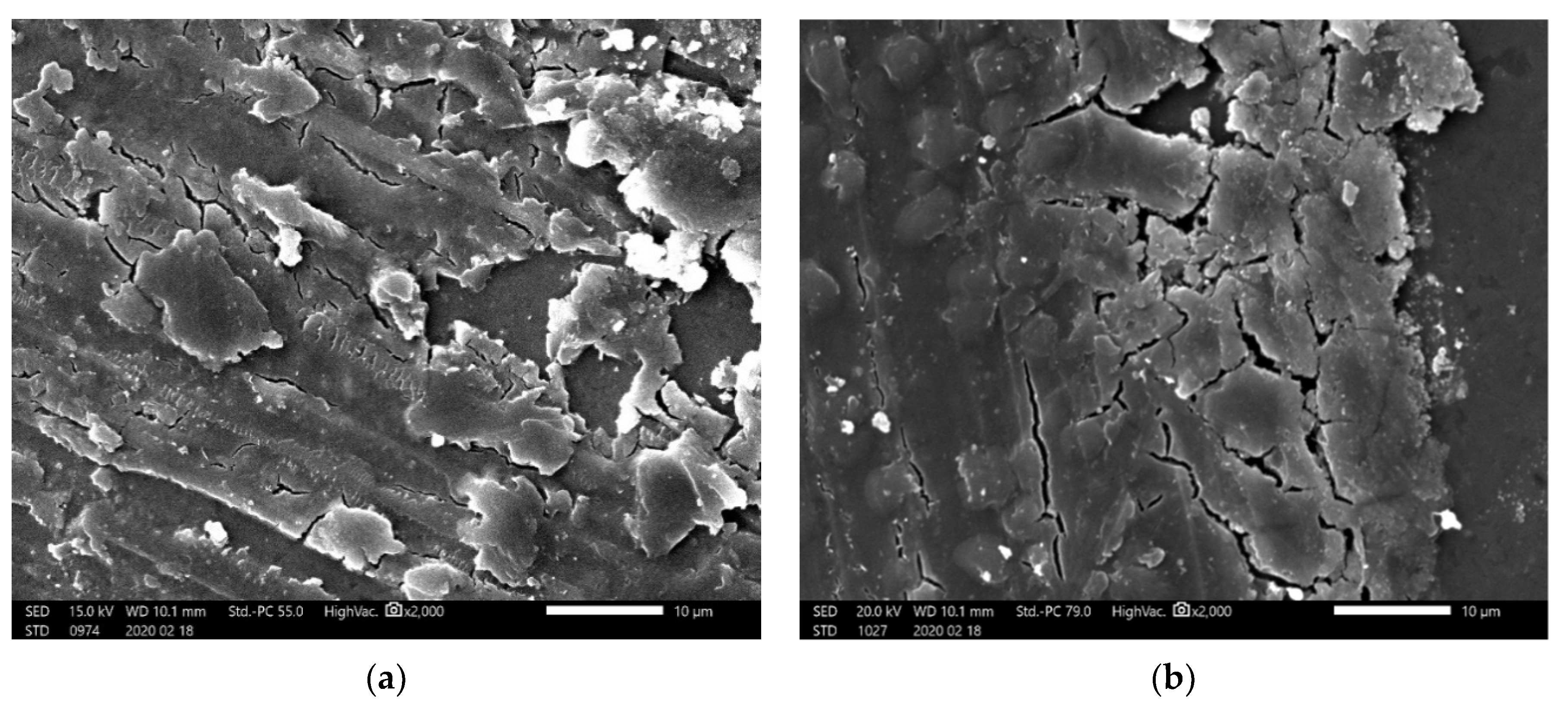
- The resistance to wear decreased with the addition of 15 vol.% of SiC. Additions of higher vol.% (20, 25, and 30) of SiC significantly improved the resistance to wear of Al2O3.
