Potential Physiological and Cellular Mechanisms of Exercise That Decrease the Risk of Severe Complications and Mortality Following SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Definitions
3. Fitness and Health Status, Immunity, and Infection
4. Deterioration of Fitness Status during a Short Period of Physical Inactivity
5. SARS-CoV-2 and COVID-19
6. Effects of Physical Activity and Exercise on Human Biology in View of COVID-19
6.1. Adipose Tissue, Obesity, MetS, and Endocrine Effects in Relation to COVID-19 and the Effects of Exercise
6.2. The Immune System in Relation to COVID-19 and the Effects of Exercise
6.3. The Cardiovascular System in Relation to COVID-19 and the Effects of Exercise
6.4. The Respiratory System in Relation to COVID-19 and the Effects of Exercise
6.5. The Kidneys and Gastrointestinal System in Relation to COVID-19 and the Effects of Exercise
6.6. Skeletal Muscle in Relation to COVID-19 and the Effects of Exercise
6.7. Epigenetics in Relation to COVID-19 and the Effects of Exercise
6.8. Mitochondrial Health in Relation to COVID-19 and the Effects of Exercise
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 31 March 2020).
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.A.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. OpenSAFELY: Factors associated with COVID-19 death in 17 million patients. Nature 2020, 584, 430. [Google Scholar] [CrossRef] [PubMed]
- Yates, T.; Razieh, C.; Zaccardi, F.; Rowlands, A.V.; Seidu, S.; Davies, M.J.; Khunti, K. Obesity, walking pace and risk of severe COVID-19 and mortality: Analysis of UK Biobank. Int. J. Obes. 2021, 45, 1155–1159. [Google Scholar] [CrossRef]
- Kerrigan, D.J.; Brawner, C.A.; Ehrman, J.K.; Keteyian, S. Cardiorespiratory Fitness Attenuates the Impact of Risk Factors Associated with COVID-19 Hospitalization. Mayo Clin. Proc. 2021, 96, 822–823. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077. [Google Scholar] [CrossRef]
- Tchang, B.G.; Askin, G.; Sahagun, A.; Hwang, J.; Huang, H.; Mendelsohn Curanaj, F.A.; Seley, J.J.; Safford, M.M.; Alonso, L.C.; Aronne, L.J.; et al. The independent risk of obesity and diabetes and their interaction in COVID-19: A retrospective cohort study. Obesity 2021, 29, 971–975. [Google Scholar] [CrossRef]
- Tavakol, Z.; Ghannadi, S.; Tabesh, M.R.; Halabchi, F.; Noormohammadpour, P.; Akbarpour, S.; Alizadeh, Z.; Nezhad, M.H.; Reyhan, S.K. Relationship between physical activity, healthy lifestyle and COVID-19 disease severity; a cross-sectional study. J. Public Health 2021, 1–9. [Google Scholar] [CrossRef]
- Xie, J.; Zu, Y.; Alkhatib, A.; Pham, T.T.; Gill, F.; Jang, A.; Radosta, S.; Chaaya, G.; Myers, L.; Zifodya, J.S.; et al. Metabolic Syndrome and COVID-19 Mortality Among Adult Black Patients in New Orleans. Diabetes Care 2020, 44, 188–193. [Google Scholar] [CrossRef]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise effects on physiological function during aging. Free Radic Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Altenburg, T.M.; et al. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.; Honce, R.; Schultz-Cherry, S. Metabolic Syndrome and Viral Pathogenesis: Lessons from Influenza and Coronaviruses. J. Virol. 2020, 94, e00665-20. [Google Scholar] [CrossRef] [PubMed]
- Honce, R.; Schultz-Cherry, S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Baik, I.; Curhan, G.C.; Rimm, E.B.; Bendich, A.; Willett, W.C.; Fawzi, W.W. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch. Intern. Med. 2000, 160, 3082–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamer, M.; O’Donovan, G.; Stamatakis, E. Lifestyle risk factors, obesity and infectious disease mortality in the general population: Linkage study of 97,844 adults from England and Scotland. Prev. Med. 2019, 123, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, J.; Askim, Å.; Mohus, R.M.; Mehl, A.; Dewan, A.; Solligård, E.; Damås, J.K.; Åsvold, B.O. Associations of obesity and lifestyle with the risk and mortality of bloodstream infection in a general population: A 15-year follow-up of 64,027 individuals in the HUNT Study. Int. J. Epidemiol. 2017, 46, 1573–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C. Coronavirus Disease-2019: A tocsin to our aging, unfit, corpulent, and immunodeficient society. J. Sport Health Sci. 2020, 9, 293–301. [Google Scholar] [CrossRef]
- Samuels, J.D. Obesity Phenotype is a Predictor of COVID-19 Disease Susceptibility. Obesity 2020, 28, 1368. [Google Scholar] [CrossRef]
- Zheng, K.I.; Gao, F.; Wang, X.B.; Sun, Q.F.; Pan, K.H.; Wang, T.Y.; Ma, H.L.; Liu, W.Y.; George, J.; Zheng, M.H. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism 2020, 108, 154244. [Google Scholar] [CrossRef] [PubMed]
- Kass, D.A.; Duggal, P.; Cingolani, O. Obesity could shift severe COVID-19 disease to younger ages. Lancet 2020, 395, 1544–1545. [Google Scholar] [CrossRef]
- Beltran-Sanchez, H.; Harhay, M.O.; Harhay, M.M.; McElligott, S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J. Am. Coll. Cardiol. 2013, 62, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-K.; Han, K.; Kim, M.K.; Koh, E.S.; Kim, E.S.; Nam, G.E.; Kwon, H.-S. Changes in metabolic syndrome and its components and the risk of type 2 diabetes: A nationwide cohort study. Sci. Rep. 2020, 10, 2313. [Google Scholar] [CrossRef]
- Ortega, F.B.; Cadenas-Sanchez, C.; Migueles, J.H.; Labayen, I.; Ruiz, J.R.; Sui, X.; Blair, S.N.; Martinez-Vizcaino, V.; Lavie, C.J. Role of Physical Activity and Fitness in the Characterization and Prognosis of the Metabolically Healthy Obesity Phenotype: A Systematic Review and Meta-analysis. Prog. Cardiovasc. Dis. 2018, 61, 190–205. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef] [Green Version]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Ekelund, U.; Tarp, J.; Steene-Johannessen, J.; Hansen, B.H.; Jefferis, B.; Fagerland, M.W.; Whincup, P.; Diaz, K.M.; Hooker, S.P.; Chernofsky, A.; et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ 2019, 366, l4570. [Google Scholar] [CrossRef] [Green Version]
- Trost, S.; Pratley, R.; Sobel, B. Impaired fibrinolysis and risk for cardiovascular disease in the metabolic syndrome and type 2 diabetes. Curr. Diab. Rep. 2006, 6, 47–54. [Google Scholar] [CrossRef]
- Fitbit. The Impact of Coronavirus on Global Activity. Available online: https://blog.fitbit.com/covid-19-global-activity/ (accessed on 5 April 2020).
- Apple. Mobility Trends Reports. Available online: https://www.apple.com/covid19/mobility (accessed on 14 May 2020).
