Virtual Reality and Physiotherapy in Post-Stroke Functional Re-Education of the Lower Extremity: A Controlled Clinical Trial on a New Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Outcome Measures
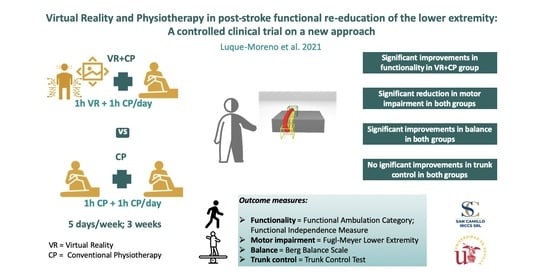
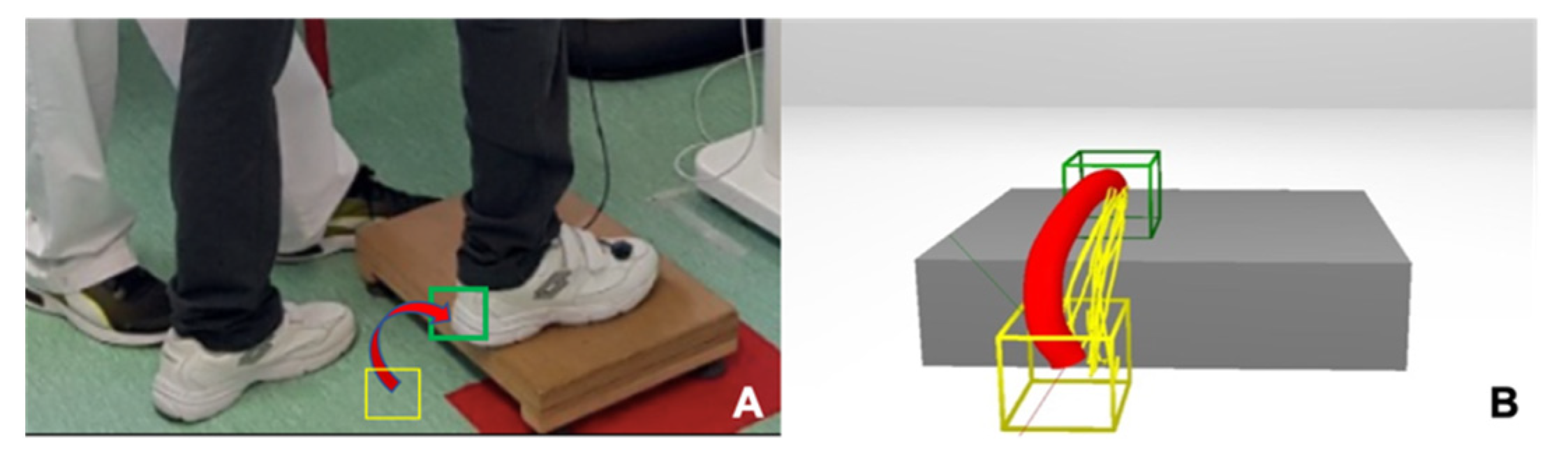
2.3. Interventions
2.4. Statistical Analysis
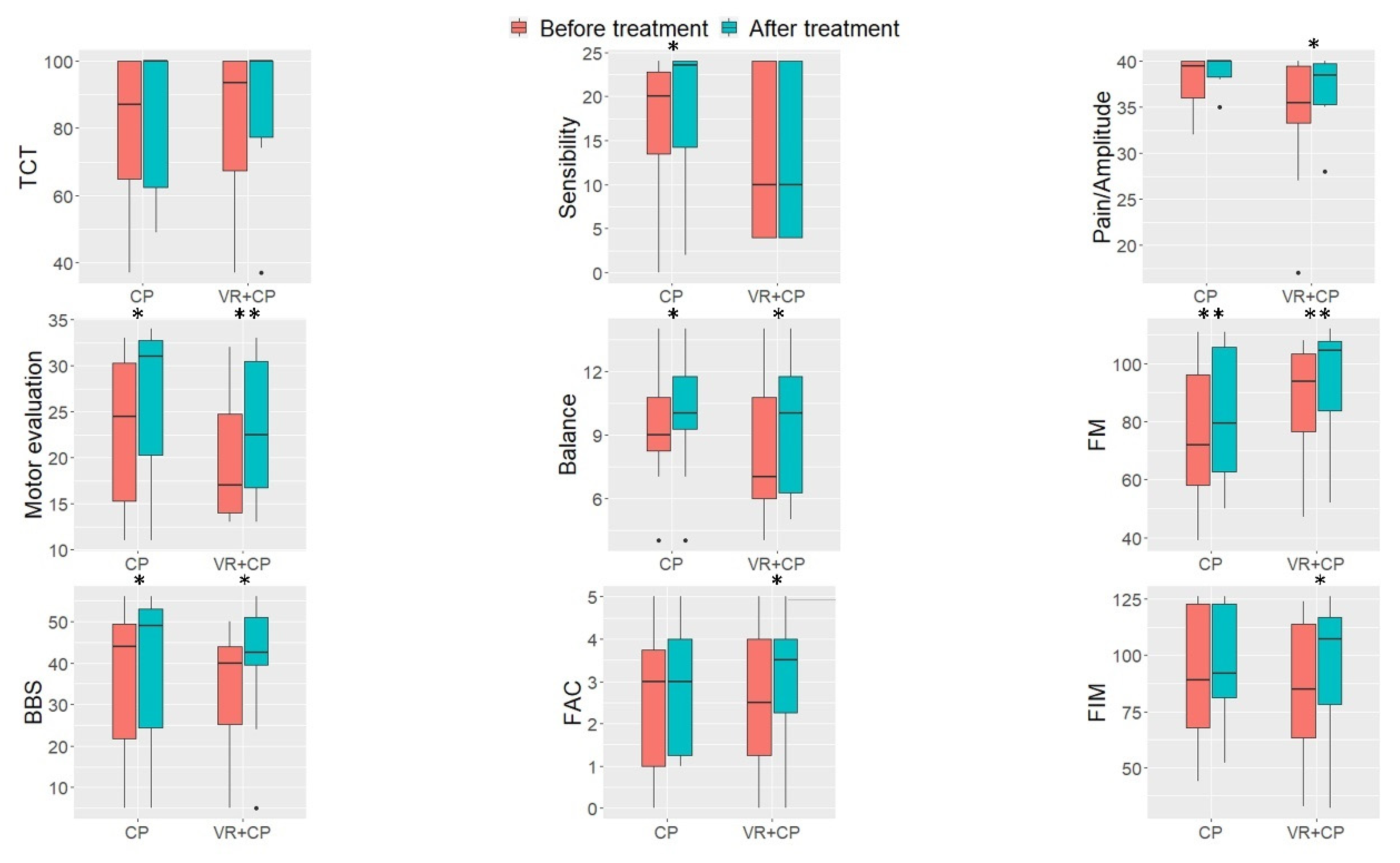
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Donnell, M.O.; Venketasubramanian, N.; et al. from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Béjot, Y.; Bailly, H.; Durier, J.; Giroud, M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med. 2016, 45, e391–e398. [Google Scholar] [CrossRef]
- Buvarp, D.; Rafsten, L.; Sunnerhagen, K.S. Predicting Longitudinal Progression in Functional Mobility After Stroke: A Prospective Cohort Study. Stroke 2020, 51, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Mayo, N.E.; Wood-Dauphinee, S.; Ahmed, S.; Gordon, C.; Higgins, J.; McEwen, S.; Salbach, N. Disablement following stroke. Disabil. Rehabil. 1999, 21, 258–268. [Google Scholar] [CrossRef]
- Menezes, K.K.; Nascimento, L.R.; Faria, C.D.; Avelino, P.R.; Scianni, A.A.; Polese, J.C.; Faria-Fortini, I.; Teixeira-Salmela, L.F. Deficits in motor coordination of the paretic lower limb best explained activity limitations after stroke. Physiother. Theory Pract. 2020, 36, 417–423. [Google Scholar] [CrossRef]
- Bower, K.; Thilarajah, S.; Pua, Y.H.; Williams, G.; Tan, D.; Mentiplay, B.; Denehy, L.; Clark, R. Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. J. Neuroeng. Rehabil. 2019, 16, 3. [Google Scholar] [CrossRef]
- Balasubramanian, C.K.; Clark, D.J.; Fox, E.J. Walking Adaptability after a Stroke and Its Assessment in Clinical Settings. Stroke Res. Treat. 2014, 2014, 591013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Criekinge, T.; Truijen, S.; Schröder, J.; Maebe, Z.; Blanckaert, K.; van der Waal, C.; Vink, M.; Saeys, W. The effectiveness of trunk training on trunk control, sitting and standing balance and mobility post-stroke: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 992–1002. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Saeys, W.; Hallemans, A.; Velghe, S.; Viskens, P.-J.; Vereeck, L.; De Hertogh, W.; Truijen, S. Trunk biomechanics during hemiplegic gait after stroke: A systematic review. Gait Posture 2017, 54, 133–143. [Google Scholar] [CrossRef]
- Balaban, B.; Tok, F. Gait Disturbances in Patients With Stroke. PM&R 2014, 6, 635–642. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Hsieh, Y.-W.; Lin, K.-C.; Chuang, L.-L.; Chang, Y.-F.; Liu, H.-L.; Chen, C.-L.; Lin, K.-H.; Wai, Y.-Y. Brain reorganization after bilateral arm training and distributed constraint-induced therapy in stroke patients: A preliminary functional magnetic resonance imaging study. Chang Gung Med. J. 2010, 33, 628–638. [Google Scholar] [PubMed]