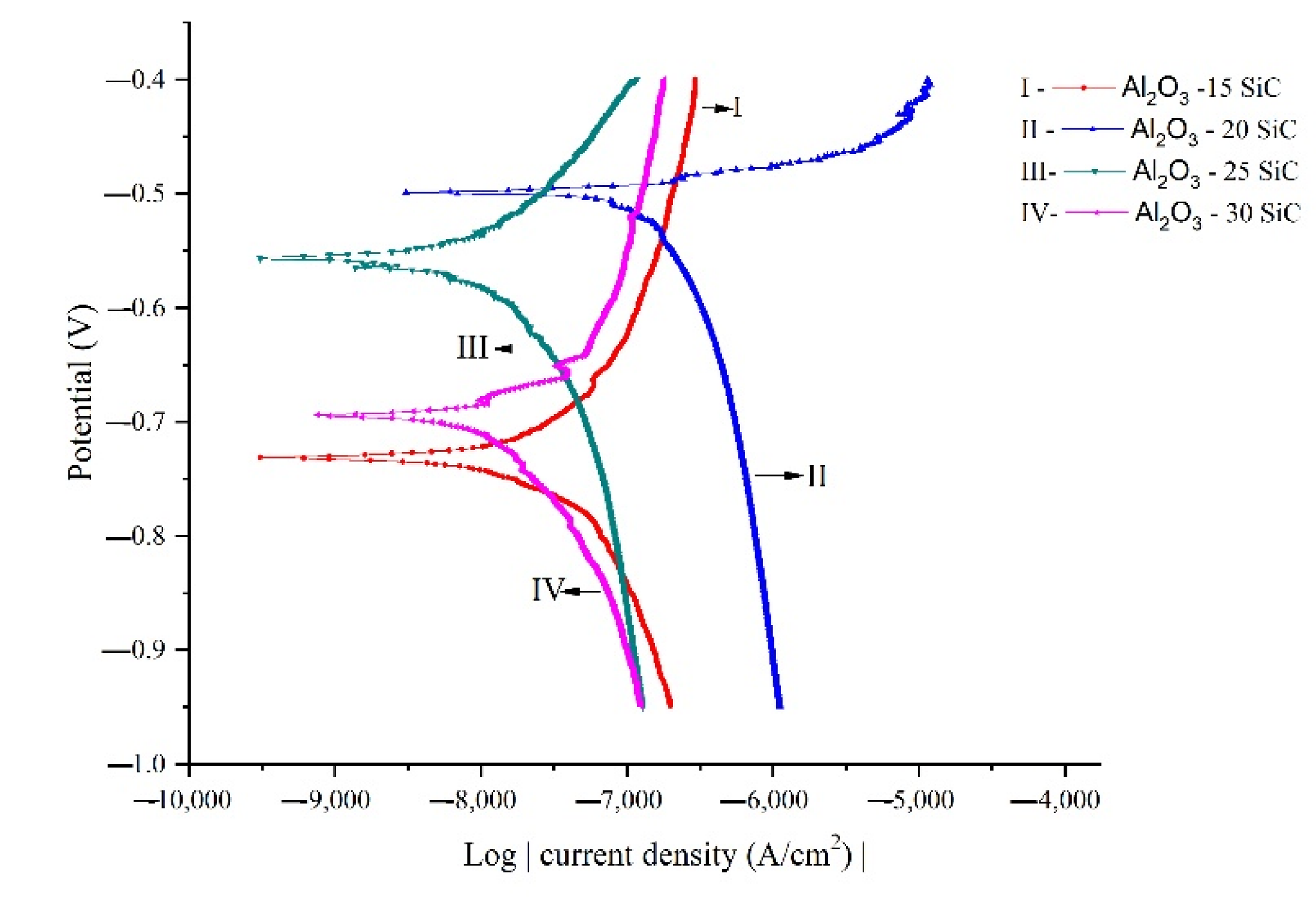
- The study’s composites showed no improvement in corrosion performance with increments in SiC additions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaafar, M.; Bonnefont, G.; Fantozzi, G.; Reveron, H. Intergranular alumina–SiC micro-nanocomposites sintered by spark plasma sintering. Mater. Chem. Phys. 2010, 124, 377–379. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Lee, J.-G. Pressureless Sintering of Alumina-Titanium Carbide Composites. J. Am. Ceram. Soc. 1989, 72, 1333–1337. [Google Scholar] [CrossRef]
- Jianxin, D.; Xing, A. Wear resistance of Al2O3/TiB2 ceramic cutting tools in sliding wear tests and in machining processes. J. Mater. Process. Technol. 1997, 72, 249–255. [Google Scholar] [CrossRef]
- Tampieri, A.; Bellosi, A. Oxidation Resistance of Alumina–Titanium Nitride and Alumina–Titanium Carbide Composites. J. Am. Ceram. Soc. 1992, 75, 1688–1690. [Google Scholar] [CrossRef]
- Gustafsson, S.; Falk, L.K.L.; Lidén, E.; Carlström, E. Pressureless sintered Al2O3–SiC nanocomposites. Ceram. Int. 2008, 34, 1609–1615. [Google Scholar] [CrossRef]
- Galusek, D.; Klement, R.; Sedláček, J.; Balog, M.; Fasel, C.; Zhang, J.; Crimp, M.A.; Riedel, R. Al2O3–SiC composites prepared by infiltration of pre-sintered alumina with a poly(allyl)carbosilane. J. Eur. Ceram. Soc. 2011, 31, 111–119. [Google Scholar] [CrossRef]
- Shi, X.L.; Xu, F.M.; Zhang, Z.J.; Dong, Y.L.; Tan, Y.; Wang, L.; Yang, J.M. Mechanical properties of hot-pressed Al2O3/SiC composites. Mater. Sci. Eng. A 2010, 527, 4646–4649. [Google Scholar] [CrossRef]