- Jakobsson, J.; Malm, C.; Furberg, M.; Ekelund, U.; Svensson, M. Physical Activity during the Coronavirus (COVID-19) Pandemic: Prevention of a Decline in Metabolic and Immunological Functions. Front. Sports Act. Living 2020, 2, 57. [Google Scholar] [CrossRef]
- Narici, M.; De Vito, G.; Franchi, M.; Paoli, A.; Moro, T.; Marcolin, G.; Grassi, B.; Baldassarre, G.; Zuccarelli, L.; Biolo, G.; et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2020, 21, 614–635. [Google Scholar] [CrossRef] [PubMed]
- Honce, R.; Schultz-Cherry, S. A tale of two pandemics: Obesity and COVID-19. J. Travel Med. 2020, 27, taaa097. [Google Scholar] [CrossRef]
- Bhutani, S.; vanDellen, M.R.; Cooper, J.A. Longitudinal Weight Gain and Related Risk Behaviors during the COVID-19 Pandemic in Adults in the US. Nutrients 2021, 13, 671. [Google Scholar] [CrossRef]
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; van Mechelen, W.; Pratt, M. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of Inactivity in Chronic Diseases: Evolutionary Insight and Pathophysiological Mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; McNamara, E.; Tainio, M.; de Sa, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef] [Green Version]
- Pulsford, R.M.; Stamatakis, E.; Britton, A.R.; Brunner, E.J.; Hillsdon, M. Associations of sitting behaviours with all-cause mortality over a 16-year follow-up: The Whitehall II study. Int. J. Epidemiol. 2015, 44, 1909–1916. [Google Scholar] [CrossRef]
- Hupin, D.; Roche, F.; Gremeaux, V.; Chatard, J.C.; Oriol, M.; Gaspoz, J.M.; Barthélémy, J.C.; Edouard, P. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥ 60 years: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1262–1267. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Krogh-Madsen, R. Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J. Appl. Physiol. 2011, 111, 1218–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowden Davies, K.A.; Sprung, V.S.; Norman, J.A.; Thompson, A.; Mitchell, K.L.; Halford, J.C.G.; Harrold, J.A.; Wilding, J.P.H.; Kemp, G.J.; Cuthbertson, D.J. Short-term decreased physical activity with increased sedentary behaviour causes metabolic derangements and altered body composition: Effects in individuals with and without a first-degree relative with type 2 diabetes. Diabetologia 2018, 61, 1282–1294. [Google Scholar] [CrossRef] [Green Version]
- Loh, R.; Stamatakis, E.; Folkerts, D.; Allgrove, J.E.; Moir, H.J. Effects of Interrupting Prolonged Sitting with Physical Activity Breaks on Blood Glucose, Insulin and Triacylglycerol Measures: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 295–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertek, S.; Cicero, A. Impact of physical activity on inflammation: Effects on cardiovascular disease risk and other inflammatory conditions. Arch. Med. Sci. AMS 2012, 8, 794–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 1–18. [Google Scholar] [CrossRef]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e1019. [Google Scholar] [CrossRef] [PubMed]
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e1015. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banu, N.; Panikar, S.S.; Leal, L.R.; Leal, A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: Therapeutic implications. Life Sci. 2020, 256, 117905. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Hellman, U.; Karlsson, M.G.; Engström-Laurent, A.; Cajander, S.; Dorofte, L.; Ahlm, C.; Laurent, C.; Blomberg, A. Presence of hyaluronan in lung alveoli in severe Covid-19: An opening for new treatment options? J. Biol. Chem. 2020, 295, 15418–15422. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef] [PubMed]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive Oxygen and Nitrogen Species Regulate Key Metabolic, Anabolic, and Catabolic Pathways in Skeletal Muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Booth, F.W.; Thomason, D.B. Molecular and cellular adaptation of muscle in response to exercise: Perspectives of various models. Physiol. Rev. 1991, 71, 541–585. [Google Scholar] [CrossRef]
- Malm, C.; Jakobsson, J.; Isaksson, A. Physical Activity and Sports—Real Health Benefits: A Review with Insight into the Public Health of Sweden. Sports 2019, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Brawner, C.A.; Ehrman, J.K.; Bole, S.; Kerrigan, D.J.; Parikh, S.S.; Lewis, B.K.; Gindi, R.M.; Keteyian, C.; Abdul-Nour, K.; Keteyian, S.J. Maximal Exercise Capacity is Inversely Related to Hospitalization Secondary to Coronavirus Disease 2019. Mayo Clin. Proc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Maltagliati, S.; Sieber, S.; Sarrazin, P.; Cullati, S.; Chalabaev, A.; Millet, G.P.; Boisgontier, M.P.; Cheval, B. Muscle Strength Explains the Protective Effect of Physical Activity against COVID-19 Hospitalization among Adults aged 50 Years and Older. medRxiv 2021. [Google Scholar] [CrossRef]
- Halabchi, F.; Mazaheri, R.; Sabeti, K.; Yunesian, M.; Alizadeh, Z.; Ahmadinejad, Z.; Aghili, S.M.; Tavakol, Z. Regular Sports Participation as a Potential Predictor of Better Clinical Outcome in Adult Patients with COVID-19: A Large Cross-Sectional Study. J. Phys. Act. Health 2020, 18, 8–12. [Google Scholar] [CrossRef]
- Gil, S.; Filho, W.J.; Shinjo, S.K.; Ferriolli, E.; Busse, A.L.; Avelino-Silva, T.J.; Longobardi, I.; de Oliveira, G.N.; Swinton, P.; Gualano, B.; et al. Muscle Strength and Muscle Mass as Predictors of Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Prospective Observational Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, J.; Moon, S.Y.; Jin, H.Y.; Yang, J.M.; Ogino, S.; Song, M.; Hong, S.H.; Abou Ghayda, R.; Kronbichler, A.; et al. Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: A nationwide cohort study. Br. J. Sports Med. 2021. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, J.J.; Beck, M.A. The impact of obesity on the immune response to infection. Proc. Nutr. Soc. 2012, 71, 298–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.K.; Feng, Y.; Yuan, M.Y.; Yuan, S.Y.; Fu, H.J.; Wu, B.Y.; Sun, G.Z.; Yang, G.R.; Zhang, X.L.; Wang, L.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Matukas, L.M.; Tomlinson, G.A.; Rachlis, A.R.; Rose, D.B.; Dwosh, H.A.; Walmsley, S.L.; Mazzulli, T.; Avendano, M.; Derkach, P.; et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003, 289, 2801–2809. [Google Scholar] [CrossRef] [Green Version]
- Badawi, A.; Ryoo, S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): A systematic review and meta-analysis. Int. J. Infect. Dis. 2016, 49, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, R.; Nishiura, H.; Kutsuna, S.; Hayakawa, K.; Ohmagari, N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): A systematic review and meta-analysis. BMC Public Health 2016, 16, 1203. [Google Scholar] [CrossRef] [Green Version]
- Van Kerkhove, M.D.; Vandemaele, K.A.H.; Shinde, V.; Jaramillo-Gutierrez, G.; Koukounari, A.; Donnelly, C.A.; Carlino, L.O.; Owen, R.; Paterson, B.; Pelletier, L.; et al. Risk Factors for Severe Outcomes following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis. PLoS Med. 2011, 8, e1001053. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goete, L. Position statement part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef]