- Kiper, P.; Szczudlik, A.; Venneri, A.; Stozek, J.; Luque-Moreno, C.; Opara, J.; Baba, A.; Agostini, M.; Turolla, A. Computational models and motor learning paradigms: Could they provide insights for neuroplasticity after stroke? An overview. J. Neurol. Sci. 2016, 369, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Kwakkel, G.; van Peppen, R.; Wagenaar, R.C.; Wood Dauphinee, S.; Richards, C.; Ashburn, A.; Miller, K.; Lincoln, N.; Partridge, C.; Wellwood, I.; et al. Effects of augmented exercise therapy time after stroke: A meta-analysis. Stroke 2004, 35, 2529–2539. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zehr, E.P. Training-Induced Neural Plasticity and Strength Are Amplified After Stroke. Exerc. Sport Sci. Rev. 2019, 47, 223–229. [Google Scholar] [CrossRef]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- French, B.; Thomas, L.H.; Coupe, J.; McMahon, N.E.; Connell, L.; Harrison, J.; Sutton, C.J.; Tishkovskaya, S.; Watkins, C.L. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst. Rev. 2016, 11, CD006073. [Google Scholar] [CrossRef] [Green Version]
- Pollock, A.; Baer, G.; Langhorne, P.; Pomeroy, V. Physiotherapy treatment approaches for the recovery of postural control and lower limb function following stroke: A systematic review. Clin. Rehabil. 2007, 21, 395–410. [Google Scholar] [CrossRef]
- Hong, Z.; Sui, M.; Zhuang, Z.; Liu, H.; Zheng, X.; Cai, C.; Jin, D. Effectiveness of Neuromuscular Electrical Stimulation on Lower Limbs of Patients With Hemiplegia After Chronic Stroke: A Systematic Review. Arch. Phys. Med. Rehabil. 2018, 99, 1011–1022.e1. [Google Scholar] [CrossRef]
- Chia, F.S.; Kuys, S.; Low Choy, N. Sensory retraining of the leg after stroke: Systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 964–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arienti, C.; Lazzarini, S.G.; Pollock, A.; Negrini, S. Rehabilitation interventions for improving balance following stroke: An overview of systematic reviews. PLoS ONE 2019, 14, e0219781. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.H.; Kim, Y.-H. Robot-assisted Therapy in Stroke Rehabilitation. J. Stroke 2013, 15, 174–181. [Google Scholar] [CrossRef]
- Major, Z.Z.; Vaida, C.; Major, K.A.; Tucan, P.; Brusturean, E.; Gherman, B.; Birlescu, I.; Craciunaș, R.; Ulinici, I.; Simori, G.; et al. Comparative Assessment of Robotic versus Classical Physical Therapy Using Muscle Strength and Ranges of Motion Testing in Neurological Diseases. J. Pers. Med. 2021, 11, 953. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Elsner, B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2017, 8, CD002840. [Google Scholar] [CrossRef] [PubMed]