- Dong, Y.L.; Xu, F.M.; Shi, X.L.; Zhang, C.; Zhang, Z.J.; Yang, J.M.; Tan, Y. Fabrication and mechanical properties of nano-/micro-sized Al2O3/SiC composites. Mater. Sci. Eng. A 2009, 504, 49–54. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Yan, H.; Jiang, K. Spark Plasma Sintering of Alumina Composites with Graphene Platelets and Silicon Carbide Nanoparticles. Adv. Eng. Mater. 2014, 16, 1111–1118. [Google Scholar] [CrossRef]
- Gao, L.; Hong, J.; Miyamoto, H.; Torre, S.D.D. Bending strength and microstructure of Al2O3 ceramics densified by spark plasma sintering. J. Eur. Ceram. Soc. 2000, 20, 2149–2152. [Google Scholar] [CrossRef]
- Parchovianský, M.; Galusek, D.; Sedláček, J.; Švančárek, P.; Kašiarová, M.; Dusza, J.; Šajgalík, P. Microstructure and mechanical properties of hot pressed Al2O3/SiC nanocomposites. J. Eur. Ceram. Soc. 2013, 33, 2291–2298. [Google Scholar] [CrossRef]
- Borsa, C.E.; Jones, N.M.; Brook, R.J.; Todd, R.I. Influence of processing on the microstructural development and flexure strength of Al2O3/SiC nanocomposites. J. Eur. Ceram. Soc. 1997, 17, 865–872. [Google Scholar] [CrossRef]
- Reveron, H.; Zaafrani, O.; Fantozzi, G. Microstructure development, hardness, toughness and creep behaviour of pressureless sintered alumina/SiC micro–nanocomposites obtained by slip-casting. J. Eur. Ceram. Soc. 2010, 30, 1351–1357. [Google Scholar] [CrossRef]
- Chuvil’deev, V.N.; Boldin, М.S.; Nokhrin, А.V.; Popov, А.А. Advanced materials obtained by Spark Plasma Sintering. Acta Astronaut. 2017, 135, 192–197. [Google Scholar] [CrossRef]