- Ahmad, R.; Al-Mass, A.; Atizado, V.; Al-Hubail, A.; Al-Ghimlas, F.; Al-Arouj, M.; Bennakhi, A.; Dermime, S.; Behbehani, K. Elevated expression of the toll like receptors 2 and 4 in obese individuals: Its significance for obesity-induced inflammation. J. Inflamm. 2012, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef] [Green Version]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P.S. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, P.M.; Caplice, N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity 2020, 28, 1191–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A. Novel Coronavirus-Induced NLRP3 Inflammasome Activation: A Potential Drug Target in the Treatment of COVID-19. Front. Immunol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; Verdiá-Báguena, C.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Castaño-Rodriguez, C.; Fernandez-Delgado, R.; Torres, J.; Aguilella, V.M.; Enjuanes, L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 2015, 485, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, S.Y.; Yuen, K.S.; Ye, Z.W.; Chan, C.P.; Jin, D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: Lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020, 9, 558–570. [Google Scholar] [CrossRef]
- Shi, C.-S.; Nabar, N.R.; Huang, N.-N.; Kehrl, J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019, 5, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 inflammasome in severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Paul, O.; Tao, J.Q.; Litzky, L.; Feldman, M.; Montone, K.; Rajapakse, C.; Bermudez, C.; Chatterjee, S. Vascular Inflammation in Lungs of Patients with Fatal Coronavirus Disease 2019 (COVID-19) Infection: Possible role for the NLRP3 inflammasome. medRxiv 2021. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959. [Google Scholar] [CrossRef]
- Kanneganti, T.-D.; Dixit, V.D. Immunological complications of obesity. Nat. Immunol. 2012, 13, 707–712. [Google Scholar] [CrossRef]
- van den Berg, D.F.; te Velde, A.A. Severe COVID-19: NLRP3 Inflammasome Dysregulated. Front. Immunol. 2020, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Ramos Muniz, M.G.; Palfreeman, M.; Setzu, N.; Sanchez, M.A.; Saenz Portillo, P.; Garza, K.M.; Gosselink, K.L.; Spencer, C.T. Obesity Exacerbates the Cytokine Storm Elicited by Francisella tularensis Infection of Females and Is Associated with Increased Mortality. BioMed Res. Int. 2018, 2018, 3412732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, W.D.; Beck, M.A. Obesity Impairs the Adaptive Immune Response to Influenza Virus. Ann. Am. Thorac. Soc. 2017, 14, S406–S409. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2021. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Obesity may hamper sars-cov-2 vaccine immunogenicity. medRxiv 2021. [Google Scholar] [CrossRef]
- Maier, H.E.; Lopez, R.; Sanchez, N.; Ng, S.; Gresh, L.; Ojeda, S.; Burger-Calderon, R.; Kuan, G.; Harris, E.; Balmaseda, A.; et al. Obesity Increases the Duration of Influenza A Virus Shedding in Adults. J. Infect. Dis. 2018, 218, 1378–1382. [Google Scholar] [CrossRef] [Green Version]
- Moriconi, D.; Masi, S.; Rebelos, E.; Virdis, A.; Manca, M.L.; De Marco, S.; Taddei, S.; Nannipieri, M. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes. Res. Clin. Pract. 2020, 14, 205–209. [Google Scholar] [CrossRef]
- Björntorp, P. Endocrine abnormalities of obesity. Metabolism 1995, 44, 21–23. [Google Scholar] [CrossRef]
- Anderson, A.J.; Andrew, R.; Homer, N.Z.M.; Hughes, K.A.; Boyle, L.D.; Nixon, M.; Karpe, F.; Stimson, R.H.; Walker, B.R. Effects of Obesity and Insulin on Tissue-Specific Recycling between Cortisol and Cortisone in Men. J. Clin. Endocrinol. Metab. 2020, 106, e1206–e1220. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.A.; Williamson, P.M.; Mangos, G.; Kelly, J.J. Cardiovascular consequences of cortisol excess. Vasc. Health Risk Manag. 2005, 1, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Leng, Y.; Zhang, Y.; Wu, K.; Ji, Y.; Lei, S.; Xia, Z. Meta-Analysis of coagulation parameters associated with disease severity and poor prognosis of COVID-19. Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. The diseasome of physical inactivity—And the role of myokines in muscle--fat cross talk. J. Physiol. 2009, 587, 5559–5568. [Google Scholar] [CrossRef] [PubMed]
- Bastolla, U. Mathematical model of SARS-Cov-2 propagation versus ACE2 fits COVID-19 lethality across age and sex and predicts that of SARS, supporting possible therapy. Front. Mol. Biosci. 2020, 8, 706122. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.; Xia, X.; Liu, K.; Yu, Z.; Tao, W.; Gong, W.; Han, J.J. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 2020, 19, e13168. [Google Scholar] [CrossRef]
- Kalinchenko, S.Y.; Tishova, Y.A.; Mskhalaya, G.J.; Gooren, L.J.G.; Giltay, E.J.; Saad, F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: The double-blinded placebo-controlled Moscow study. Clin. Endocrinol. 2010, 73, 602–612. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Malkin, C.J.; Pugh, P.J.; Jones, R.D.; Kapoor, D.; Channer, K.S.; Jones, T.H. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metab. 2004, 89, 3313–3318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, L.D.; Herbert, P.; Sculthorpe, N.F.; Grace, F.M. Exercise training improves free testosterone in lifelong sedentary aging men. Endocr. Connect. 2017, 6, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Ari, Z.; Kutlu, N.; Uyanik, B.S.; Taneli, F.; Buyukyazi, G.; Tavli, T. Serum testosterone, growth hormone, and insulin-like growth factor-1 levels, mental reaction time, and maximal aerobic exercise in sedentary and long-term physically trained elderly males. Int. J. Neurosci. 2004, 114, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, V.N.; Foster-Schubert, K.; Chubak, J.; Sorensen, B.; Ulrich, C.M.; Stancyzk, F.Z.; Plymate, S.; Stanford, J.; White, E.; Potter, J.D.; et al. Effect of exercise on serum sex hormones in men: A 12-month randomized clinical trial. Med. Sci. Sports Exerc. 2008, 40, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Cutolo, M.; Sulli, A.; Capellino, S.; Villaggio, B.; Montagna, P.; Seriolo, B.; Straub, R.H. Sex hormones influence on the immune system: Basic and clinical aspects in autoimmunity. Lupus 2004, 13, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Foo, Y.Z.; Nakagawa, S.; Rhodes, G.; Simmons, L.W. The effects of sex hormones on immune function: A meta-analysis. Biol. Rev. Camb. Philos. Soc. 2017, 92, 551–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peretz, J.; Pekosz, A.; Lane, A.P.; Klein, S.L. Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L415–L425. [Google Scholar] [CrossRef]
- Robinson, D.P.; Hall, O.J.; Nilles, T.L.; Bream, J.H.; Klein, S.L. 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J. Virol. 2014, 88, 4711–4720. [Google Scholar] [CrossRef] [Green Version]
- Çayan, S.; Uğuz, M.; Saylam, B.; Akbay, E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: A cohort study. Aging Male 2020, 23, 1493–1503. [Google Scholar] [CrossRef]
- Rastrelli, G.; Di Stasi, V.; Inglese, F.; Beccaria, M.; Garuti, M.; Di Costanzo, D.; Spreafico, F.; Greco, G.F.; Cervi, G.; Pecoriello, A.; et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 2021, 9, 88–98. [Google Scholar] [CrossRef]
- Trumble, B.C.; Blackwell, A.D.; Stieglitz, J.; Thompson, M.E.; Suarez, I.M.; Kaplan, H.; Gurven, M. Associations between male testosterone and immune function in a pathogenically stressed forager-horticultural population. Am. J. Phys. Anthropol. 2016, 161, 494–505. [Google Scholar] [CrossRef]
