Abstract
The prevalence of inflammatory bowel disease (IBD) has increased worldwide. The prevalence of metabolic dysfunction associated fatty liver disease (MAFLD) has also risen. However, there is limited research on the connection between MAFLD and IBD in the Asian population. This study aims to analyze the prevalence and clinical significance of MAFLD in Taiwanese IBD patients with clinical remission. We retrospectively analyzed IBD patients who received transient elastography for liver fibrosis and controlled attenuation parameter evaluation for liver steatosis. This study enrolled 120 patients with IBD, including 45 Crohn’s disease (CD) and 75 ulcerative colitis (UC). MAFLD prevalence in IBD was 29.2%. Patients with MAFLD had a shorter disease duration (2.8 years vs. 5.3 years, p = 0.017), higher alanine aminotransferase levels (24 U/L vs. 17 U/L, p = 0.003), a lower estimated glomerular filtration rate (91.37 mL/min/1.73 m2 vs. 103.92 mL/min/1.73 m2, p = 0.004), and higher γ-glutamyl transferase (γ-GT) (24 mg/dL vs. 13 mg/dL, p < 0.001). The prevalence of significant fibrosis in IBD with MAFLD was 17.1%. Significant fibrosis was found in older age (58.5 years vs. 40 years, p = 0.004) and the high type 2 diabetes mellitus proportion (50.0% vs. 10.3%, p = 0.049). A trend of longer disease duration was found in significant fibrosis (4.9 years vs. 1.6 years, p = 0.051). The prevalence of MALFD in IBD was 29.2%. and 17.1% of them had significant fibrosis. In addition to the intestinal manifestation, the study findings remind clinicians that they should be aware of the possibility of hepatic complications for IBD patients.
1. Introduction
Inflammatory bowel diseases (IBDs), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory disorders mostly affecting the gastrointestinal tract causing immunologic dysregulation with genetic factors, gut microbiota, and environmental factors [1]. Recently, IBD prevalence has increased globally, initially in Western countries as well as in Asian countries, including Taiwan, which has likely been related to environmental factors and the Westernized diet and lifestyle [2,3].
A similar increased prevalence was noted in nonalcoholic fatty liver diseases (NAFLD) [4,5]. NAFLD, also known as metabolic dysfunction-associated fatty liver disease (MAFLD), was defined as intracellular fat deposition in the liver of >5% without excessive alcohol consumption, toxin, or a viral cause of hepatitis, including a hepatitis B virus and hepatitis C virus infection [6]. MAFLD, which was first introduced in 2019 with an expert’s consensus that precisely represented the pathogenesis of fatty liver, was associated with metabolic dysfunction, including overweight, obesity, type 2 diabetes mellitus (T2DM), or normal weight with metabolic syndrome or lipid disorders [7]. MAFLD was also associated with IBD, with marked prevalence in different regions. A meta-analysis reported the prevalence of NAFLD in IBD at 36.9%, 17.2%, and 11.8% in European, Western Pacific, and Eastern Mediterranean countries, respectively [8]. Meanwhile, MAFLD-associated liver fibrosis in patients with IBD, which was represented in few studies, demonstrated an estimated prevalence of 1.2%–16% which differed due to different methods, such as noninvasive biomarkers, a Fibrosis-4 index, transient elastography (TE), or a histopathology diagnosis [9,10,11].
The risk factors of developing MAFLD in patients with IBD remain undetermined [10]. Some studies have supported traditional risk factors, such as T2DM, weight gain, or obesity, to contribute to MAFLD development in patients with IBD [6,12,13]. Other studies have highlighted the involvement of disease activity, duration, and drug-induced liver injury in MAFLD progression [9,14].
This study aims to analyze the prevalence of MAFLD and fibrosis in patients with IBD in our cohort by TE and to identify the associated risk factors with liver steatosis and fibrosis.