- Gama, G.L.; Celestino, M.L.; Barela, J.A.; Forrester, L.; Whitall, J.; Barela, A.M. Effects of Gait Training With Body Weight Support on a Treadmill Versus Overground in Individuals With Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Lewek, M.D.; Feasel, J.; Wentz, E.; Brooks, F.P.; Whitton, M.C. Use of Visual and Proprioceptive Feedback to Improve Gait Speed and Spatiotemporal Symmetry Following Chronic Stroke: A Case Series. Phys. Ther. 2012, 92, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Luque-Moreno, C.; Oliva-Pascual-Vaca, A.; Kiper, P.; Rodríguez-Blanco, C.; Agostini, M.; Turolla, A. Virtual Reality to Assess and Treat Lower Extremity Disorders in Post-stroke Patients. Methods Inf. Med. 2016, 55, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, S.; Ambrosini, E.; Ravelli, P.; Guanziroli, E.; Molteni, F.; Ferrigno, G.; Pedrocchi, A. A biofeedback cycling training to improve locomotion: A case series study based on gait pattern classification of 153 chronic stroke patients. J. Neuroeng. Rehabil. 2011, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Maier, M.; Rubio Ballester, B.; Duff, A.; Duarte Oller, E.; Verschure, P.F.M.J. Effect of Specific Over Nonspecific VR-Based Rehabilitation on Poststroke Motor Recovery: A Systematic Meta-analysis. Neurorehabil. Neural Repair 2019, 33, 112–129. [Google Scholar] [CrossRef]
- Luque-Moreno, C.; Cano-Bravo, F.; Kiper, P.; Solís-Marcos, I.; Moral-Munoz, J.A.; Agostini, M.; Oliva-Pascual-Vaca, Á.; Turolla, A. Reinforced Feedback in Virtual Environment for Plantar Flexor Poststroke Spasticity Reduction and Gait Function Improvement. BioMed Res. Int. 2019, 2019, 6295263. [Google Scholar] [CrossRef] [Green Version]
- Kiper, P.; Agostini, M.; Luque-Moreno, C.; Tonin, P.; Turolla, A. Reinforced feedback in virtual environment for rehabilitation of upper extremity dysfunction after stroke: Preliminary data from a randomized controlled trial. BioMed Res. Int. 2014, 2014, 752128. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque-Moreno, C. A Decade of Progress Using Virtual Reality for Poststroke Lower Extremity Rehabilitation: Systematic Review of the Intervention Methods. BioMed Res. 2015, 2015, 352529. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iruthayarajah, J.; McIntyre, A.; Cotoi, A.; Macaluso, S.; Teasell, R. The use of virtual reality for balance among individuals with chronic stroke: A systematic review and meta-analysis. Top. Stroke Rehabil. 2017, 24, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Cano Porras, D.; Siemonsma, P.; Inzelberg, R.; Zeilig, G.; Plotnik, M. Advantages of virtual reality in the rehabilitation of balance and gait: Systematic review. Neurology 2018, 90, 1017–1025. [Google Scholar] [CrossRef]
- Mohammadi, R.; Semnani, A.V.; Mirmohammadkhani, M.; Grampurohit, N. Effects of Virtual Reality Compared to Conventional Therapy on Balance Poststroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, Y.J.; Park, S.W. The Effects of Virtual Reality Training on Function in Chronic Stroke Patients: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 2019, 7595639. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Lo, W.L.A.; Mao, Y.R.; Ding, M.H.; Lin, Q.; Li, H.; Zhao, J.L.; Xu, Z.Q.; Bian, R.H.; Huang, D.F. Effect of Virtual Reality on Postural and Balance Control in Patients with Stroke: A Systematic Literature Review. BioMed Res. Int. 2016, 2016, 7309272. [Google Scholar] [CrossRef]