- Auerbach, J.M.; Khera, M. Testosterone’s Role in COVID-19. J. Sex. Med. 2021, 18, 843–848. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e1039. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Pence, B.D. Exercise immunology: Future directions. J. Sport Health Sci. 2020, 9, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Ascaso-del-Río, A.; Mielgo-Ayuso, J. Physical Exercise as a Multimodal Tool for COVID-19: Could It Be Used as a Preventive Strategy? Int. J. Environ. Res. Public Health 2020, 17, 8496. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Weng, N.P. Aging of the immune system: How much can the adaptive immune system adapt? Immunity 2006, 24, 495–499. [Google Scholar] [CrossRef] [Green Version]
- Goronzy, J.J.; Weyand, C.M. Immune aging and autoimmunity. Cell. Mol. Life Sci. 2012, 69, 1615–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goronzy, J.J.; Weyand, C.M. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity—catalysts of autoimmunity and chronic inflammation. Arthritis Res. Ther. 2003, 5, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.-J.; Lee, J.K.; Shin, O.S. Aging and the Immune System: The Impact of Immunosenescence on Viral Infection, Immunity and Vaccine Immunogenicity. Immune Netw. 2019, 19, e37. [Google Scholar] [CrossRef]
- Cavanagh, M.M.; Weyand, C.M.; Goronzy, J.J. Chronic inflammation and aging: DNA damage tips the balance. Curr. Opin. Immunol. 2012, 24, 488–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Turner, J.E. Is immunosenescence influenced by our lifetime “dose” of exercise? Biogerontology 2016, 17, 581–602. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.E.; Brum, P.C. Does Regular Exercise Counter T Cell Immunosenescence Reducing the Risk of Developing Cancer and Promoting Successful Treatment of Malignancies? Oxid. Med. Cell. Longev. 2017, 2017, 4234765. [Google Scholar] [CrossRef]
- Collao, N.; Rada, I.; Francaux, M.; Deldicque, L.; Zbinden-Foncea, H. Anti-Inflammatory Effect of Exercise Mediated by Toll-Like Receptor Regulation in Innate Immune Cells—A Review. Int. Rev. Immunol. 2020, 39, 39–52. [Google Scholar] [CrossRef] [PubMed]
- da Luz Scheffer, D.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Et Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Antiviral signaling through pattern recognition receptors. J. Biochem. 2007, 141, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Panda, A.; Joshi, S.R.; Qian, F.; Allore, H.G.; Montgomery, R.R. Dysregulation of human Toll-like receptor function in aging. Ageing Res. Rev. 2011, 10, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleeson, M.; McFarlin, B.; Flynn, M. Exercise and Toll-like receptors. Exerc. Immunol. Rev. 2006, 12, 34–53. [Google Scholar] [PubMed]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Flynn, M.G.; McFarlin, B.K. Toll-like receptor 4: Link to the anti-inflammatory effects of exercise? Exerc. Sport Sci. Rev. 2006, 34, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kuang, M.; Li, J.; Zhu, L.; Jia, Z.; Guo, X.; Hu, Y.; Kong, J.; Yin, H.; Wang, X.; et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021, 1–3. [Google Scholar] [CrossRef]
- Brandão, S.C.S.; Ramos, J.O.X.; Dompieri, L.T.; Godoi, E.; Figueiredo, J.L.; Sarinho, E.S.C.; Chelvanambi, S.; Aikawa, M. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev. 2020. [Google Scholar] [CrossRef]
- Olejnik, J.; Hume, A.J.; Mühlberger, E. Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathog. 2018, 14, e1007390. [Google Scholar] [CrossRef] [Green Version]
- Aboudounya, M.M.; Heads, R.J. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediat. Inflamm. 2021, 2021, 8874339. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Markoff, P.A.; Balk-Lamberton, A.J.; Yang, H.; Chritton, D.B.; Lee, J.W.; Arabatzis, K. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int. J. Sports Med. 1990, 11, 467–473. [Google Scholar] [CrossRef]
- Kohut, M.L.; Arntson, B.A.; Lee, W.; Rozeboom, K.; Yoon, K.J.; Cunnick, J.E.; McElhaney, J. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine 2004, 22, 2298–2306. [Google Scholar] [CrossRef]
- Woods, J.A.; Keylock, K.T.; Lowder, T.; Vieira, V.J.; Zelkovich, W.; Dumich, S.; Colantuano, K.; Lyons, K.; Leifheit, K.; Cook, M.; et al. Cardiovascular exercise training extends influenza vaccine seroprotection in sedentary older adults: The immune function intervention trial. J. Am. Geriatr. Soc. 2009, 57, 2183–2191. [Google Scholar] [CrossRef]
- Pascoe, A.R.; Fiatarone Singh, M.A.; Edwards, K.M. The effects of exercise on vaccination responses: A review of chronic and acute exercise interventions in humans. Brain. Behav. Immun. 2014, 39, 33–41. [Google Scholar] [CrossRef]
- Simpson, R.J.; Hussain, M.; Baker, F.; Bigley, A.B.; Peek, M.K.; Stowe, R.P. Cardiorespiratory fitness is associated with better control of latent herpesvirus infections in a large ethnically diverse community sample: Evidence from the Texas City Stress and Health Study. Brain. Behav. Immun. 2017, 66, e35. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.-Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal. Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef] [PubMed]
- Rundqvist, H.; Veliça, P.; Barbieri, L.; Gameiro, P.A.; Bargiela, D.; Gojkovic, M.; Mijwel, S.; Reitzner, S.M.; Wulliman, D.; Ahlstedt, E.; et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. Elife 2020, 9, e59996. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, G.P.; da Silva, I.M.; Santos, M.A.; Elsner, V.R.; Fonseca, S.G.; Peres, A.; Romão, P.R.T. Immunoregulation induced by autologous serum collected after acute exercise in obese men: A randomized cross-over trial. Sci. Rep. 2020, 10, 21735. [Google Scholar] [CrossRef] [PubMed]
- Simões, H.G.; Rosa, T.S.; Sousa, C.V.; Aguiar, S.d.S.; Motta-Santos, D.; Degens, H.; Korhonen, M.T.; Campbell, C.S.G. Does Longer Leukocyte Telomere Length and Higher Physical Fitness Protect Master Athletes From Consequences of Coronavirus (SARS-CoV-2) Infection? Front. Sports Act. Living 2020, 2, 87. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, P.; Fisher, R.; Kallings, L.; Svenson, U.; Roos, G.; Hellénius, M.L. Stand up for health--avoiding sedentary behaviour might lengthen your telomeres: Secondary outcomes from a physical activity RCT in older people. Br. J. Sports Med. 2014, 48, 1407–1409. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Green, G.; Zhuo, H.; Liu, K.D.; Kangelaris, K.N.; Gomez, A.; Jauregui, A.; Vessel, K.; Ke, S. Peripheral blood leukocyte telomere length is associated with survival of sepsis patients. Eur. Respir. J. 2020, 55, 1901044. [Google Scholar] [CrossRef]
- Wang, Q.; Codd, V.; Raisi-Estabragh, Z.; Musicha, C.; Bountziouka, V.; Kaptoge, S.; Allara, E.; Angelantonio, E.D.; Butterworth, A.S.; Wood, A.M.; et al. Older biological age is associated with adverse COVID-19 outcomes: A cohort study in UK Biobank. medRxiv 2021. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, R.; Guío-Carrión, A.; Zapatero-Gaviria, A.; Martínez, P.; Blasco, M.A. Shorter telomere lengths in patients with severe COVID-19 disease. Aging 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise Attenuates the Major Hallmarks of Aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.A.; Sagot, Y.; Hedou, G.; Grek, C.; Wilkes, T.; Vinik, A.I.; Ghatnekar, G. Low-Dose Pulsatile Interleukin-6 As a Treatment Option for Diabetic Peripheral Neuropathy. Front. Endocrinol. 2017, 8, 89. [Google Scholar] [CrossRef]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol.-Cell Physiol. 2010, 298, C807–C816. [Google Scholar] [CrossRef] [Green Version]