2. Materials and Methods
2.1. Inclusion Criteria
We retrospectively analyzed patients with IBD from January 2019 to April 2023 at our institution. We involved patients with IBD from January 2019 in an in-hospital liver disease surveillance program, including abdominal ultrasound examination and TE encompassing liver stiffness measurements (LSM) for evaluating liver fibrosis and controlled attenuation parameters (CAPs) for liver steatosis (Figure 1). The liver stiffness was divided into four stages with cut-off values of LSM for staging as F0–1, F2, F3, and F4 at <7.2 kPa, <9.7 kPa, <12.5 kPa, and ≥12.5 kPa, respectively, based on previous literature [15,16]. F2, F3, and F4 were defined as significant fibrosis, advanced fibrosis, and standard for cirrhosis, respectively. We clarify steatosis in the liver as the median value of the CAP of ≥248 dB/m and staging as follows: CAP of ≥248 dB/m, ≥268 dB/m, and ≥288 dB/m as S1, S2, and S3, respectively [15].
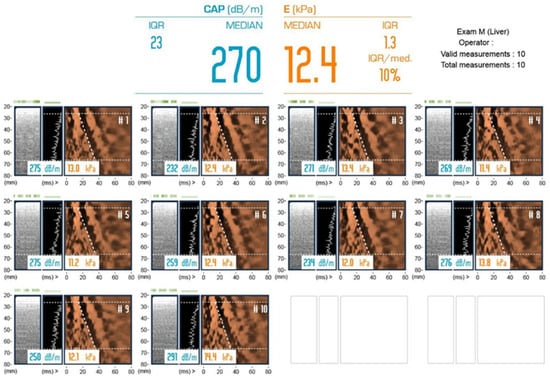
Figure 1.
The LSMs and CAP measurements were performed using FibroScan (Echosens, Paris, France) by an experienced operator who has performed more than 5000 FibroScan examinations. The finding LSM:12.4 kPa/CAP:270 dB/m was judged as S2 and F3.
Regular abdominal ultrasound for steatosis was defined as normal, mild, moderate, or severe fatty liver and judged by a hepatologist [17]. Body mass index (BMI) was defined by the World Health Organization consensus for the Asia group as underweight (<18.5 kg/m2), normal weight (18.5–23 kg/m2), overweight (23–27.5 kg/m2), and obesity (≥27.5 kg/m2) [18]. Inclusion criteria were definite CD or UC diagnosis and National Health Insurance-certified major illness at age >20 years old and having received a transabdominal ultrasound and TE examination with LSM and CAP at clinical remission status. Lack or failure of LSM and CAP examination were excluded. We retrospectively review the chart for variable factors, such as age, gender, BMI, bowel resection history, laboratory measurements, such as white blood cell (WBC) counts, hemoglobin (Hb), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (γ-GT), alkaline phosphatase (ALK-P), fasting glucose, fasting insulin, glycated hemoglobin (HbA1c), creatine, triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), total cholesterol, high-density lipoprotein cholesterol (HDL-C), albumin, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and medical history of hypertension, T2DM, and biologic products used.
2.2. Metabolic Dysfunction-Associated Fatty Liver Diseases
We clarify steatosis in the liver as the median value of a CAP at ≥248 dB/m [15]. The MAFLD diagnosis was defined as hepatic steatosis and concomitant with T2DM, BMI of ≥23 kg/m2, or presenting with at least two of the following metabolic abnormalities: waist circumference of ≥90 in the male and ≥80 in the female, blood pressure of ≥130/85 mmHg, TG of ≥150 mg/dLl, HDL-C of <40 mg/dL in the male and <50 mg/dL in the female, or under controlled medication for blood pressure and dyslipidemia, prediabetes, fasting glucose of >100 mg/dL or HbA1c of 5.7%–6.4%, homeostasis model assessment of insulin resistance score (HOMA-IR) of ≥2.5 in the female and serum CRP level of >2 mg/L.
