1. Introduction
Salt-affected habitats are complex environments in which subtle changes in ecological factors determine plant occurrence, communities’ richness and distribution [
1]. In this sense, differences in levels of germination success linked to salt tolerance and biotic interactions during seedling emergence and establishment are important in plant composition and salt marsh zonation patterns [
2]. Therefore, the knowledge of biological traits related to reproductive behaviour is essential for the development of conservation strategies and the management of threatened species [
3] and ecosystems, such as salt marshes.
Water/osmotic stress caused by a decrease in osmotic potential under salinity and drought inhibits various plant processes, especially seed germination that determines plants dispersal and abundance [
1,
4]. In addition to osmotic effect, salinity stress leads to an accumulation of ions, which can produce ionic stress [
4]. In both cases, salinity limits plant growth and development required for survival by reducing turgor pressure and photosynthesis [
5]. These water/osmotic stresses activate diverse physiological and biochemical response mechanisms in plants linked to seed traits, morphology and anatomy, water relation, or antioxidative metabolism [
6,
7].
Evidences suggest that the exposure of seeds to osmotic stresses such as salinity and drought can inflict enhanced production of potentially toxic reactive oxygen species (ROS), which if not quenched efficiently through cellular antioxidants can induce oxidative damage to important cellular components, such as membrane lipids, proteins and nucleic acids [
8,
9,
10]. This is particularly true for the seeds of halophytes which experience large variations in soil moisture and salinity; therefore, the success of their seed germination and seedling establishment depends on minimizing the damages resulting from enhanced ROS under the aforementioned stresses [
11,
12]. The antioxidant machinery of the plant cells is composed of many enzymatic and non-enzymatic components [
11,
12]. Superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) are key antioxidant enzymes, whereas ascorbic acid (AA) and glutathione (GSH) are common non-enzymatic antioxidants [
8,
13,
14]. Most information on ROS homeostasis in plants under environmental stresses is based on vegetative tissues and any knowledge about ROS production and quenching in germinating seeds, particularly of halophytes, is generally scant [
8,
11,
12].
This study examined germination behaviour, seed micromorphology and biochemical responses of two species of the genus
Halopeplis; viz.,
H. amplexicaulis and
H. perfoliata.
Halopeplis is a characteristic genus of the leaf succulent eu-halophyte Amaranthaceae [
15], which includes only three species,
H. perfoliata (Forssk.) Bunge,
H. pygmaea (Pall.) Bunge and
H. amplexicaulis (Vahl) Ung.-Sternb.
Halopeplis amplexicaulis is an annual found in salty habitats, throughout the Mediterranean basin and other territories in Africa, including southern Africa where the populations were presumably introduced years ago [
16], but are currently cited as a native plant [
17]. It is the only European and Mediterranean representative species of this genus. Whereas
H. perfoliata is a chamephyte species which inhabits coastal salt marshes of the Arabian Peninsula [
18]. It is an example of a species with a typical circum-Arabian distribution in the Nubo-Sindian zone of the Sahara-Sindian phytochorion [
19].
Some earlier studies have reported different ecological aspects of the seed germination of the
Halopeplis species individually [
20,
21,
22,
23,
24]. However, a comparison of the osmotic stresses and biochemical aspects of the seed germination of the
Halopeplis species has not been done. In this context, this study deals with the comparative seed germination strategies and biochemical responses to osmotic stresses (NaCl and PEG) of two species of
Halopeplis with different life-forms and which grow in different environmental conditions. Furthermore, studies involving biochemical analysis coupled with the germination ecology of congeneric species differing in life-form and occurrence are limited but crucial to understand species abundance/distribution in a changing climate. In this sense, our study attempted to approach these aspects, which have so far not been sufficiently clarified, through the following objectives:
- (1)
To study whether the different environmental conditions of the sample sites, especially a more stressful maternal environment, direct the plasticity of seed responses to temperature, salt and drought tolerance.
- (2)
To test whether ionic toxicity is a relevant aspect that affects germination in saline conditions.
- (3)
To confirm the relation of life-form and germination responses.
- (4)
To evaluate ROS production and quenching responses of the germinating seeds of the two species under osmotic stresses (NaCl and PEG).
- (5)
To unveil similarities in ROS production and quenching responses of germinating seeds of the two species.
- (6)
To assess if species from a more stressful environment have a greater ability to survive in a global warming scenario.
Additionally, seed features were characterized in order to determine a potential relationship between germination behaviours under different osmotic stresses.