- Rinnov, A.; Yfanti, C.; Nielsen, S.; Akerström, T.C.; Peijs, L.; Zankari, A.; Fischer, C.P.; Pedersen, B.K. Endurance training enhances skeletal muscle interleukin-15 in human male subjects. Endocrine 2014, 45, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.L.; Bérard, M.; Soares, M.V.; Oldham, J.; Cook, J.E.; Akbar, A.N.; Tough, D.F.; Beverley, P.C. Prolonged exposure of naïve CD8+ T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length. Immunology 2006, 119, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.-Y.; Lichy, J.H.; Waldmann, T.A.; Perera, L.P. The role of interleukin-15 in inflammation and immune responses to infection: Implications for its therapeutic use. Microbes Infect. 2012, 14, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Attar, A.; Presnell, S.R.; Clasey, J.L.; Long, D.E.; Walton, R.G.; Sexton, M.; Starr, M.E.; Kern, P.A.; Peterson, C.A.; Lutz, C.T. Human Body Composition and Immunity: Visceral Adipose Tissue Produces IL-15 and Muscle Strength Inversely Correlates with NK Cell Function in Elderly Humans. Front. Immunol. 2018, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Mahenthiran, A.K.; Mahenthiran, A.K.; Mahenthiran, J. Cardiovascular system and COVID-19: Manifestations and therapeutics. Rev. Cardiovasc. Med. 2020, 21, 399–409. [Google Scholar] [CrossRef]
- Wilson, M.G.; Ellison, G.M.; Cable, N.T. Basic science behind the cardiovascular benefits of exercise. Br. J. Sports Med. 2016, 50, 93–99. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr. An “Exercise” in Cardiac Metabolism. Front. Cardiovasc. Med. 2018, 5, 66. [Google Scholar] [CrossRef]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B.; Ludwig, D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020, 16, 341–342. [Google Scholar] [CrossRef] [Green Version]
- Borges, J.P.; da Silva Verdoorn, K. Cardiac Ischemia/Reperfusion Injury: The Beneficial Effects of Exercise. Adv. Exp. Med. Biol. 2017, 999, 155–179. [Google Scholar] [CrossRef]
- Penna, C.; Alloatti, G.; Crisafulli, A. Mechanisms Involved in Cardioprotection Induced by Physical Exercise. Antioxid Redox Signal. 2020, 32, 1115–1134. [Google Scholar] [CrossRef]
- Pranata, R.; Lim, M.A.; Yonas, E.; Vania, R.; Lukito, A.A.; Siswanto, B.B.; Meyer, M. Body mass index and outcome in patients with COVID-19: A dose-response meta-analysis. Diabetes Metab. 2020, 47, 101178. [Google Scholar] [CrossRef]
- Paton, C.M.; Brandauer, J.; Weiss, E.P.; Brown, M.D.; Ivey, F.M.; Roth, S.M.; Hagberg, J.M. Hemostatic response to postprandial lipemia before and after exercise training. J. Appl. Physiol. 2006, 101, 316–321. [Google Scholar] [CrossRef]
- Schnohr, P.; O’Keefe, J.H.; Lange, P.; Jensen, G.B.; Marott, J.L. Impact of persistence and non-persistence in leisure time physical activity on coronary heart disease and all-cause mortality: The Copenhagen City Heart Study. Eur. J. Prev. Cardiol. 2017, 24, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yu, C.; Guo, Y.; Bian, Z.; Li, X.; Yang, L.; Chen, Y.; Li, M.; Li, X.; Chen, J.; et al. Effect of total, domain-specific, and intensity-specific physical activity on all-cause and cardiovascular mortality among hypertensive adults in China. J. Hypertens. 2018, 36, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Jung, W.S.; Park, W.; Park, H.Y. Twelve Weeks of Combined Resistance and Aerobic Exercise Improves Cardiometabolic Biomarkers and Enhances Red Blood Cell Hemorheological Function in Obese Older Men: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 5020. [Google Scholar] [CrossRef] [Green Version]
- Warner, F.J.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Angiotensin-converting enzyme-2: A molecular and cellular perspective. Cell. Mol. Life Sci. 2004, 61, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Frantz, E.; Dalla, C.; Prodel, E.; Braz, I.D.; Giori, I.G.; Bargut, T.; Cristina, L.; Magliano, D.; Angelo, C.; Nobrega, A.; et al. Modulation of the renin–angiotensin system in white adipose tissue and skeletal muscle: Focus on exercise training. Clin. Sci. 2018, 132, 1487–1507. [Google Scholar] [CrossRef]
- Evangelista, F.S. Physical Exercise and the Renin Angiotensin System: Prospects in the COVID-19. Front. Physiol. 2020, 11, 1282. [Google Scholar] [CrossRef]
- Labò, N.; Ohnuki, H.; Tosato, G. Vasculopathy and Coagulopathy Associated with SARS-CoV-2 Infection. Cells 2020, 9, 1583. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.J.; Xie, J.; Liu, Y.M.; Zhao, Y.C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients with Hypertension Hospitalized with COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Zbinden-Foncea, H.; Francaux, M.; Deldicque, L.; Hawley, J.A. Does high cardiorespiratory fitness confer some protection against pro-inflammatory responses after infection by SARS-CoV-2? Obesity 2020, 28, 1378–1381. [Google Scholar] [CrossRef]
- Motta-Santos, D.; Santos, R.A.S.; Santos, S.H.S. Angiotensin-(1-7) and Obesity: Role in Cardiorespiratory Fitness and COVID-19 Implications. Obesity 2020, 28, 1786. [Google Scholar] [CrossRef]
- Magalhaes, G.S.; Rodrigues-Machado, M.D.G.; Motta-Santos, D.; Campagnole-Santos, M.J.; Santos, R.A.S. Activation of Ang-(1-7)/Mas Receptor Is a Possible Strategy to Treat Coronavirus (SARS-CoV-2) Infection. Front. Physiol. 2020, 11, 730. [Google Scholar] [CrossRef]
- Lo, K.B.; Bhargav, R.; Salacup, G.; Pelayo, J.; Albano, J.; McCullough, P.A.; Rangaswami, J. Angiotensin converting enzyme inhibitors and angiotensin II receptor blockers and outcomes in patients with COVID-19: A systematic review and meta-analysis. Expert Rev. Cardiovasc. Ther. 2020, 18, 919–930. [Google Scholar] [CrossRef]
- McKenzie, D.C. Respiratory physiology: Adaptations to high-level exercise. Br. J. Sports Med. 2012, 46, 381. [Google Scholar] [CrossRef] [PubMed]
- Tedjasaputra, V.; Bouwsema, M.M.; Stickland, M.K. Effect of aerobic fitness on capillary blood volume and diffusing membrane capacity responses to exercise. J. Physiol. 2016, 594, 4359–4370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffman, K.E.; Carlson, A.R.; Miller, A.D.; Johnson, B.D.; Taylor, B.J. The effect of aging and cardiorespiratory fitness on the lung diffusing capacity response to exercise in healthy humans. J. Appl. Physiol. 2017, 122, 1425–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis Goncalves, C.T.; Reis Goncalves, C.G.; de Almeida, F.M.; Lopes, F.D.; dos Santos Durao, A.C.; dos Santos, F.A.; da Silva, L.F.; Marcourakis, T.; Castro-Faria-Neto, H.C.; Vieira, R.P.; et al. Protective effects of aerobic exercise on acute lung injury induced by LPS in mice. Crit. Care 2012, 16, R199. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Liu, T.; Nieman, D.C.; Cui, Y.; Li, F.; Yang, L.; Shi, H.; Chen, P. Aerobic Exercise Attenuates Acute Lung Injury through NET Inhibition. Front. Immunol. 2020, 11, 409. [Google Scholar] [CrossRef]
- Toledo, A.C.; Magalhaes, R.M.; Hizume, D.C.; Vieira, R.P.; Biselli, P.J.C.; Moriya, H.T.; Mauad, T.; Lopes, F.D.T.Q.S.; Martins, M.A. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur. Respir. J. 2012, 39, 254. [Google Scholar] [CrossRef] [Green Version]