- Omori, M. Sintering, consolidation, reaction and crystal growth by the spark plasma system (SPS). Mater. Sci. Eng. A 2000, 287, 183–188. [Google Scholar] [CrossRef]
- Hulbert, D.M.; Anders, A.; Andersson, J.; Lavernia, E.J.; Mukherjee, A.K. A discussion on the absence of plasma in spark plasma sintering. Scr. Mater. 2009, 60, 835–838. [Google Scholar] [CrossRef]
- Song, S.-X.; Wang, Z.; Shi, G.-P. Heating mechanism of spark plasma sintering. Ceram. Int. 2013, 39, 1393–1396. [Google Scholar] [CrossRef]
- Cuenca-Álvarez, R.; de la Torre, S.D.; López, F.J. Mechanical dispersion of platinum particles and its effect on the microstructure of MCrAlY alloy prepared by SPS. Powder Technol. 2016, 291, 193–200. [Google Scholar] [CrossRef]
- Matizamhuka, W.R. Spark plasma sintering (SPS)—An advanced sintering technique for structural nanocomposite materials. J. South. Afr. Inst. Min. Metall. 2016, 116, 1171–1180. [Google Scholar] [CrossRef]
- Álvarez, I.; Torrecillas, R.; Solis, W.; Peretyagin, P.; Fernández, A. Microstructural design of Al2O3–SiC nanocomposites by Spark Plasma Sintering. Ceram. Int. 2016, 42, 17248–17253. [Google Scholar] [CrossRef]
- Chae, J.H.; Kim, K.H.; Choa, Y.H.; Matsushita, J.; Yoon, J.-W.; Shim, K.B. Microstructural evolution of Al2O3–SiC nanocomposites during spark plasma sintering. J. Alloys Compd. 2006, 413, 259–264. [Google Scholar] [CrossRef]
- Galusková, D.; Šajgalík, P.; Galusek, D.; Hnatko, M. Corrosion of Alumina Ceramics in an Aqueous Solution of Sodium Chloride. Key Eng. Mater. 2009, 409, 283–286. [Google Scholar] [CrossRef]