4. Discussion
Sensitivity to environmental fluctuations is an important physiological characteristic that allows seeds to germinate in specific environmental conditions [
44,
45]. Indeed, environmental regulation of germination is a multifaceted process that allows seeds to only germinate when environmental stresses do not surpass their limits of tolerance [
46]. Among the two compared species,
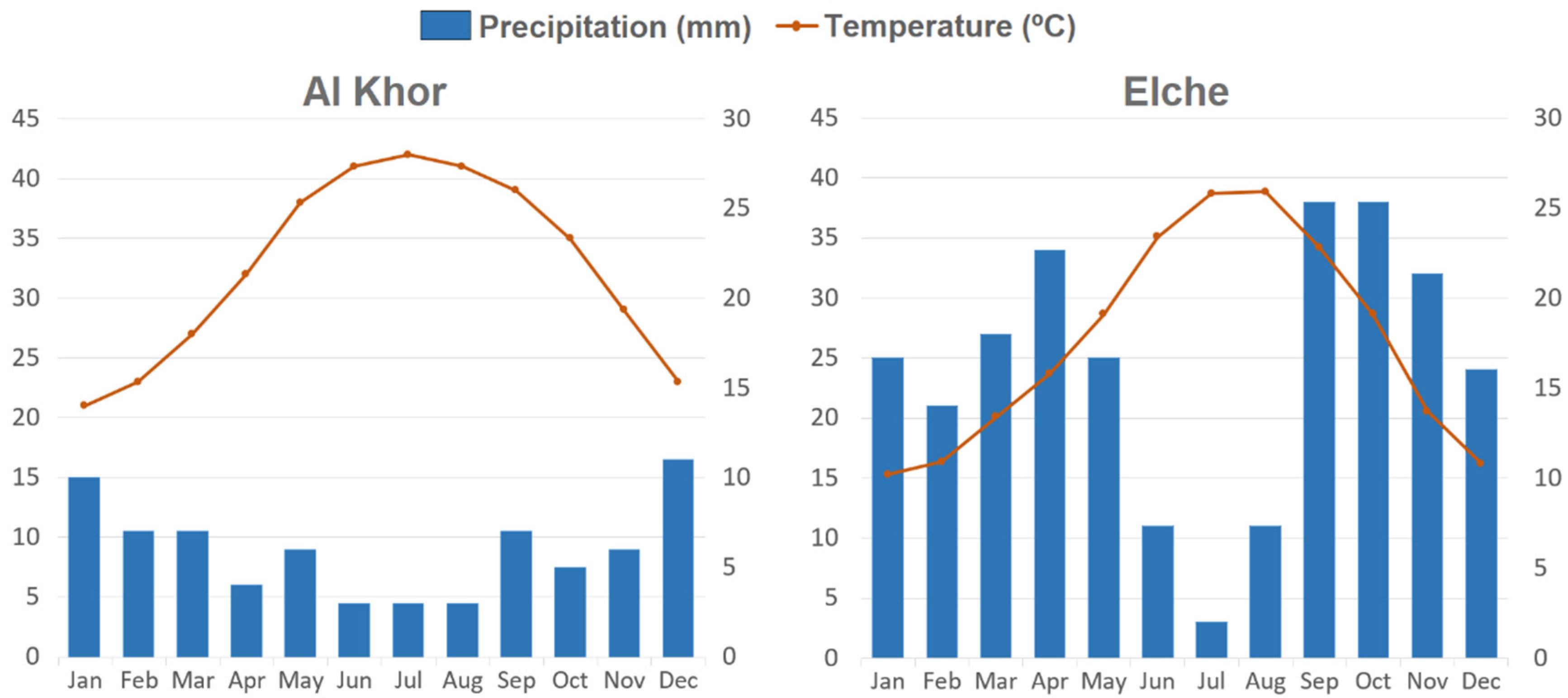
H. perfoliata reached high germination percentages at a wide range of temperatures, including the lowest temperature regime. This is a different result compared with other subtropical halophytes which prefer higher temperatures for germination [
47,
48], and is also an unexpected response for a plant living in a desert area with higher temperatures most of the year. This response could be a niche partitioning adaptation of the test species to confine its germination to the brief period of water availability rather than the temperature regime that regulates germination of most co-occurring species. The majority of rainfall in the natural habitat of
H. perfoliata corresponds to the period of lower temperatures (from December to January), causing less soil water deficit and a less edaphic salt concentration, thus germinability under low temperatures will broaden the germination window of test species. Indeed, germination at the highest temperatures would be linked to osmotic stresses and therefore to a lower chance of success for seedlings. In this sense, Ref. [
49] reported an increased germination percentage and rate at low temperature regimes in seeds of another halophyte,
Suaeda fruticosa, under high maternal salinity. Comparatively,
H. amplexicaulis reached high germination percentage at the intermediate temperature regimes (25/15 °C) that corresponds to the period of higher precipitation (April, September and October) in the growing area.