- You, S.H.; Jang, S.H.; Kim, Y.H.; Hallett, M.; Ahn, S.H.; Kwon, Y.H.; Kim, J.H.; Lee, M.Y. Virtual reality-induced cortical reorganization and associated locomotor recovery in chronic stroke: An experimenter-blind randomized study. Stroke 2005, 36, 1166–1171. [Google Scholar] [CrossRef] [Green Version]
- Kiper, P.; Szczudlik, A.; Mirek, E.; Nowobilski, R.; Opara, J.; Agostini, M.; Tonin, P.; Turolla, A. The application of virtual reality in neuro-rehabilitation: Motor re-learning supported by innovative technologies. Rehabil. Med. 2013, 17, 29–36. [Google Scholar] [CrossRef]
- Kiper, P.; Szczudlik, A.; Agostini, M.; Opara, J.; Nowobilski, R.; Ventura, L.; Tonin, P.; Turolla, A. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 834–842.e4. [Google Scholar] [CrossRef]
- MacPherson, H. Pragmatic clinical trials. Complementary Ther. Med. 2004, 12, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Turolla, A.; Dam, M.; Ventura, L.; Tonin, P.; Agostini, M.; Zucconi, C.; Kiper, P.; Cagnin, A.; Piron, L. Virtual reality for the rehabilitation of the upper limb motor function after stroke: A prospective controlled trial. J. Neuroeng. Rehabil. 2013, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Polli, A.; Moseley, G.L.; Gioia, E.; Beames, T.; Baba, A.; Agostini, M.; Tonin, P.; Turolla, A. Graded motor imagery for patients with stroke: A non-randomized controlled trial of a new approach. Eur. J. Phys. Rehabil. Med. 2017, 53, 14–23. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- De Renzi, E.; Motti, F.; Nichelli, P. Imitating gestures. A quantitative approach to ideomotor apraxia. Arch. Neurol. 1980, 37, 6–10. [Google Scholar] [CrossRef]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [Green Version]
- Cecchi, F.; Carrabba, C.; Bertolucci, F.; Castagnoli, C.; Falsini, C.; Gnetti, B.; Hochleitner, I.; Lucidi, G.; Martini, M.; Mosca, I.E.; et al. Transcultural translation and validation of Fugl–Meyer assessment to Italian. Disabil. Rehabil. 2020, 1–6. [Google Scholar] [CrossRef]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meissner, D.; Pohl, M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef]
- Keith, R.A.; Granger, C.V.; Hamilton, B.B.; Sherwin, F.S. The functional independence measure: A new tool for rehabilitation. Adv. Clin. Rehabil. 1987, 1, 6–18. [Google Scholar] [CrossRef] [Green Version]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Franchignoni, F.P.; Tesio, L.; Ricupero, C.; Martino, M.T. Trunk control test as an early predictor of stroke rehabilitation outcome. Stroke 1997, 28, 1382–1385. [Google Scholar] [CrossRef]