- Sudha, P.N.; Sangeetha, K.; Jisha Kumari, A.V.; Vanisri, N.; Rani, K. Corrosion of ceramic materials. In Fundamental Biomaterials: Ceramics; Woodhead Publishing: Cambridge, UK, 2018; ISBN 9780081022047. [Google Scholar]
- Momohjimoh, I.; Hussein, M.; Al-Aqeeli, N. Recent Advances in the Processing and Properties of Alumina–CNT/SiC Nanocomposites. Nanomaterials 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedláček, J.; Galusek, D.; Švančárek, P.; Riedel, R.; Atkinson, A.; Wang, X. Abrasive wear of Al2O3–SiC and Al2O3–(SiC)–C composites with micrometer- and submicrometer-sized alumina matrix grains. J. Eur. Ceram. Soc. 2008, 28, 2983–2993. [Google Scholar] [CrossRef]
- Anya, C.C. Wet erosive wear of alumina and its composites with SiC nano-particles. Ceram. Int. 1998, 24, 533–542. [Google Scholar] [CrossRef]
- Rodríguez, J.; Martín, A.; Pastor, J.Y.; LLorca, J.; Bartolomé, J.F.; Moya, J.S. Sliding Wear of Alumina/Silicon Carbide Nanocomposites. J. Am. Ceram. Soc. 1999, 82, 2252–2254. [Google Scholar] [CrossRef]
- Borsa, C.E.; Jiao, S.; Todd, R.I.; Brook, R.J. Processing and properties of Al2O3/SiC nanocomposites. J. Microsc. 1995, 177, 305–312. [Google Scholar] [CrossRef]
- Ishitsuka, M.; Tamai, M. Fabrication and Mechanical Properties of Pressureless Sintered Al2O3/SiC Nanocomposites. J. Jpn. Soc. Powder Powder Metall. 1992, 39, 1109–1112. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Niihara, K. Microstructure and mechanical properties of pressureless sintered Al2O3/Sic nanocomposites. Nanostructured Mater. 1997, 9, 193–196. [Google Scholar] [CrossRef]
- Ji, Y.; Yeomans, J.A. Processing and mechanical properties of Al2O3–5 vol.% Cr nanocomposites. J. Eur. Ceram. Soc. 2002, 22, 1927–1936. [Google Scholar] [CrossRef]