2.3. Statistical Analysis
The demographic and other clinical data of patients were expressed as frequency (%), median (interquartile range [IQR], 25th–75th percentile), or mean ± standard deviation (SD). The one-sample Kolmogorov–Smirnov Test evaluated the distribution of continuous variables. We used a Mann–Whitney U test or the Student’s t-test for continuous data. We used Fisher’s exact test or chi-square test for categorical data. We used multivariable logistic regression analysis to analyze factors associated with the risk of MAFLD. We discarded diagnostic criteria for MAFLD and selected variables with p-values of <0.05 from the crude model with backward elimination to enter multivariate adjustment. The statistical significance was defined as p-values of <0.05 with two sides. We used an IBM Statistical Package for the Social Sciences version 22.0 (IBM Corp., Armonk, NY, USA) for statistical analyses.
3. Results
3.1. Baseline Characteristics of IBD, including CD and UC
This study enrolled 120 patients with IBD, including 45 with CD and 75 with UC, as shown in Table 1. The male was more predominant in IBD accounting for 67.5%, including 73.3% with CD and 64% with UC. The median age at diagnosis was 43.5 years, which was younger for CD than for UC (37 years vs. 45 years, p = 0.089). Patients with CD demonstrated more gallstone (26.7% vs. 6.7%, p = 0.002) and bowel resection history (46.7% vs. 2.7%, p < 0.001). BMI, abdominal circumference, AST, ALT, fasting glucose, and HbA1c demonstrated no difference. However, CD had a significantly higher neutrophil-to-lymphocyte ratio (3.42 vs. 2.53, p = 0.027), low albumin (4.2 g/dL vs. 4.4 g/dL, p = 0.023), a higher CRP level (0.24 mg/dL vs. 0.12 mg/dL, p = 0.045), and higher ALK-P (72 vs. 56 U/L, p = 0.003). Furthermore, liver steatosis, the CAP, and fibrosis, including Fib-4 or LSM, demonstrated no difference.

Table 1.
Patient baseline characteristics.
3.2. Comparison of MAFLD with Non-MAFLD
Liver steatosis was detected in 39 patients as shown in Table 2. The definition of MAFLD was fulfilled in 35 patients, and the prevalence was 29.2% in patients with IBD. MAFLD patients had a higher BMI (26.3 kg/m2 vs. 21.5 kg/m2, p < 0.001), greater abdominal circumference (93 cm vs. 76 cm, p < 0.001), higher TG (132 mg/dL vs. 74 mg/dL, p < 0.001), lower HDL (44 mg/dL vs. 49 mg/dL, p = 0.026), higher HOMA-IR (1.91 vs. 0.9, p = 0.003), and higher LDL (117 mg/dL vs. 98 mg/dL, p = 0.016) compared with non-MAFLD patients due to enrolled factors for diagnosis. However, patients with MAFLD had a shorter disease duration (2.8 years vs. 5.3 years, p = 0.017), higher ALT levels (24 U/L vs. 17 U/L, p = 0.003), a lower estimate of the glomerular filtration rate (eGFR) (91.37 mL/min/1.73 m2 vs. 103.92 mL/min/1.73 m2, p = 0.004), and higher γ-GT (24 mg/dL vs. 13 mg/dL, p < 0.001). Furthermore, MAFLD patients demonstrated a higher LSM value (5.3 kpa vs. 4.9 kpa, p = 0.024) and significant fibrosis (17.1% vs. 2.4%, p = 0.008). Otherwise, WBC counts (6.9 × 103/μL vs. 5.7 × 103/μL, p = 0.090), CRP (0.17 mg/dL vs. 0.11 mg/dL, p = 0.161), or ESR (11 mm/h vs. 12 mm/h, p = 0.943) demonstrated no difference.

Table 2.
Comparison of patient characteristics with and without MAFLD.
3.3. Multivariate Analysis of Factors Associated with MAFLD
Table 3 shows the multivariate analysis of factors associated with MAFLD. We excluded metabolic factors, such as BMI, lipid profile, CRP, fasting glucose, or HOMA-IR, because they are conditions for MAFLD diagnosis. We selected crude model variables with p-values of <0.05 into a multivariate adjustment. MAFLD was associated with higher Hb (adjusted odds ratio [aOR]: 1.91, 95% confidence interval [CI]: 1.17–3.13, p = 0.010), elevated γ-GT (aOR: 1.11, 95% CI: 1.02–1.21, p = 0.013), and significant liver fibrosis, namely F2, F3, and F4 (aOR: 31.25, 95% CI: 1.2–815.55, p = 0.039).