- Murugan, A.T.; Sharma, G. Obesity and respiratory diseases. Chron. Respir. Dis. 2008, 5, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Chen, M.-S.; Yu, H. The relationship between obstructive sleep apnea and obesity hypoventilation syndrome: A systematic review and meta-analysis. Oncotarget 2017, 8, 93168–93178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, L.; Zou, X.; Shen, Y.; Hu, D.; Hu, X.; Li, Z.; Kamel, I.R. Visceral Adiposity and High Intramuscular Fat Deposition Independently Predict Critical Illness in Patients with Sars-COV-2. Obesity 2020, 28, 2040–2048. [Google Scholar] [CrossRef]
- Petersen, A.; Bressem, K.; Albrecht, J.; Thieß, H.M.; Vahldiek, J.; Hamm, B.; Makowski, M.R.; Niehues, A.; Niehues, S.M.; Adams, L.C. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism 2020, 110, 154317. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Q.; Zhang, W.; Yu, S.; Cheng, X.; Wang, L.; Chen, X.; Chen, Y. The Involvement of Chronic Kidney Disease and Acute Kidney Injury in Disease Severity and Mortality in Patients with COVID-19: A Meta-Analysis. Kidney Blood Press. Res. 2021, 46, 17–30. [Google Scholar] [CrossRef]
- Hapca, S.; Siddiqui, M.K.; Kwan, R.S.Y.; Lim, M.; Matthew, S.; Doney, A.S.F.; Pearson, E.R.; Palmer, C.N.A.; Bell, S. The Relationship between AKI and CKD in Patients with Type 2 Diabetes: An Observational Cohort Study. J. Am. Soc. Nephrol. 2021, 32, 138. [Google Scholar] [CrossRef]
- Wang, S.; Ma, P.; Zhang, S.; Song, S.; Wang, Z.; Ma, Y.; Xu, J.; Wu, F.; Duan, L.; Yin, Z.; et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: A multi-centre retrospective study. Diabetologia 2020, 63, 2102–2111. [Google Scholar] [CrossRef]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- Armaly, Z.; Kinaneh, S.; Skorecki, K. Renal Manifestations of Covid-19: Physiology and Pathophysiology. J. Clin. Med. 2021, 10, 1216. [Google Scholar] [CrossRef]
- Costa, L.B.; Perez, L.G.; Palmeira, V.A.; Macedo, E.C.T.; Ribeiro, V.T.; Lanza, K.; Simões, E.S.A.C. Insights on SARS-CoV-2 Molecular Interactions With the Renin-Angiotensin System. Front. Cell Dev. Biol. 2020, 8, 559841. [Google Scholar] [CrossRef]
- Stump, C.S. Physical Activity in the Prevention of Chronic Kidney Disease. Cardiorenal Med. 2011, 1, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kuro, O.M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, Aging, and the Failing Kidney. Front. Endocrinol. 2020, 11, 560. [Google Scholar] [CrossRef]
- Saar-Kovrov, V.; Donners, M.M.P.C.; van der Vorst, E.P.C. Shedding of Klotho: Functional Implications in Chronic Kidney Disease and Associated Vascular Disease. Front. Cardiovasc. Med. 2021, 7, 407. [Google Scholar] [CrossRef]
- Nakanishi, K.; Nishida, M.; Taneike, M.; Yamamoto, R.; Moriyama, T.; Yamauchi-Takihara, K. Serum Klotho Levels Contribute to the Prevention of Disease Progression. Int. J. Gen. Med. 2021, 14, 229–236. [Google Scholar] [CrossRef]
- Mancusi, C.; Izzo, R.; di Gioia, G.; Losi, M.A.; Barbato, E.; Morisco, C. Insulin Resistance the Hinge Between Hypertension and Type 2 Diabetes. High. Blood Press. Cardiovasc. Prev. 2020, 27, 515–526. [Google Scholar] [CrossRef]
- Rosa, T.D.S.; Corrêa, H.L.; Barbosa, L.P.; Santos, P.A.D.; Leite, P.L.A.; Aguiar, S.S.; Deus, L.A.; Maciel, L.A.; Neves, R.V.P.; Simoes, H.G. Age-related Decline in Renal Function is Attenuated in Master Athletes. Int. J. Sports Med. 2021. [Google Scholar] [CrossRef]
- Mostafidi, E.; Moeen, A.; Nasri, H.; Ghorbani Hagjo, A.; Ardalan, M. Serum Klotho Levels in Trained Athletes. Nephrourol. Mon. 2016, 8, e30245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middelbeek, R.J.W.; Motiani, P.; Brandt, N.; Nigro, P.; Zheng, J.; Virtanen, K.A.; Kalliokoski, K.K.; Hannukainen, J.C.; Goodyear, L.J. Exercise intensity regulates cytokine and klotho responses in men. Nutr. Diabetes 2021, 11, 5. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la, O.A.; Jurado-Fasoli, L.; Ruiz, J.R.; Castillo, M.J. Association of basal metabolic rate and fuel oxidation in basal conditions and during exercise, with plasma S-klotho: The FIT-AGEING study. Aging 2019, 11, 5319–5333. [Google Scholar] [CrossRef]
- Ji, N.; Luan, J.; Hu, F.; Zhao, Y.; Lv, B.; Wang, W.; Xia, M.; Zhao, X.; Lao, K. Aerobic exercise-stimulated Klotho upregulation extends life span by attenuating the excess production of reactive oxygen species in the brain and kidney. Exp. Ther. Med. 2018, 16, 3511–3517. [Google Scholar] [CrossRef] [Green Version]
- Rao, Z.; Zheng, L.; Huang, H.; Feng, Y.; Shi, R. α-Klotho Expression in Mouse Tissues Following Acute Exhaustive Exercise. Front. Physiol. 2019, 10, 1498. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, T.; Miyaki, A.; Akazawa, N.; Choi, Y.; Ra, S.G.; Tanahashi, K.; Kumagai, H.; Oikawa, S.; Maeda, S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H348–H355. [Google Scholar] [CrossRef] [Green Version]
- Conn, V.S.; Koopman, R.J.; Ruppar, T.M.; Phillips, L.J.; Mehr, D.R.; Hafdahl, A.R. Insulin Sensitivity Following Exercise Interventions: Systematic Review and Meta-Analysis of Outcomes Among Healthy Adults. J. Prim. Care Community Health 2014, 5, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Alberca, G.G.F.; Solis-Castro, R.L.; Solis-Castro, M.E.; Alberca, R.W. Coronavirus disease-2019 and the intestinal tract: An overview. World J. Gastroenterol. 2021, 27, 1255–1266. [Google Scholar] [CrossRef]
- Harmer, D.; Gilbert, M.; Borman, R.; Clark, K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002, 532, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.D.; Allen, J.M.; Pence, B.D.; Wallig, M.A.; Gaskins, H.R.; White, B.A.; Woods, J.A. Exercise and gut immune function: Evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunol. Cell Biol. 2016, 94, 158–163. [Google Scholar] [CrossRef]
- Guo, M.; Tao, W.; Flavell, R.A.; Zhu, S. Potential intestinal infection and faecal–oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Corsetti, G.; Assanelli, D.; Testa, C.; Romano, C.; Dioguardi, F.S.; Aquilani, R. Effects of chronic exercise on gut microbiota and intestinal barrier in human with type 2 diabetes. Minerva Med. 2019, 110, 3–11. [Google Scholar] [CrossRef]
- Kim, Y.J.; Womble, J.T.; Gunsch, C.K.; Ingram, J.L. The Gut/Lung Microbiome Axis in Obesity, Asthma, and Bariatric Surgery: A Literature Review. Obesity 2021, 29, 636–644. [Google Scholar] [CrossRef]
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut-lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017, 43, 81–95. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Coyle, E.F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2016, 2, e000143. [Google Scholar] [CrossRef] [Green Version]
- Gemmink, A.; Schrauwen, P.; Hesselink, M.K.C. Exercising your fat (metabolism) into shape: A muscle-centred view. Diabetologia 2020, 63, 1453–1463. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Exercise Metabolism: Fuels for the Fire. Cold Spring Harb. Perspect. Med. 2018, 8, a029744. [Google Scholar] [CrossRef]
- Coyle, E.F. Physical activity as a metabolic stressor. Am. J. Clin. Nutr. 2000, 72, 512s–520s. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D.; Kang, C.; Zhang, T. The role of mitochondria in redox signaling of muscle homeostasis. J. Sport Health Sci. 2020, 9, 386–393. [Google Scholar] [CrossRef]
- Phillips, S.A.; Das, E.; Wang, J.; Pritchard, K.; Gutterman, D.D. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J. Appl. Physiol. 2011, 110, 1013–1020. [Google Scholar] [CrossRef] [Green Version]