- Jiao, S.; Jenkins, M.L.; Davidge, R.W. Electron microscopy of crack/particle interactions in Al2O3/SiC nanocomposites. J. Microsc. 1997, 185, 259–264. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Hong, J.; Miyamoto, H.; Miyamoto, K.; Nishikawa, Y.; Torre, S.D.D. Mechanical Properties and Microstructure of Nano-SiC–Al2O3 Composites Densified by Spark Plasma Sintering. J. Eur. Ceram. Soc. 1999, 19, 609–613. [Google Scholar] [CrossRef]
- Piciacchio, A.; Lee, S.-H.; Messing, G.L. Processing and Microstructure Development in Alumina–Silicon Carbide Intragranular Particulate Composites. J. Am. Ceram. Soc. 1994, 77, 2157–2164. [Google Scholar] [CrossRef]
- Hsueh, C.-H.; Evans, A.G.; Mcmeeking, R.M. Influence of Multiple Heterogeneities on Sintering Rates. J. Am. Ceram. Soc. 1986, 69, C-64–C-66. [Google Scholar] [CrossRef]
- Zhao, J.; Stearns, L.C.; Harmer, M.P.; Chan, H.M.; Miller, G.A.; Cook, R.F. Mechanical Behavior of Alumina–Silicon Carbide „Nanocomposites”. J. Am. Ceram. Soc. 1993, 76, 503–510. [Google Scholar] [CrossRef]
- Xu, Y.; Zangvil, A.; Kerber, A. SiC nanoparticle-reinforced Al2O3 matrix composites: Role of intra- and intergranular particles. J. Eur. Ceram. Soc. 1997, 17, 921–928. [Google Scholar] [CrossRef]
- Limpichaipanit, A.; Todd, R.I. The relationship between microstructure, fracture and abrasive wear in Al2O3/SiC nanocomposites and microcomposites containing 5 and 10% SiC. J. Eur. Ceram. Soc. 2009, 29, 2841–2848. [Google Scholar] [CrossRef]
