The Influence of Depression on Biased Diagnosis of Premenstrual Syndrome and Premenstrual Dysphoric Disorder by the PSST Inventory
Abstract
:1. Introduction
2. Materials and Methods
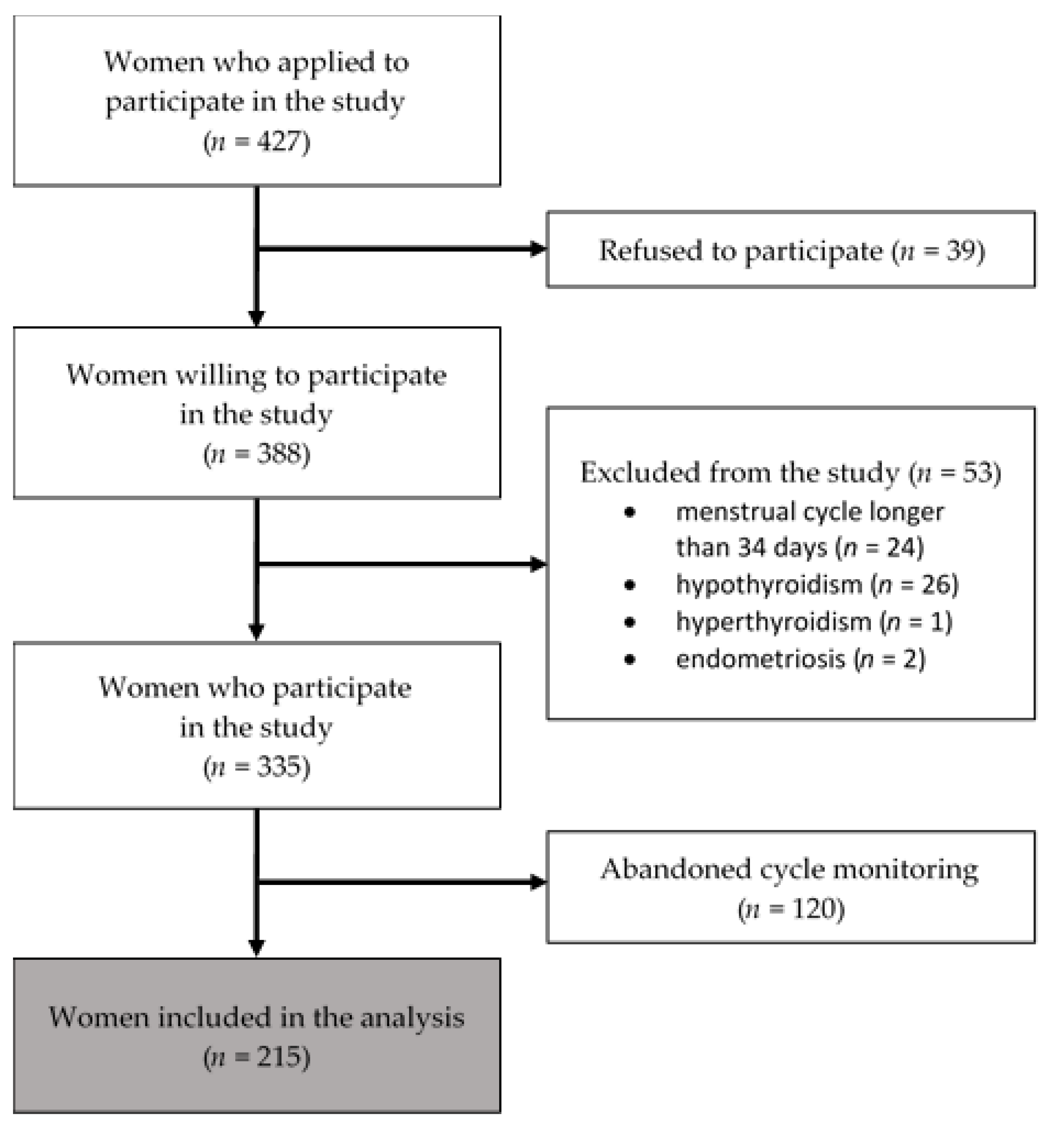
2.1. Procedure and Participants
2.2. Instruments
2.2.1. Depression
2.2.2. Prospective PMS/PMDD Diagnosis
2.2.3. Retrospective PMS/PMDD Diagnosis
2.3. Data Analysis
3. Results
3.1. Item Response Theory Analysis
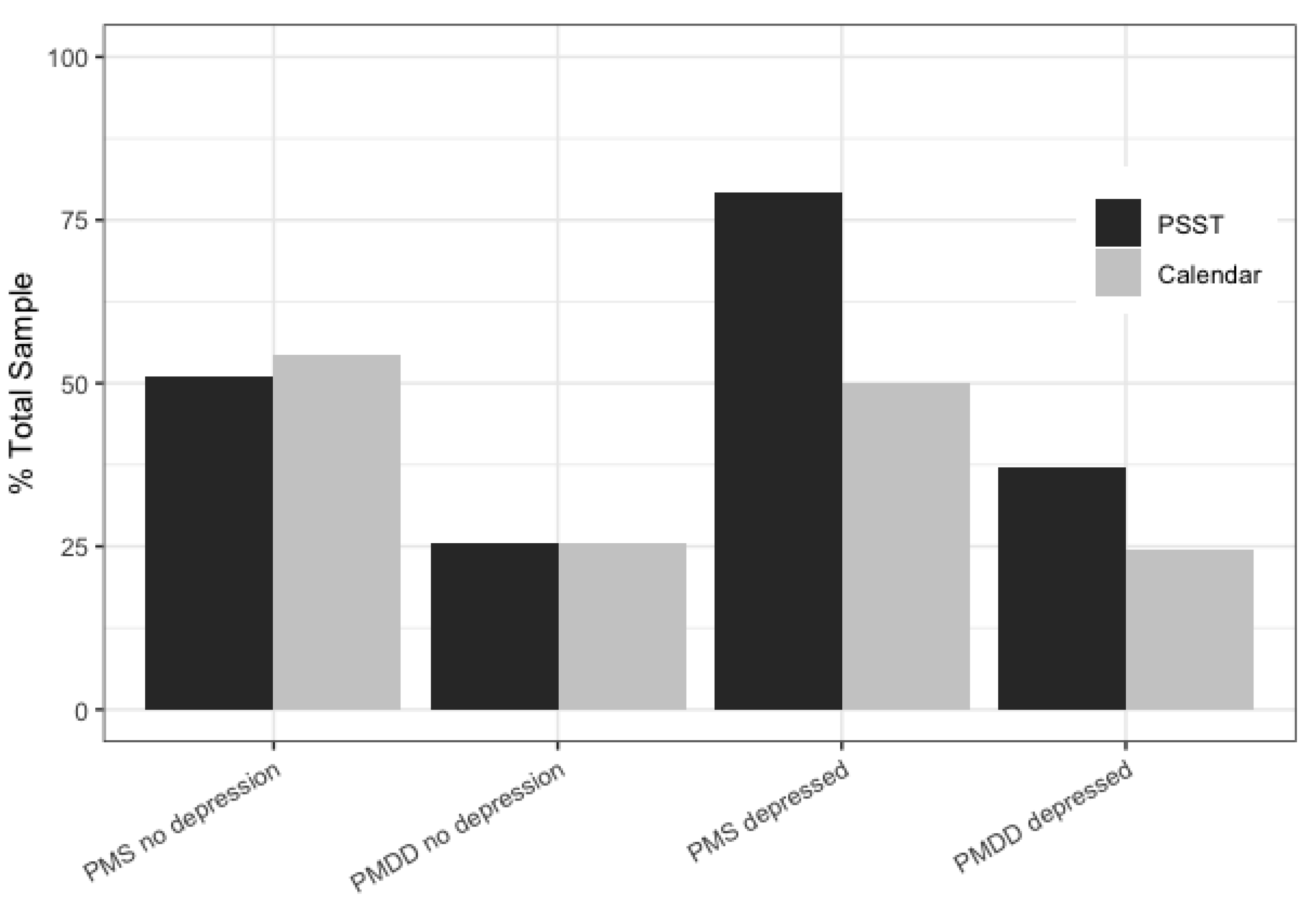
3.2. Discriminant Validity, Sensitivity, and Specificity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freeman, E.W. Premenstrual syndrome and premenstrual dysphoric disorder: Definitions and diagnosis. Psychoneuroendocrinology 2003, 28, 25–37. [Google Scholar] [CrossRef]
- Ryu, A.; Kim, T.-H. Premenstrual syndrome: A mini review. Maturitas 2015, 82, 436–440. [Google Scholar] [CrossRef]
- Freeman, E.W.; Halberstadt, S.M.; Rickels, K.; Legler, J.M.; Lin, H.; Sammel, M.D. Core symptoms that discriminate premenstrual syndrome. J. Womens Health 2011, 20, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonkers, K.A.; Simoni, M.K. Premenstrual disorders. Am. J. Obs. Gynecol. 2018, 218, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lustyk, M.K.B.; Gerrish, W.G. Premenstrual syndrome and premenstrual dysphoric disorder: Issues of quality of life, stress and exercise. In Handbook of Disease Burdens and Quality of Life Measures; Preedy, V.R., Watson, R.R., Eds.; Springer: New York, NY, USA, 2010. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Weisz, G.; Knaapen, L. Diagnosing and treating premenstrual syndrome in five western nations. Soc. Sci. Med. 2009, 68, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Halbreich, U.; Borenstein, J.; Pearlstein, T.; Kahn, L.S. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology 2003, 28, 1–23. [Google Scholar] [CrossRef]
- Gehlert, S.; Song, I.H.; Chang, C.-H.; Hartlage, S.A. The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychol. Med. 2009, 39, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Mortola, J.F.; Girton, L.; Beck, L.; Yen, S.S.C. Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: The calendar of premenstrual experiences. Obstet. Gynecol. 1990, 76, 302–307. [Google Scholar] [CrossRef]
- Endicott, J.; Nee, J.; Harrison, W. Daily record of severity of problems (DRSP): Reliability and validity. Arch. Womens Ment. Health 2006, 9, 41–49. [Google Scholar] [CrossRef]
- Steiner, M.; Macdougall, M.; Brown, E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch. Womens Ment. Health 2003, 6, 203–209. [Google Scholar] [CrossRef]
- El-Gizawy, Z.A.; O’Brien, P.M.S. Premenstrual syndrome. In Dewhurst’s Textbook of Obstetrics & Gynaecology; Edmonds, D.K., Lees, C., Bourne, T., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- Yonkers, K.A.; O’Brien, S.; Eriksson, E. Premenstrual Syndrome. Lancet 2008, 371, 1200–1210. [Google Scholar] [CrossRef]