Table 3.
Multivariable analysis for factors associated with MAFLD.
3.4. Factors Associated with Significant Fibrosis
The distribution of the liver fibrosis stage estimated by LSM is illustrated in Figure 2. The prevalence of significant fibrosis (fibrosis stage ≥ F3) in patients with MAFLD was 17.1% compared with 2.1% in patients without MAFLD (p = 0.008) (Figure 3).
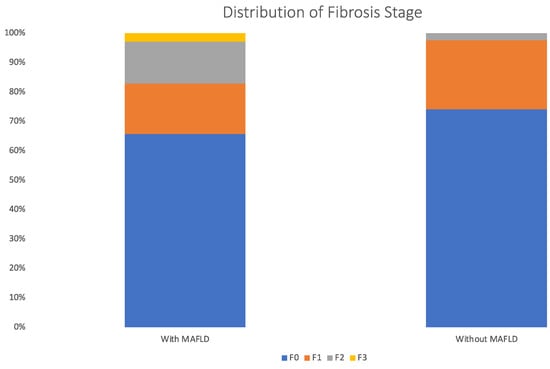
Figure 2.
Distribution of liver fibrosis stage by LSM in patients with and without MAFLD.
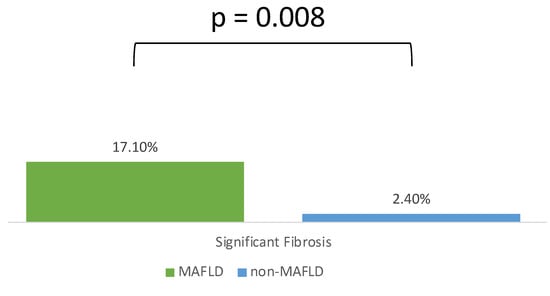
Figure 3.
MAFLD patients have a higher proportion of significant fibrosis than those without MAFLD.
Table 4 shows the comparison of features of significant fibrosis among the MAFLD population. Significant fibrosis was associated with older age (58.5 years vs. 40 years, p = 0.004), a high T2DM history proportion (50.0% vs. 10.3%, p = 0.049), and lower platelet counts (195 × 103/μL vs. 261 × 103/μL, p = 0.049). Furthermore, we noticed the trend of a longer disease duration with significant fibrosis (4.9 years vs. 1.6 years, p = 0.051).

Table 4.
MAFLD patient with and without significant fibrosis.
4. Discussion
The prevalence of MAFLD in patients with IBD in our cohort was 29.2%. Conditions for diagnosis, such as a higher BMI, insulin resistance, and metabolic syndrome, patients with MAFLD having higher ALT and γ-GT, lower eGFR and significant fibrosis were excluded from the study. MAFLD was associated with elevated γ-GT and significant fibrosis after being adjusted with multivariate analysis.
The prevalence of MAFLD in IBD was mostly analyzed in America and Europe and was 28.2% (95% CI: 22.2–34.3), and 36.9% (95% CI: 31.2–42.6), respectively [8]. A few studies addressed the prevalence of MAFLD in IBD in the Asia-Pacific region (Table 5). The MAFLD prevalence was 10.7% in patients with IBD in China, 16.7% in patients with IBD in South Korea, and 21.8% in CD patients in Japan [19,20,21]. The discordance of prevalence was affected by the measurement methods and regional differences. We had previously published and reported a MAFLD prevalence of 29.6% in IBD [10]. On this occasion, we introduced a new definition of MAFLD with the extended database which showed the prevalence of MAFLD in IBD at 29.2%. Liver steatosis was detected in 39 patients, and 90% of patients had metabolic disorders and were diagnosed with MAFLD. Our TE database does not contain any information regarding the prevalence of MAFLD in the general population for comparison, but our previous ultrasound-based report found that the prevalence of it in the Taiwanese population was 26% [22].