- MacDougall, J.D.; Tuxen, D.; Sale, D.G.; Moroz, J.R.; Sutton, J.R. Arterial blood pressure response to heavy resistance exercise. J. Appl. Physiol. 1985, 58, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazeminia, M.; Daneshkhah, A.; Jalali, R.; Vaisi-Raygani, A.; Salari, N.; Mohammadi, M. The Effect of Exercise on the Older Adult’s Blood Pressure Suffering Hypertension: Systematic Review and Meta-Analysis on Clinical Trial Studies. Int. J. Hypertens. 2020, 2020, 2786120. [Google Scholar] [CrossRef]
- Oliver-Martínez, P.A.; Ramos-Campo, D.J.; Martínez-Aranda, L.M.; Martínez-Rodríguez, A.; Rubio-Arias, J. Chronic effects and optimal dosage of strength training on SBP and DBP: A systematic review with meta-analysis. J. Hypertens. 2020, 38, 1909–1918. [Google Scholar] [CrossRef]
- Green, D.J.; Hopman, M.T.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef]
- Barretti, D.L.; Magalhães Fde, C.; Fernandes, T.; do Carmo, E.C.; Rosa, K.T.; Irigoyen, M.C.; Negrão, C.E.; Oliveira, E.M. Effects of aerobic exercise training on cardiac renin-angiotensin system in an obese Zucker rat strain. PLoS ONE 2012, 7, e46114. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.Z.; Yang, Y.H.; Sun, J.C.; Wu, Z.T.; Zhang, R.W.; Shen, D.; Wang, Y.K. Exercise Training Improves the Altered Renin-Angiotensin System in the Rostral Ventrolateral Medulla of Hypertensive Rats. Oxid. Med. Cell. Longev. 2016, 2016, 7413963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinshi, Y.; Higashiura, K.; Yoshida, D.; Togashi, N.; Yoshida, H.; Miyazaki, Y.; Ura, N.; Shimamoto, K. Angiotensin II inhibits glucose uptake of skeletal muscle via the adenosine monophosphate-activated protein kinase pathway. J. Am. Soc. Hypertens. 2007, 1, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, J.C.; Wilson, R.J.; Yan, Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016, 30, 13–22. [Google Scholar] [CrossRef]
- Hall, E.C.R.; Murgatroyd, C.; Stebbings, G.K.; Cunniffe, B.; Harle, L.; Salter, M.; Ramadass, A.; Westra, J.W.; Hunter, E.; Akoulitchev, A.; et al. The Prospective Study of Epigenetic Regulatory Profiles in Sport and Exercise Monitored Through Chromosome Conformation Signatures. Genes 2020, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Sany, M.R.U.; Islam, M.S.; Islam, A. Epigenetic Regulator miRNA Pattern Differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 World-Wide Isolates Delineated the Mystery Behind the Epic Pathogenicity and Distinct Clinical Characteristics of Pandemic COVID-19. Front. Genet. 2020, 11, 765. [Google Scholar] [CrossRef]
- Mitash, N.; Donovan, J.E.; Swiatecka-Urban, A. The Role of MicroRNA in the Airway Surface Liquid Homeostasis. Int. J. Mol. Sci. 2020, 21, 3848. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Donaldson, A.; Natanek, S.A.; Lewis, A.; Man, W.D.; Hopkinson, N.S.; Polkey, M.I.; Kemp, P.R. Increased skeletal muscle-specific microRNA in the blood of patients with COPD. Thorax 2013, 68, 1140–1149. [Google Scholar] [CrossRef] [Green Version]
- Sardar, R.; Satish, D.; Birla, S.; Gupta, D. Integrative analyses of SARS-CoV-2 genomes from different geographical locations reveal unique features potentially consequential to host-virus interaction, pathogenesis and clues for novel therapies. Heliyon 2020, 6, e04658. [Google Scholar] [CrossRef]
- Schmitz, B.; Breulmann, F.L.; Jubran, B.; Rolfes, F.; Thorwesten, L.; Krüger, M.; Klose, A.; Schnittler, H.J.; Brand, S.M. A three-step approach identifies novel shear stress-sensitive endothelial microRNAs involved in vasculoprotective effects of high-intensity interval training (HIIT). Oncotarget 2019, 10, 3625–3640. [Google Scholar] [CrossRef]
- Camera, D.M.; Ong, J.N.; Coffey, V.G.; Hawley, J.A. Selective Modulation of MicroRNA Expression with Protein Ingestion Following Concurrent Resistance and Endurance Exercise in Human Skeletal Muscle. Front. Physiol. 2016, 7, 87. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, R.F.; Markworth, J.F.; Aasen, K.M.M.; Zeng, N.; Cameron-Smith, D.; Mitchell, C.J. Acute resistance exercise modulates microRNA expression profiles: Combined tissue and circulatory targeted analyses. PLoS ONE 2017, 12, e0181594. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Woodhead, J.S.T.; Zeng, N.; Blenkiron, C.; Merry, T.L.; Cameron-Smith, D.; Mitchell, C.J. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E723–E733. [Google Scholar] [CrossRef]
- Silver, J.L.; Alexander, S.E.; Dillon, H.T.; Lamon, S.; Wadley, G.D. Extracellular vesicular miRNA expression is not a proxy for skeletal muscle miRNA expression in males and females following acute, moderate intensity exercise. Physiol. Rep. 2020, 8, e14520. [Google Scholar] [CrossRef]
- McGee, S.L.; Hargreaves, M. Histone modifications and exercise adaptations. J. Appl. Physiol. 2011, 110, 258–263. [Google Scholar] [CrossRef]
- Tian, H.; Liu, S.; Ren, J.; Lee, J.K.W.; Wang, R.; Chen, P. Role of Histone Deacetylases in Skeletal Muscle Physiology and Systemic Energy Homeostasis: Implications for Metabolic Diseases and Therapy. Front. Physiol. 2020, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Javaid, N.; Choi, S. Acetylation- and Methylation-Related Epigenetic Proteins in the Context of Their Targets. Genes 2017, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Turner, D.C.; Gorski, P.P.; Maasar, M.F.; Seaborne, R.A.; Baumert, P.; Brown, A.D.; Kitchen, M.O.; Erskine, R.M.; Dos-Remedios, I.; Voisin, S.; et al. DNA methylation across the genome in aged human skeletal muscle tissue and muscle-derived cells: The role of HOX genes and physical activity. Sci. Rep. 2020, 10, 15360. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Shimizu, J.; Kawano, F.; Kim, H.J.; Kim, C.K. Adaptive responses of histone modifications to resistance exercise in human skeletal muscle. PLoS ONE 2020, 15, e0231321. [Google Scholar] [CrossRef] [Green Version]
- Seaborne, R.A.; Strauss, J.; Cocks, M.; Shepherd, S.; O’Brien, T.D.; van Someren, K.A.; Bell, P.G.; Murgatroyd, C.; Morton, J.P.; Stewart, C.E.; et al. Human Skeletal Muscle Possesses an Epigenetic Memory of Hypertrophy. Sci. Rep. 2018, 8, 1898. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Shaath, H.; Alajez, N.M. Protein Coding and Long Noncoding RNA (lncRNA) Transcriptional Landscape in SARS-CoV-2 Infected Bronchial Epithelial Cells Highlight a Role for Interferon and Inflammatory Response. Genes 2020, 11, 760. [Google Scholar] [CrossRef]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 2019, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Bonilauri, B.; Dallagiovanna, B. Long Non-coding RNAs Are Differentially Expressed after Different Exercise Training Programs. Front. Physiol. 2020, 11, 567614. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M. Exercise and Gene Expression. Prog. Mol. Biol. Transl. Sci. 2015, 135, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Howlett, K.F.; McGee, S.L. Epigenetic regulation of skeletal muscle metabolism. Clin. Sci. 2016, 130, 1051–1063. [Google Scholar] [CrossRef]
- Jacques, M.; Hiam, D.; Craig, J.; Barrès, R.; Eynon, N.; Voisin, S. Epigenetic changes in healthy human skeletal muscle following exercise- a systematic review. Epigenetics 2019, 14, 633–648. [Google Scholar] [CrossRef]