- Henz, A.; Ferreira, C.; Oderich, C.; Gallon, C.; Castro, J.; Conzatti, M.; de Almeida Fleck, M.P. Premenstrual syndrome diagnosis: A comparative study between the daily record of severity of problems (DRSP) and the premenstrual symptoms screening tool (PSST). Rev. Bras. Ginecol. Obstetrícia RBGO Gynecol. Obstet. 2018, 40, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, T.S.; Barrett, L.F. Trends in ambulatory self-report: The role of momentary experience in psychosomatic medicine. Psychosom Med. 2012, 74, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosman, R.C.; Jung, S.E.; Miloserdov, K.; Schoevers, R.A.; aan het Rot, M. Daily symptom ratings for studying premenstrual dysphoric disorder: A review. J. Affect Disord. 2016, 189, 43–53. [Google Scholar] [CrossRef]
- Forrester-Knauss, C.; Stutz, E.Z.; Weiss, C.; Tschudin, S. The interrelation between premenstrual syndrome and major depression: Results from a population-based sample. BMC Public Health 2011, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, L.S.; Soares, C.N.; Otto, M.W.; Sweeney, B.H.; Liberman, R.F.; Harlow, B.L. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women The Harvard Study of Moods and Cycles. J. Affect Disord. 2002, 70, 125–132. [Google Scholar] [CrossRef]
- Kepple, A.L.; Lee, E.E.; Haq, N.; Rubinow, D.R.; Schmidt, P.J. History of Postpartum Depression in a Clinic-Based Sample of Women With Premenstrual Dysphoric Disorder. J. Clin. Psychiatry 2016, 77, e415–e420. [Google Scholar] [CrossRef] [PubMed]
- Wittchen, H.-U.; Becker, E.; Lieb, R.; Krause, P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol. Med. 2002, 32, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Bloch, M. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry 2000, 157, 924–930. [Google Scholar] [CrossRef]