In many cases, salt tolerance under laboratory conditions may not correlate with plant responses under field conditions [
50]. This fact could be explained in terms of the physiological responses to salinity, which are complex and vary with factors such as temperature, drought and soil texture. In the case of the two species of
Halopeplis compared in this study, a higher tolerance was observed under laboratory conditions in
H. perfoliata. This species reached higher germination percentages than those obtained in the tests carried out for the same species by other authors [
51]. Nevertheless, salinity affected seed germination of both species, as reported in other species of the same family [
52,
53].
Halopeplis perfoliata reached a higher germination percentage in a shorter germination period, and it took less number of days until the first germination, which could be an adaptation to the environmental conditions of habitats with short and irregular rainfall periods that cause a decrease in soil salt concentration and reduce soil temperature, both suitable conditions for germination and seedling survival [
51,
54,
55]. Moreover, a short germination period ensures an adequate quantity of seedlings under favourable conditions [
56]. Most of the plants showing very fast germination grow in high-stress arid or saline habitats, and belong to the family Amaranthaceae, which is characterized by a generally higher salt tolerance in the germination phase [
47]. The better germination percentage and speed in
H. perfoliata seeds could also be ascribed to a maternal effect. A number of authors have observed that a maternal effect derived from the growth of plants in a saline environment can improve salt tolerance in the germination period, through a transmission of information to subsequent generations. This maternal effect has been observed in many halophytes [
57,
58], and their positive effect in improving a tolerance to high temperature regimes have also been probed in other species [
49]. Conversely, some authors indicated better results in germination success for plants from non-saline environments when compared with plants growing in saline conditions [
59]. Hence, effects of maternal environment on seed germination responses may vary among species.
Germination inhibition in halophytes, derived from an increase in salt concentration, can be the result of ionic toxicity and/or osmotic stress [
60,
61,
62,
63] depending on the species [
64,
65]. In this study, recovery data demonstrated that the inhibitory effect observed in PEG treatment is greater than that observed in NaCl in both species. These findings hint at the main role of osmotic stress in germination inhibition compared with specific ion effects. These results agree with those obtained in other species [
48,
66,
67,
68,
69]. In this study, relative water uptake (Wr) data also indicated a similar decline under isotonic NaCl and PEG treatments compared with the control for both test species as reported for
Suaeda fruticosa and
Limonium stocksii [
8]. This also hints at osmotic constraint for germination inhibition in test species under salt/osmotic solutions. However, Ref. [
70] observed a greater inhibition of germination in seeds of several halophytes when treated with mannitol and/or PEG, compared with those treated with different concentrations of NaCl. Contrarily, in other species, such as
Suaeda heterophylla [
63] or
Atriplex halimus [
65] salinity caused ionic toxicity.
Reactive oxygen species (ROS) such as H
2O
2 are produced in germinating seeds during the imbibitional reactivation of mitochondrial oxygen metabolism and their production may enhance under environmental stresses such as salinity [
8,
12,
71]. Such information about halophyte seeds is generally scant. In addition, only a few studies coupled biochemical analysis to explain the germination ecology responses of seeds. In this study, the H
2O
2 content of germinating seeds of two tested
Halopeplis species in NaCl or PEG treatments were comparable to that in the control treatment. Likewise, the H
2O
2 content of brown seeds of
Arthrocnemum macrostachyum and large seeds of
A. indicum did not vary across salinity during germination [
72]. The nearly static levels of H
2O
2 in halophyte seeds, including our test species, could be ascribed to unaltered mitochondrial activity, which also indicates that mitochondrial function was not compromised under NaCl/PEG treatment in the tested halophyte seeds. Unaltered or even decreased activities of H
2O
2 detoxification enzymes (CAT and APX) and non-enzymatic antioxidants (ascorbate and glutathione) in germinating seeds of both test species also hint at no excessive H
2O
2 production. Similarly, activities of antioxidant enzymes were either comparable or decreased under salinity compared with the control in germinating seeds of the halophyte
Gypsophila oblanceolate [
73]. Furthermore, the unaltered MDA (oxidative membrane damage marker) content of
H. perfoliata in both NaCl and PEG compared with the control and that of
H. amplexicaulis in NaCl compared with the control also indicate a lack of oxidative damage under stress conditions. Likewise, the MDA content of germinating seeds of halophytes
A. macrostachyum [
72] and
Salsola drummondii [
19] also did not increase under saline conditions. An increase in the MDA content of germinating
H. amplexicaulis seeds in PEG solution, despite an unaltered H
2O
2 content, might be related to superoxide accumulation, which is indicated by the higher SOD activity. However, this increase in the MDA content was probably not too damaging, as most un-germinated seeds showed recovery of germination when transferred to the water. Hence, high recovery coupled with a lack of substantial oxidative damage indicates that germination inhibition under NaCl and isotonic PEG treatments in the two test halophytes probably resulted from a lack of sufficient imbibition owing to osmotic constraint rather than damages resulting from ionic toxicity.