- Widmann, M.; Nieß, A.M.; Munz, B. Physical Exercise and Epigenetic Modifications in Skeletal Muscle. Sports Med. 2019, 49, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Soplinska, A.; Zareba, L.; Wicik, Z.; Eyileten, C.; Jakubik, D.; Siller-Matula, J.M.; De Rosa, S.; Malek, L.A.; Postula, M. MicroRNAs as Biomarkers of Systemic Changes in Response to Endurance Exercise-A Comprehensive Review. Diagnostics 2020, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Seaborne, R.A.; Sharples, A.P. The Interplay Between Exercise Metabolism, Epigenetics, and Skeletal Muscle Remodeling. Exerc. Sport Sci. Rev. 2020, 48, 188–200. [Google Scholar] [CrossRef]
- Ferrari, L.; Vicenzi, M.; Tarantini, L.; Barretta, F.; Sironi, S.; Baccarelli, A.A.; Guazzi, M.; Bollati, V. Effects of Physical Exercise on Endothelial Function and DNA Methylation. Int. J. Environ. Res. Public Health 2019, 16, 2530. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.S.; Sapp, R.M.; Kim, K.I.; Heilman, J.M.; Hagberg, J.; Prior, S.J. Effects of Exercise Training on the Paracrine Function of Circulating Angiogenic Cells. Int. J. Sports Med. 2020. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refolo, G.; Vescovo, T.; Piacentini, M.; Fimia, G.M.; Ciccosanti, F. Mitochondrial Interactome: A Focus on Antiviral Signaling Pathways. Front. Cell Dev. Biol. 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Bilet, L.; Phielix, E.; van de Weijer, T.; Gemmink, A.; Bosma, M.; Moonen-Kornips, E.; Jorgensen, J.A.; Schaart, G.; Zhang, D.; Meijer, K.; et al. One-leg inactivity induces a reduction in mitochondrial oxidative capacity, intramyocellular lipid accumulation and reduced insulin signalling upon lipid infusion: A human study with unilateral limb suspension. Diabetologia 2020, 63, 1211–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Aging, Male Sex, Obesity, and Metabolic Inflammation Create the Perfect Storm for COVID-19. Diabetes 2020, 69, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Trott, D.W.; Fadel, P.J. Inflammation as a mediator of arterial ageing. Exp. Physiol. 2019, 104, 1455–1471. [Google Scholar] [CrossRef]
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020, 21, 315–319. [Google Scholar] [CrossRef]
- Devries, M.C.; Samjoo, I.A.; Hamadeh, M.J.; McCready, C.; Raha, S.; Watt, M.J.; Steinberg, G.R.; Tarnopolsky, M.A. Endurance training modulates intramyocellular lipid compartmentalization and morphology in skeletal muscle of lean and obese women. J. Clin. Endocrinol. Metab. 2013, 98, 4852–4862. [Google Scholar] [CrossRef] [Green Version]
- Meex, R.C.; Schrauwen-Hinderling, V.B.; Moonen-Kornips, E.; Schaart, G.; Mensink, M.; Phielix, E.; van de Weijer, T.; Sels, J.P.; Schrauwen, P.; Hesselink, M.K. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 2010, 59, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.K.; Chaubey, G.; Chen, J.Y.; Suravajhala, P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol.-Cell Physiol. 2020, 319, C258–C267. [Google Scholar] [CrossRef]
- Ajaz, S.; McPhail, M.J.; Singh, K.K.; Mujib, S.; Trovato, F.M.; Napoli, S.; Agarwal, K. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am. J. Physiol.-Cell Physiol. 2021, 320, C57–C65. [Google Scholar] [CrossRef]
- Burtscher, J.; Cappellano, G.; Omori, A.; Koshiba, T.; Millet, G.P. Mitochondria: In the Cross Fire of SARS-CoV-2 and Immunity. iScience 2020, 23, 101631. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Millet, G.P.; Burtscher, M. Low cardiorespiratory and mitochondrial fitness as risk factors in viral infections: Implications for COVID-19. Br. J. Sports Med. 2020. [Google Scholar] [CrossRef]
- Park, S.Y.; Rossman, M.J.; Gifford, J.R.; Bharath, L.P.; Bauersachs, J.; Richardson, R.S.; Abel, E.D.; Symons, J.D.; Riehle, C. Exercise training improves vascular mitochondrial function. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H821–H829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somani, S.M.; Husain, K. Exercise training alters kinetics of antioxidant enzymes in rat tissues. Biochem. Mol. Biol. Int. 1996, 38, 587–595. [Google Scholar] [PubMed]
- Chang, C.H.; Kao, C.H.; Chio, C.C.; Lin, C.H.; Lin, M.T.; Chang, C.P. Attenuating heatstroke-induced acute lung inflammation, edema, and injury in rats by exercise preconditioning. J. Trauma. Acute Care Surg. 2013, 74, 1052–1059. [Google Scholar] [CrossRef] [Green Version]
- Trangmar, S.J.; Chiesa, S.T.; Kalsi, K.K.; Secher, N.H.; González-Alonso, J. Whole body hyperthermia, but not skin hyperthermia, accelerates brain and locomotor limb circulatory strain and impairs exercise capacity in humans. Physiol. Rep. 2017, 5, e13108. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, D.C.; Febbraio, M.A.; Hargreaves, M. Heat shock proteins and exercise adaptations. Our knowledge thus far and the road still ahead. J. Appl. Physiol. 2016, 120, 683–691. [Google Scholar] [CrossRef]
- Krause, M.; Heck, T.G.; Bittencourt, A.; Scomazzon, S.P.; Newsholme, P.; Curi, R.; Homem de Bittencourt, P.I., Jr. The chaperone balance hypothesis: The importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat. Inflamm. 2015, 2015, 249205. [Google Scholar] [CrossRef]
- Heck, T.G.; Ludwig, M.S.; Frizzo, M.N.; Rasia-Filho, A.A.; Homem de Bittencourt, P.I. Suppressed anti-inflammatory heat shock response in high-risk COVID-19 patients: Lessons from basic research (inclusive bats), light on conceivable therapies. Clin. Sci. 2020, 134, 1991–2017. [Google Scholar] [CrossRef]
- Moreno Fernández-Ayala, D.J.; Navas, P.; Lόpez-Lluch, G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp. Gerontol. 2020, 142, 111147. [Google Scholar] [CrossRef]
- da Silva Gomes Dias, S.; Soares, V.C.; Ferreira, A.C.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Teixeira, L.; Barreto, E.; Mattos, M.; de Freitas, C.S.; et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. bioRxiv 2020. [Google Scholar] [CrossRef]
- Silvas, J.A.; Jureka, A.S.; Nicolini, A.M.; Chvatal, S.A.; Basler, C.F. Inhibitors of VPS34 and lipid metabolism suppress SARS-CoV-2 replication. bioRxiv Prepr. Serv. Biol. 2020. [Google Scholar] [CrossRef]
- Hsu, C.C.; Tsai, H.H.; Fu, T.C.; Wang, J.S. Exercise Training Enhances Platelet Mitochondrial Bioenergetics in Stroke Patients: A Randomized Controlled Trial. J. Clin. Med. 2019, 8, 2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, C.H.; Fu, T.C.; Tsai, H.H.; Hsu, C.C.; Wang, C.H.; Wang, J.S. High-intensity interval training enhances mitochondrial bioenergetics of platelets in patients with heart failure. Int. J. Cardiol. 2019, 274, 214. [Google Scholar] [CrossRef]
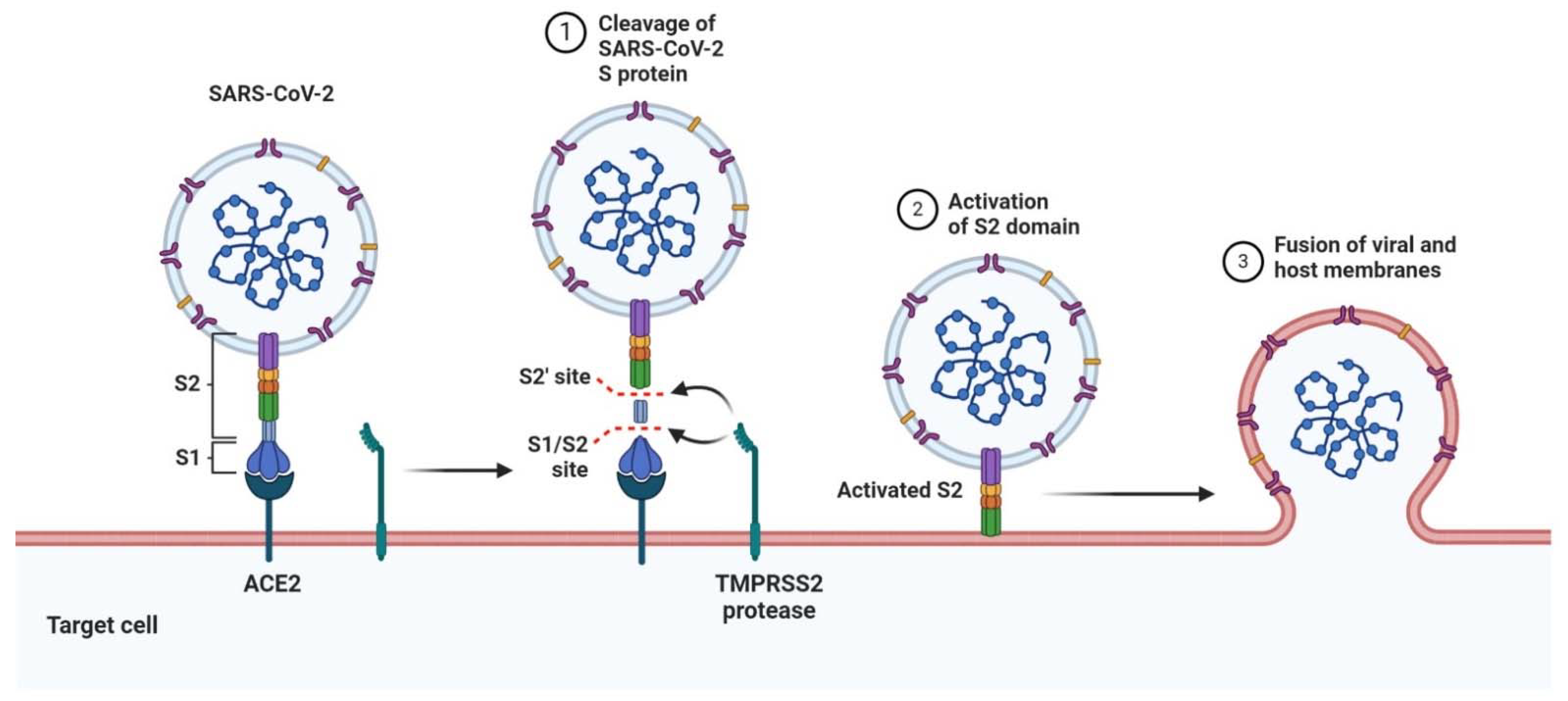



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobsson, J.; Cotgreave, I.; Furberg, M.; Arnberg, N.; Svensson, M. Potential Physiological and Cellular Mechanisms of Exercise That Decrease the Risk of Severe Complications and Mortality Following SARS-CoV-2 Infection. Sports 2021, 9, 121. https://doi.org/10.3390/sports9090121
Jakobsson J, Cotgreave I, Furberg M, Arnberg N, Svensson M. Potential Physiological and Cellular Mechanisms of Exercise That Decrease the Risk of Severe Complications and Mortality Following SARS-CoV-2 Infection. Sports. 2021; 9(9):121. https://doi.org/10.3390/sports9090121
Chicago/Turabian StyleJakobsson, Johan, Ian Cotgreave, Maria Furberg, Niklas Arnberg, and Michael Svensson. 2021. "Potential Physiological and Cellular Mechanisms of Exercise That Decrease the Risk of Severe Complications and Mortality Following SARS-CoV-2 Infection" Sports 9, no. 9: 121. https://doi.org/10.3390/sports9090121