- Payne, J.L.; Palmer, J.T.; Joffe, H. A reproductive subtype of depression: Conceptualizing models and moving toward etiology. Harv. Rev. Psychiatry 2009, 17, 72–86. [Google Scholar] [CrossRef] [Green Version]
- Kaniasty, K. Klęska żywiołowa czy katastrofa społeczna? Psychospołeczne konsekwencje polskiej powodzi 1997 roku. Gdańsk Gdańskie Wydaw. Psychol. 2003. [Google Scholar]
- First, M.B.; Popiel, A.; Zawadzki, B.; Habrat-Pragłowska, E.; Lazarowicz, H. SCID-I. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version; Pracownia Testów Psychologicznych PTP: Warszawa, Poland, 2014. [Google Scholar]
- Gregory, R.J. Psychological Testing: History, Principles, and Applications, 7th ed.; Pearson: Boston, MA, USA, 2014; 624p. [Google Scholar]
- Brzezińska, J. A polytomous item response theory models using R. Econometrics 2016, 2, 43–53. [Google Scholar]
- Rizopoulos, D. An R Package for Latent Variable Modeling and Item Response Theory Analyses. Available online: http://www.jstatsoft.org/v17/i05/ (accessed on 17 December 2018).
- Schivinski, B.; Brzozowska-Woś, M.; Buchanan, E.M.; Griffiths, M.D.; Pontes, H.M. Psychometric assessment of the Internet Gaming Disorder diagnostic criteria: An Item Response Theory study. Addict. Behav. Rep. 2018, 8, 176–184. [Google Scholar] [CrossRef]
- Choi, S.W.; Gibbons, L.E.; Crane, P.K. An R Package for Detecting Differential Item Functioning Using Iterative Hybrid Ordinal Logistic Regression/Item Response Theory and Monte Carlo Simulations. Available online: http://www.jstatsoft.org/v39/i08/ (accessed on 15 July 2019).