- Fil Balkan, A.; Salcı, Y.; Keklicek, H.; Çetin, B.; Adın, R.M.; Armutlu, K. The trunk control: Which scale is the best in very acute stroke patients? Top. Stroke Rehabil. 2019, 26, 359–365. [Google Scholar] [CrossRef]
- Piron, L.; Turolla, A.; Tonin, P.; Piccione, F.; Lain, L.; Dam, M. Satisfaction with care in post-stroke patients undergoing a telerehabilitation programme at home. J. Telemed. Telecare 2008, 14, 257–260. [Google Scholar] [CrossRef]
- Davies, P. Pasos a Seguir. Tratamiento Integrado de Pacientes con Hemiplejía, 2nd ed.; Medica Panamericana: Madrid, Spain, 2012. [Google Scholar]
- Vaughan-Graham, J.; Cott, C.; Wright, F.V. The Bobath (NDT) concept in adult neurological rehabilitation: What is the state of the knowledge? A scoping review. Part I: Conceptual perspectives. Disabil. Rehabil. 2015, 37, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Chanubol, R.; Wongphaet, P.; Chavanich, N.; Werner, C.; Hesse, S.; Bardeleben, A.; Merholz, J. A randomized controlled trial of Cognitive Sensory Motor Training Therapy on the recovery of arm function in acute stroke patients. Clin. Rehabil. 2012, 26, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.H.; Shepherd, R.B. Enhancing physical activity and brain reorganization after stroke. Neurol. Res. Int. 2011, 2011, 515938. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.; Whitall, J.; Kwakkel, G.; Mehrholz, J.; Ewings, S.; Burridge, J. The effect of time spent in rehabilitation on activity limitation and impairment after stroke. Cochrane Database Syst. Rev. 2021, 10, CD012612. [Google Scholar] [CrossRef]
- Gomez-Cuaresma, L.; Lucena-Anton, D.; Gonzalez-Medina, G.; Martin-Vega, F.J.; Galan-Mercant, A.; Luque-Moreno, C. Effectiveness of Stretching in Post-Stroke Spasticity and Range of Motion: Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1074. [Google Scholar] [CrossRef]
- Gage, J.R. Surgical treatment of knee dysfunction in cerebral palsy. Clin. Orthop. Relat. Res. 1990, 253, 45–54. [Google Scholar] [CrossRef]
- Rech, K.D.; Salazar, A.P.; Marchese, R.R.; Schifino, G.; Cimolin, V.; Pagnussat, A.S. Fugl-Meyer Assessment Scores Are Related With Kinematic Measures in People with Chronic Hemiparesis after Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104463. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.H.; You, S.H.; Hallett, M.; Cho, Y.W.; Park, C.-M.; Cho, S.-H.; Lee, H.-Y.; Kim, T.-H. Cortical reorganization and associated functional motor recovery after virtual reality in patients with chronic stroke: An experimenter-blind preliminary study. Arch. Phys. Med. Rehabil. 2005, 86, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Verschure, P.F.M.J. Neuroscience, virtual reality and neurorehabilitation: Brain repair as a validation of brain theory. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2254–2257. [Google Scholar] [CrossRef]