- Ortiz-Merino, J.L.; Todd, R.I. Relationship between wear rate, surface pullout and microstructure during abrasive wear of alumina and alumina/SiC nanocomposites. Acta Mater. 2005, 53, 3345–3357. [Google Scholar] [CrossRef]
- Kara, H.; Roberts, S.G. Polishing Behavior and Surface Quality of Alumina and Alumina/Silicon Carbide Nanocomposites. J. Am. Ceram. Soc. 2000, 83, 1219–1225. [Google Scholar] [CrossRef]
- Guicciardi, S.; Sciti, D.; Melandri, C.; Bellosi, A. Dry Sliding Wear Behavior of Al2O3–SiC Submicro-and Nano-Composites. J. Am. Ceram. Soc. 2005, 88, 179–183. [Google Scholar] [CrossRef]
- Cherif, K.; Gueroult, B.; Rigaud, M. Al2O3–ZrO2 debris life cycle during wear: Effects of the third body on wear and friction. Wear 1997, 208, 161–168. [Google Scholar] [CrossRef]
- Gee, M.G. The formation of aluminium hydroxide in the sliding wear of alumina. Wear 1992, 153, 201–227. [Google Scholar] [CrossRef]
- Roepstorff, S.; Maahn, E. Corrosion Resistance of Aluminum-Silicon Carbide Composite Materials. In Proceedings of the 12th Scandinavian Corrosion Congress and Eurocorr, Espoo, Finland, 31 May–4 June 1992. [Google Scholar]
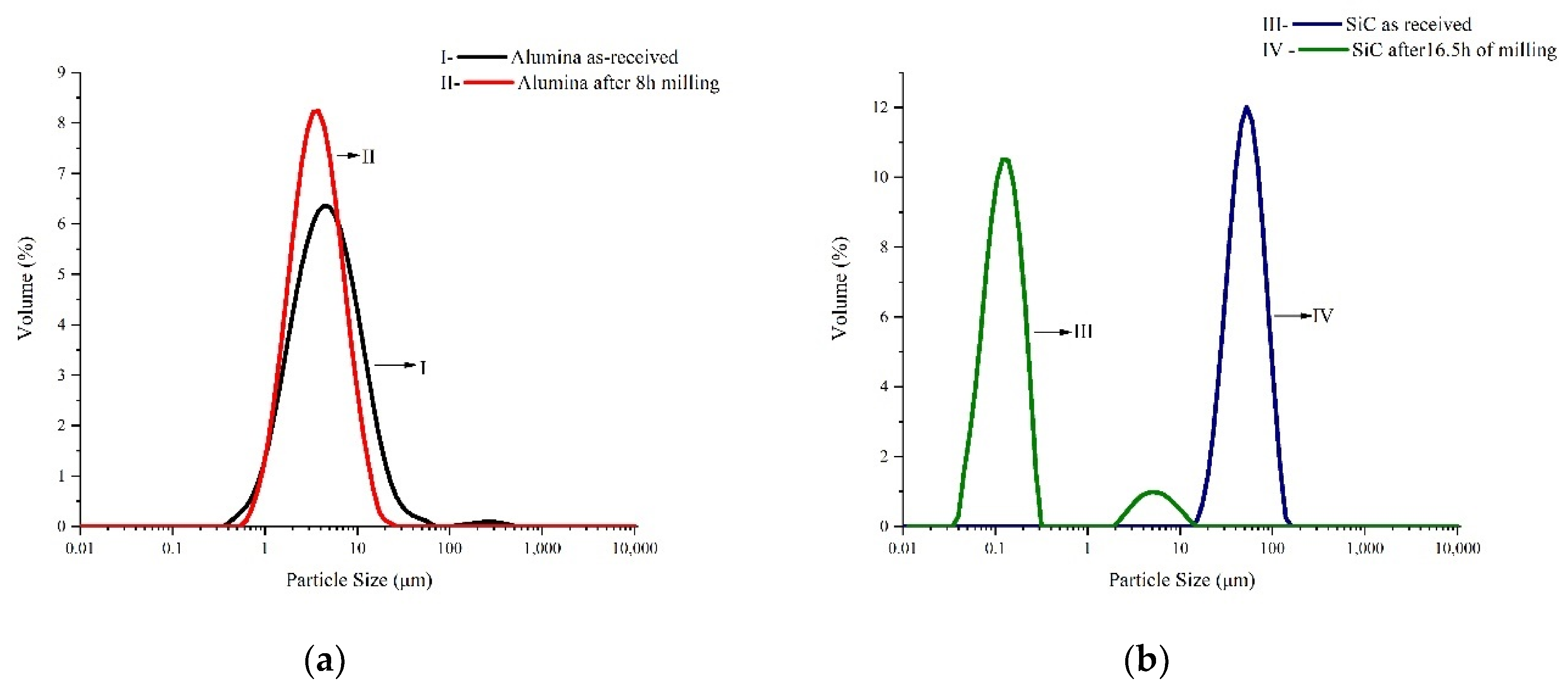

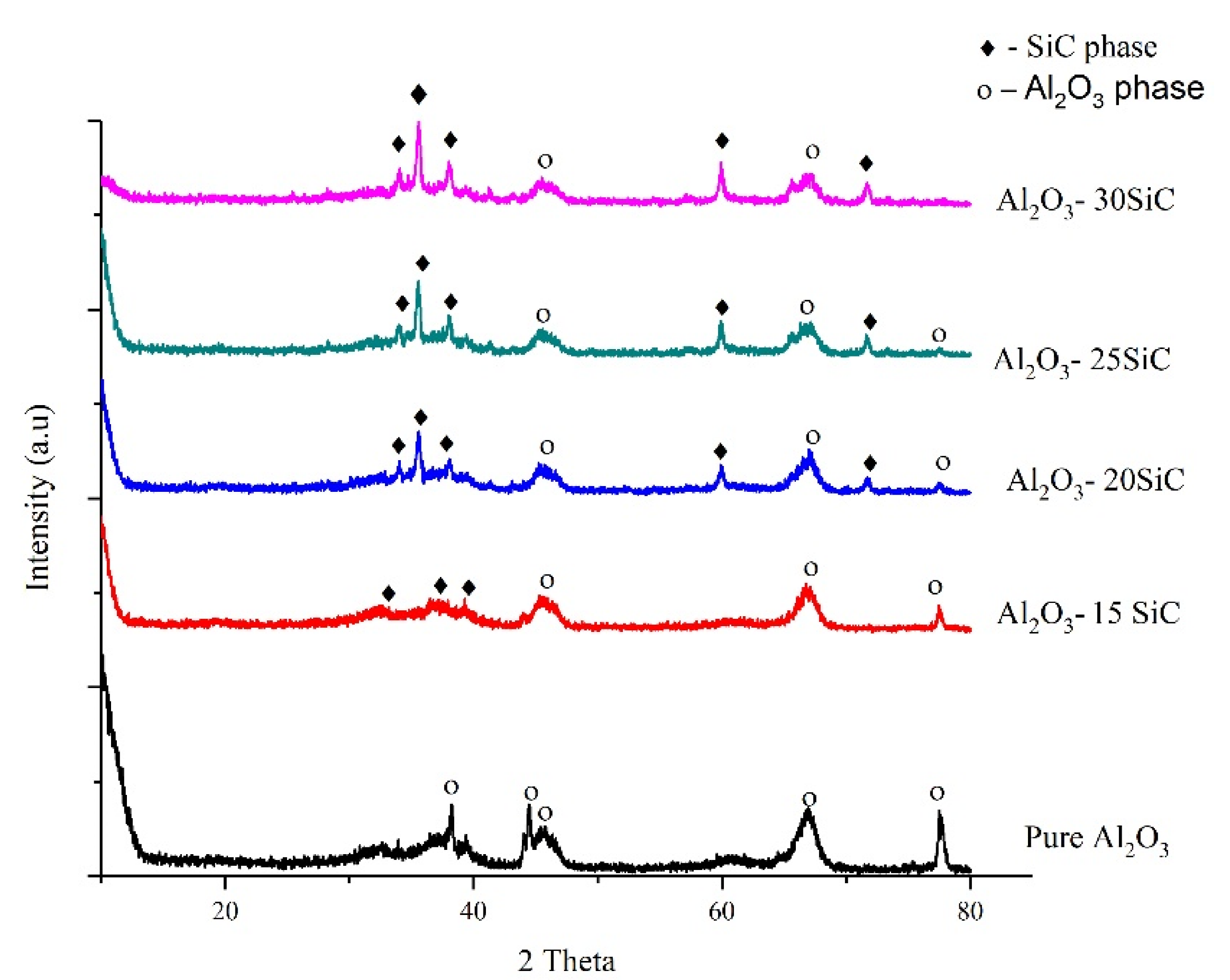
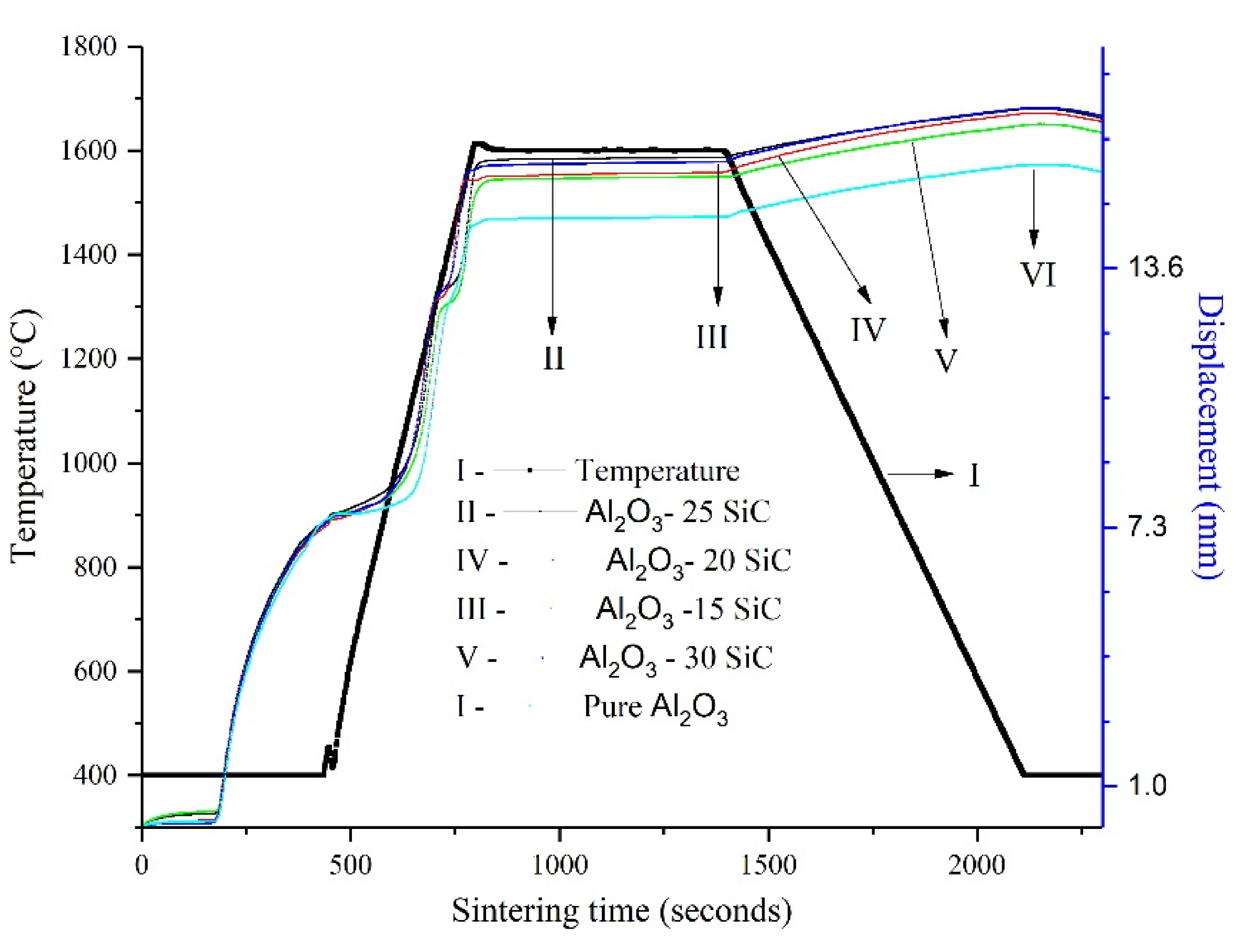
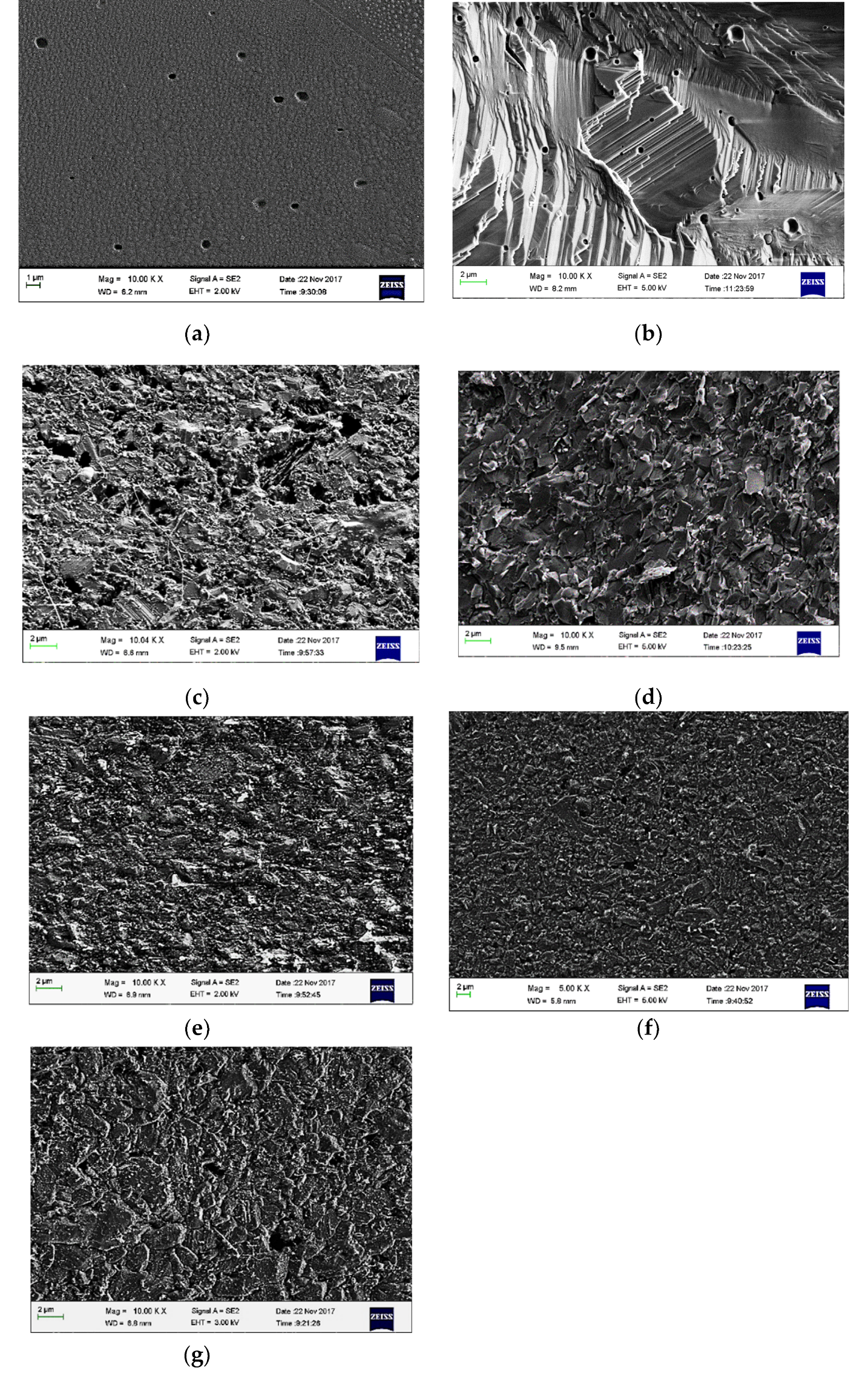
| Material | d10 (μm) | d50 (μm) | d90 (μm) |
|---|---|---|---|
| Before milling | |||
| Al2O3 | 2.172 | 6.37 | 18.76 |
| SiC | 26.77 | 48.01 | 82.63 |
| After milling | |||
| Al2O3 | 1.448 | 3.344 | 7.712 |
| SiC | 0.063 | 0.120 | 0.245 |
| Sample | Crystallite Size (nm) |
|---|---|
| Pure Al2O3 | 0.049 |
| Al2O3-15SiC | 0.315 |
| Al2O3-20SiC | 1.107 |
| Al2O3-25SiC | 0.109 |