Recovery tests showed a strong priming effect in
H. amplexicaulis, while in
H. perfoliata this effect was not noteworthy. Accordingly, some authors have reported better germination and an increased stress tolerance in seeds primed with NaCl or PEG, through a shortening of time for seed emergence, an increase in germination percentage and rate, an increase of chlorophyll content and root viability and the contribution in maintaining RWC under low soil moisture [
74,
75]. In this regard, plants living in saline environments have two different strategies (i.e., salt tolerance or salt avoidance), to survive in environments with high salt concentrations [
4,
76,
77]. Salt avoidance mechanisms include phenological adaptations, such as short life cycles [
78], and physiological adaptations, such as seed dormancy or germination timing. The high percentage of recovery germination after the alleviation of salinity corresponds to one of the salt-avoidance strategies of halophytes. This mechanism supplies a viable seed bank with new individuals able to germinate once the salinity decreases [
79,
80] and prevents seedling mortality in unfavourable periods [
44]. Both strategies were found in the two studied species.
H. amplexicaulis could be categorized as a salt-avoider species, compared with the salt-tolerant
H. perfoliata, according to [
81] who proved the use of one or both of these strategies in species belonging to the family Amaranthaceae. Moreover, the higher salt tolerance of
H. perfoliata seems to be related to the climate conditions of its habitat (high temperature and low rainfall), that do not allow for a significant decrease in soil salinity concentration throughout the year.
Halophytes have different life-forms, from annuals to perennials, therefore one can expect different germination responses to salinity and drought depending on their life cycles. Accordingly, some authors demonstrated a greater salt tolerance in the germination phase for annual plants than in perennials and explained it in terms of their pioneer character, differences in the habitat colonized and the ability to complete the life cycles rapidly in an unpredictable environment [
82]. In contrast, other authors identified annuals as the most sensitive to salinity [
1], with stress escaping strategies [
83,
84]. Our results are in accordance with latter findings as they show a higher tolerance in the perennial
H. perfoliata than in the annual
H. amplexicaulis. In many cases, annual plants could be more demanding in terms of germination requirements, and their stress tolerance is lower because the survival of future generations depends exclusively on the survival of the seedlings after the germination of the seeds. According to the aforementioned results, it is expected that
H. perfoliata will be less affected by global warming than
H. amplexicaulis.
Research findings concerning seed size related to germination percentage and speed in stressful environments are not yet clear and conclusive enough [
85]. However, generally a better adaptation to adverse conditions would be expected for larger seeds [
86,
87,
88]. In this sense, Ref. [
89] have evidenced a positive relationship between seed size and tolerance to water stress. However, some authors have evidenced opposing results [
57,
90], and in many cases, the absence of a relationship between seed size and germination response has been found [
91,
92]. Germination velocity can be affected in large seeds by the necessity of more water absorption and thus, a longer time for imbibition [
85,
93,
94]. However, different authors have demonstrated fast germination in plants living in high stress environments as an adaptive mechanism that allows seeds to take advantage of short favourable periods and ensure the survival of seedlings [
54,
95]. In the case of the two
Halopeplis species studied, there is no clear evidence of the relevance of this factor in germination response. According to our study and those from diverse authors, germination response in drought-affected habitats is more related to the environmental conditions of the growth area or genetic heterogeneity than other factors, e.g., seed size [
92,
96,
97,
98].