- Lord, S.E.; McPherson, K.; McNaughton, H.K.; Rochester, L.; Weatherall, M. Community Ambulation after Stroke: How Important and Obtainable Is It and What Measures Appear Predictive? Arch. Phys. Med. Rehabil. 2004, 85, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; Baer, G.; Campbell, P.; Choo, P.L.; Forster, A.; Morris, J.; Pomeroy, V.M.; Langhorne, P. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst. Rev. 2014, 4, CD001920. [Google Scholar] [CrossRef] [Green Version]
- Kiper, P.; Luque-Moreno, C.; Pernice, S.; Maistrello, L.; Agostini, M.; Turolla, A. Functional changes in the lower extremity after non-immersive virtual reality and physiotherapy following stroke. J. Rehabil. Med. 2020, 52, jrm00122. [Google Scholar] [CrossRef]
| ID Patient | Sex | Age (Years) | Post-Stroke Months | Hemisphere of Stroke | Type of Stroke |
|---|---|---|---|---|---|
| 1 | Man | 74 | 2.03 | Left | Ischemic |
| 2 | Man | 77 | 10 | Left | Ischemic |
| 3 | Man | 64 | 4.7 | Right | Ischemic |
| 4 | Man | 58 | 7.3 | Left | Hemorrhagic |
| 5 | Man | 50 | 2.8 | Right | Hemorrhagic |
| 6 | Man | 59 | 4.6 | Right | Ischemic |
| 7 | Man | 59 | 10 | Left | Ischemic |
| 8 | Man | 45 | 18.3 | Right | Ischemic |
| 9 | Man | 68 | 5.1 | Left | Hemorrhagic |
| 10 | Man | 73 | 4.1 | Right | Hemorrhagic |
| 11 | Woman | 76 | 6 | Left | Ischemic |
| 12 | Woman | 59 | 1 | Left | Ischemic |
| 13 | Man | 65 | 1.4 | Left | Hemorrhagic |
| 14 | Woman | 56 | 1.1 | Left | Ischemic |
| 15 | Man | 69 | 1.4 | Left | Hemorrhagic |
| 16 | Woman | 29 | 0.8 | Left | Ischemic |
| 17 | Man | 62 | 4 | Left | Ischemic |
| 18 | Man | 59 | 7.5 | Left | Ischemic |
| 19 | Man | 67 | 3 | Left | Ischemic |
| 20 | Woman | 80 | 1.5 | Left | Ischemic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque-Moreno, C.; Kiper, P.; Solís-Marcos, I.; Agostini, M.; Polli, A.; Turolla, A.; Oliva-Pascual-Vaca, A. Virtual Reality and Physiotherapy in Post-Stroke Functional Re-Education of the Lower Extremity: A Controlled Clinical Trial on a New Approach. J. Pers. Med. 2021, 11, 1210. https://doi.org/10.3390/jpm11111210
Luque-Moreno C, Kiper P, Solís-Marcos I, Agostini M, Polli A, Turolla A, Oliva-Pascual-Vaca A. Virtual Reality and Physiotherapy in Post-Stroke Functional Re-Education of the Lower Extremity: A Controlled Clinical Trial on a New Approach. Journal of Personalized Medicine. 2021; 11(11):1210. https://doi.org/10.3390/jpm11111210
Chicago/Turabian StyleLuque-Moreno, Carlos, Pawel Kiper, Ignacio Solís-Marcos, Michela Agostini, Andrea Polli, Andrea Turolla, and Angel Oliva-Pascual-Vaca. 2021. "Virtual Reality and Physiotherapy in Post-Stroke Functional Re-Education of the Lower Extremity: A Controlled Clinical Trial on a New Approach" Journal of Personalized Medicine 11, no. 11: 1210. https://doi.org/10.3390/jpm11111210