| Al2O3-30SiC | 0.169 |
| Sample | Theoretical Density (g/cm3) | Bulk Density (g/cm3) | Relative Density (%) | Average Grain Size (µm) |
|---|---|---|---|---|
| Pure Al2O3 | 3.97 | 3.939 | 99.2 | 15.5 |
| Al2O3-15SiC | 3.82 | 3.797 | 99.4 | 2.0 |
| Al2O3-20SiC | 3.78 | 3.789 | 100 | 2.3 |
| Al2O3-25SiC | 3.73 | 3.724 | 99.8 | 49.8 |
| Al2O3-30SiC | 3.73 | 3.638 | 97.5 | 43.3 |
| Sample | Approximate Wear Track Size (µm) |
|---|---|
| Pure Al2O3 | 2324 |
| Al2O3-15SiC | 375 |
| Al2O3-20SiC | 637 |
| Al2O3-25SiC | 823 |
| Al2O3-30SiC | 1242 |
| Sample | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|
| Al2O3-15SiC | −0.731 | 2.433 × 10−8 |
| Al2O3-20SiC | −0.499 | 3.841 × 10−9 |
| Al2O3-25SiC | −0.556 | 9.577 × 10−9 |
| Al2O3-30SiC | −0.694 | 9.577 × 10−9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogale, N.F.; Matizamhuka, W.R. A Study on the Effect of Ultrafine SiC Additions on Corrosion and Wear Performance of Alumina-Silicon Carbide Composite Material Produced by SPS Sintering. Metals 2020, 10, 1337. https://doi.org/10.3390/met10101337
Mogale NF, Matizamhuka WR. A Study on the Effect of Ultrafine SiC Additions on Corrosion and Wear Performance of Alumina-Silicon Carbide Composite Material Produced by SPS Sintering. Metals. 2020; 10(10):1337. https://doi.org/10.3390/met10101337
Chicago/Turabian StyleMogale, Ntebogeng F., and Wallace R. Matizamhuka. 2020. "A Study on the Effect of Ultrafine SiC Additions on Corrosion and Wear Performance of Alumina-Silicon Carbide Composite Material Produced by SPS Sintering" Metals 10, no. 10: 1337. https://doi.org/10.3390/met10101337