Table 5.
Prevalence of NAFLD/MAFLD in the Asia-Pacific region.
The patients gained weight and the prevalence of obesity was approximately 32.7% with more effective therapy, such as biologics and well-controlled disease with persistent remission [23]. Subsequently, the increased BMI in IBD was associated with more metabolic syndrome, NAFLD development, and liver fibrosis [24]. One recent meta-analysis revealed that increased age and higher BMI were associated with the risk of NAFLD development with aOR of 1.03 (95% CI: 1.01–1.05) and 1.27 (95% CI: 1.22–1.32), respectively [8]. However, a statistically significant increased risk of NAFLD development, including diabetes, hypertension, dyslipidemia, and surgical history, was not achieved, with aORs of 1.84 (95% CI: 0.86–2.83), 1.15 (95% CI: 0.25–2.06), 2.00 (95% CI: 0.00–4.48), and 1.22 (95% CI: 0.51–1.93), respectively. The statistically insignificant and wide 95% CI with a vertical line at 1.0 was due to the small number of studies. Some authors indicated metabolic syndrome as a risk factor for developing NAFLD in IBD as well as the general population [25]. However, one recent study involving two medical centers analyzed the risk factors of developing MAFLD in patients with IBD compared with healthy control matched by age, sex, T2DM, and BMI, which revealed IBD as a predictor for MAFLD development and liver fibrosis with an OR of 1.99 (p < 0.001) and 5.55 (p < 0.001), respectively [26]. Our cohort was lacking a controlled group for comparison. We performed multivariate adjustment after excluding diagnosed conditions for MAFLD, and MAFLD was associated with elevated γ-GT and significant fibrosis.
A few studies addressed liver fibrosis in patients with IBD with fatty liver. NAFLD prevalence with liver fibrosis differed. Veltkamp reported a prevalence of 8% in patients with IBD defined as TE of >7 kPa, and mostly in patients with CD [27]. Ritaccio reported a prevalence of 4% in IBD defined as an NAFLD fibrosis score (NFS) of >0.675 [28]. Bessissow reported a prevalence of 2.2% in IBD defined as FIB-4 of ≥2.67 [29]. A recent study in Korea reports a prevalence of 5.3% in IBD with FIB-4 of ≥1.45 [21]. Ritaccio reported that 16% of patients had progressed to a NFS during 5-year follow-ups. Despite a small cohort, 56 of 138, at 5-year follow-up, the author emphasized the result representing pattern of disease fluctuation [28]. Our cohort demonstrates a 17.1% prevalence of significant fibrosis, with TE of ≥7.2 kPa. This significant fibrosis was observed to be more common in older individuals and those with a higher portion of T2DM. Additionally, we observed a trend indicating that longer disease duration was associated with a higher likelihood of significant fibrosis. These findings may indicate that advancing age and prolonged disease duration may contribute to significant fibrosis development and progression. It also emphasizes the need for regular monitoring and follow-up over an extended period.
Our study had several limitations. First, this single-center study had a limited sample size which may affect the statistical power and precision of the results. Second, histopathology diagnosis, which remains a gold standard for the diagnosis of NAFLD and NASH, was lacking. Instead, we utilized TE as a more objective method to detect steatosis and fibrosis and employed a new inclusive criterion for MAFLD diagnosis to account for the possible impact of a concomitant with chronic hepatitis B and C infection. Third, we did not analyze the specific medications for IBD such as biologics [30], cumulative dosage of steroids, 5-aminosalicylates (5-ASA), and immunomodulators, such as azathioprine. Moreover, we did not investigate the role of gut microbiota which also plays a crucial role in both IBD and MAFLD.
5. Conclusions
Our cohort demonstrated 90% of patients with IBD and liver steatosis to be associated with metabolic dysfunction and was diagnosed with MAFLD. Additionally, we noted that MAFLD in patients with IBD was associated with shortened IBD duration, a higher Hb, an elevated GPT level, a decreased eGFR, an elevated γ-GT, and significant fibrosis. Moreover, we revealed that MAFLD was associated with elevated γ-GT and significant fibrosis. Significant fibrosis was associated with older age, as well as a noticeable trend indicating a correlation with a longer disease duration. Further studies are needed for a long-term follow-up for fibrosis progression in patients with IBD and MAFLD.
Author Contributions
Conceptualization, S.-W.H. and H.-H.Y.; methodology, S.-P.H.; software, S.-P.H.; formal analysis, H.-H.Y. and S.-W.H.; investigation, S.-W.H. and T.-C.C.; resources, H.-H.Y.; data curation, S.-W.H. and H.-H.Y.; writing—original draft preparation, P.-Y.S., C.-T.Y. and Y.-Y.C.; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received funding for this manuscript from the Changhua Christian Hospital (110-CCH-IRP-020, 111-CCH-IRP-011 and 112-CCH-IRP-033).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Changhua Christian Hospital (Protocol Code 230523, approval date 1 June 2023).
Informed Consent Statement
Patient consent was waived due to the retrospective analysis of the study.
Data Availability Statement
The data are available on reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.F. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef]
- Yen, H.H.; Weng, M.T.; Tung, C.C.; Wang, Y.T.; Chang, Y.T.; Chang, C.H.; Shieh, M.J.; Wong, J.M.; Wei, S.C. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: A nationwide populationbased study. Intest. Res. 2019, 17, 54–62. [Google Scholar] [CrossRef]
- Ong, J.; Alswat, K.; Hamid, S.; El-Kassas, M. Nonalcoholic Fatty Liver Disease in Asia, Africa, and Middle East Region. Clin. Liver Dis. 2023, 27, 287–299. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Shen, B.; Fan, J.G. Systematic Review with Meta-analysis: Epidemiology of Nonalcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1764–1772. [Google Scholar] [CrossRef]
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.S.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Singh, S.; Loomba, R. Meta-analysis: Prevalence of, and risk factors for, non-alcoholic fatty liver disease in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2022, 55, 894–907. [Google Scholar] [CrossRef]
- Sourianarayanane, A.; Garg, G.; Smith, T.H.; Butt, M.I.; McCullough, A.J.; Shen, B. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, e279–e285. [Google Scholar] [CrossRef]
- Yen, H.H.; Su, P.Y.; Huang, S.P.; Wu, L.; Hsu, T.C.; Zeng, Y.H.; Chen, Y.Y. Evaluation of non-alcoholic fatty liver disease in patients with inflammatory bowel disease using controlled attenuation parameter technology: A Taiwanese retrospective cohort study. PLoS ONE 2021, 16, e0252286. [Google Scholar] [CrossRef]
- Principi, M.; Iannone, A.; Losurdo, G.; Mangia, M.; Shahini, E.; Albano, F.; Rizzi, S.F.; La Fortezza, R.F.; Lovero, R.; Contaldo, A.; et al. Nonalcoholic Fatty Liver Disease in Inflammatory Bowel Disease: Prevalence and Risk Factors. Inflamm. Bowel Dis. 2018, 24, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Haas, L.; Chevalier, R.; Major, B.T.; Enders, F.; Kumar, S.; Tung, J. Biologic Agents Are Associated with Excessive Weight Gain in Children with Inflammatory Bowel Disease. Dig. Dis. Sci. 2017, 62, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Magrì, S.; Paduano, D.; Chicco, F.; Cingolani, A.; Farris, C.; Delogu, G.; Tumbarello, F.; Lai, M.; Melis, A.; Casula, L.; et al. Nonalcoholic fatty liver disease in patients with inflammatory bowel disease: Beyond the natural history. World J. Gastroenterol. 2019, 25, 5676–5686. [Google Scholar] [CrossRef]
- Lapumnuaypol, K.; Kanjanahattakij, N.; Pisarcik, D.; Thongprayoon, C.; Wijarnpreecha, K.; Cheungpasitporn, W. Effects of inflammatory bowel disease treatment on the risk of nonalcoholic fatty liver disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 854–860. [Google Scholar] [CrossRef]
- Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chan, H.L.; Le Bail, B.; Choi, P.C.; Kowo, M.; Chan, A.W.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef]
- Wong, V.W.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH—Current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Joseph, R.; Lopez, R.; McCullough, A.J. Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. J. Hepatol. 2009, 51, 1061–1067. [Google Scholar] [CrossRef]
- Consultation, W.E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Sagami, S.; Ueno, Y.; Tanaka, S.; Fujita, A.; Hayashi, R.; Oka, S.; Hyogo, H.; Chayama, K. Significance of non-alcoholic fatty liver disease in Crohn’s disease: A retrospective cohort study. Hepatol. Res. 2017, 47, 872–881. [Google Scholar] [CrossRef]
- Li, D.; Lu, C.; Yu, C. High incidence of non-alcoholic fatty liver disease in patients with Crohn’s disease but not ulcerative colitis. Int. J. Clin. Exp. Pathol. 2017, 10, 10633–10639. [Google Scholar]
- Hyun, H.K.; Lee, H.W.; Park, J.; Park, S.J.; Park, J.J.; Kim, T.I.; Lee, J.S.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; et al. Hepatic Steatosis but Not Fibrosis Is Independently Associated with Poor Outcomes in Patients with Inflammatory Bowel Disease. Gut Liver 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lin, C.Y.; Yen, H.H.; Su, P.Y.; Zeng, Y.H.; Huang, S.P.; Liu, I.L. Machine-Learning Algorithm for Predicting Fatty Liver Disease in a Taiwanese Population. J. Pers. Med. 2022, 12, 1026. [Google Scholar] [CrossRef]
- Flores, A.; Burstein, E.; Cipher, D.J.; Feagins, L.A. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig. Dis. Sci. 2015, 60, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Saroli Palumbo, C.; Restellini, S.; Chao, C.Y.; Aruljothy, A.; Lemieux, C.; Wild, G.; Afif, W.; Lakatos, P.L.; Bitton, A.; Cocciolillo, S.; et al. Screening for Nonalcoholic Fatty Liver Disease in Inflammatory Bowel Diseases: A Cohort Study Using Transient Elastography. Inflamm. Bowel Dis. 2019, 25, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Duque, J.C.; Calleja, J.L.; Iruzubieta, P.; Hernández-Conde, M.; Rivas-Rivas, C.; Vera, M.I.; Garcia, M.J.; Pascual, M.; Castro, B.; García-Blanco, A.; et al. Increased risk of MAFLD and Liver Fibrosis in Inflammatory Bowel Disease Independent of Classic Metabolic Risk Factors. Clin. Gastroenterol. Hepatol. 2023, 21, 406–414.e407. [Google Scholar] [CrossRef]
- Veltkamp, C.; Lan, S.; Korompoki, E.; Weiss, K.H.; Schmidt, H.; Seitz, H.K. Hepatic Steatosis and Fibrosis in Chronic Inflammatory Bowel Disease. J. Clin. Med. 2022, 11, 2623. [Google Scholar] [CrossRef]
- Ritaccio, G.; Stoleru, G.; Abutaleb, A.; Cross, R.K.; Shetty, K.; Sakiani, S.; Wong, U. Nonalcoholic Fatty Liver Disease Is Common in IBD Patients However Progression to Hepatic Fibrosis by Noninvasive Markers Is Rare. Dig. Dis. Sci. 2021, 66, 3186–3191. [Google Scholar] [CrossRef]
- Bessissow, T.; Le, N.H.; Rollet, K.; Afif, W.; Bitton, A.; Sebastiani, G. Incidence and Predictors of Nonalcoholic Fatty Liver Disease by Serum Biomarkers in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1937–1944. [Google Scholar] [CrossRef]
- Yen, H.-H.; Hsu, Y.-C.; Kuo, C.-H.; Hsu, T.-C.; Chen, Y.-Y. Real-world experience of adalimumab therapy for patients with ulcerative colitis: A single tertiary medical center experience in Central Taiwan. Adv. Dig. Med. 2023, 10, 28–33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).