- Stevenson, M. epiR: Tools for the Analysis of Epidemiological Data; R-Studio Package; 2019. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. Boston; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Hartlage, S.A.; Arduino, K.E. Toward the content validity of premenstrual dysphoric disorder: Do anger and irritability more than depressed mood represent treatment-seekers’ experiences? Psychol. Rep. 2002, 90, 189–202. [Google Scholar] [CrossRef]
- Nicolau, Z.F.M.; Bezerra, A.G.; Polesel, D.N.; Andersen, M.L.; Bittencourt, L.; Tufik, S.; Hachu, H. Premenstrual syndrome and sleep disturbances: Results from the Sao Paulo Epidemiologic Sleep Study. Psychiatry Res. 2018, 264, 427–431. [Google Scholar] [CrossRef]
- Haywood, A.; Slade, P.; King, H. Assessing the assessment measures for menstrual cycle symptoms A guide for researchers and clinicians. J. Psychosom Res. 2002, 52, 223–237. [Google Scholar] [CrossRef]
- Kannisto, K.A.; Korhonen, J.; Adams, C.E.; Koivunen, M.H.; Vahlberg, T.; Välimäki, M.A. Factors Associated With Dropout During Recruitment and Follow-Up Periods of a mHealth-Based Randomized Controlled Trial for Mobile. Net to Encourage Treatment Adherence for People With Serious Mental Health Problems. J. Med. Internet Res. 2017, 19, e46. [Google Scholar] [CrossRef]

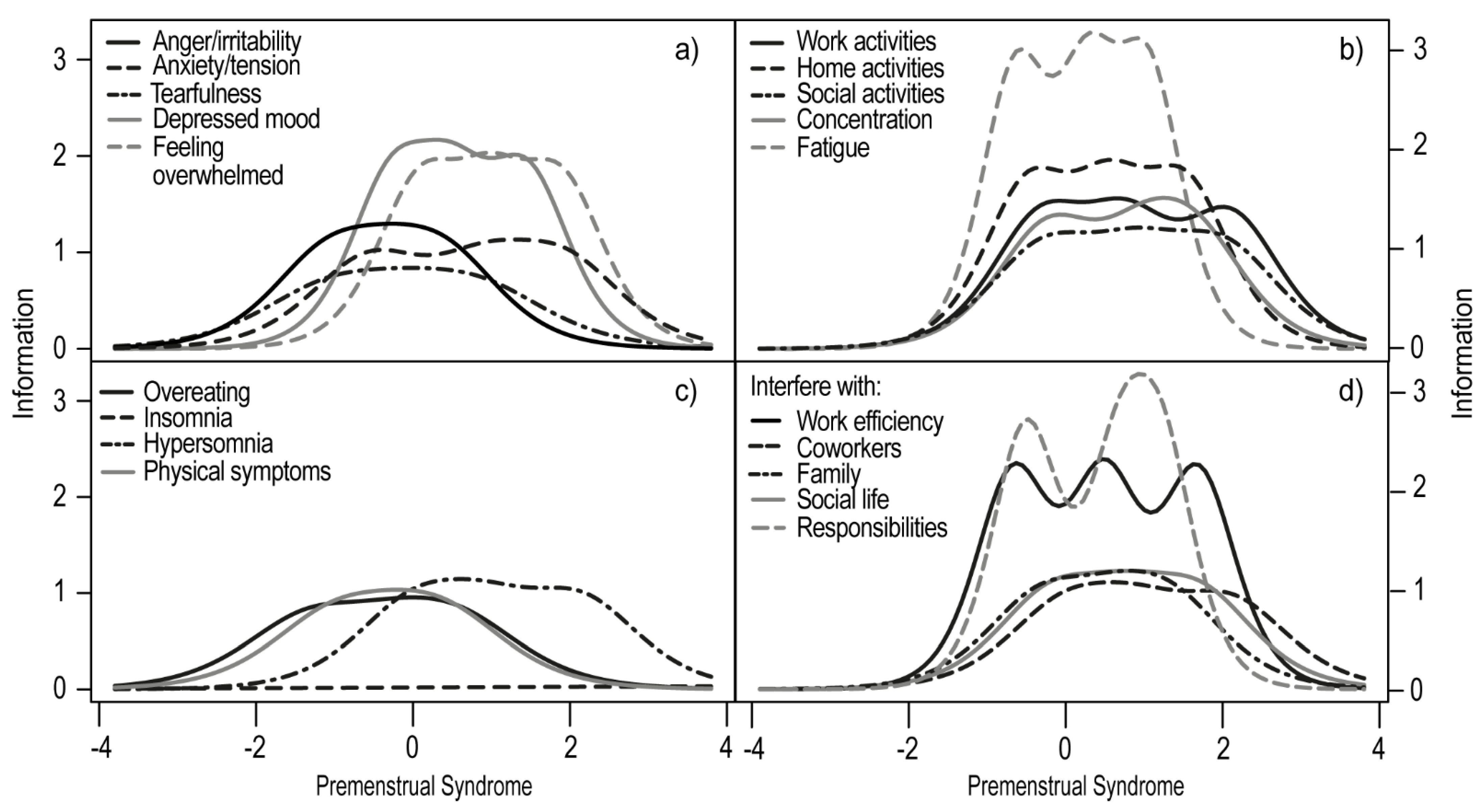
| Discrimination Level | Extremity Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | Depressed | Non-Depressed | Extrmt1 | Extrmt2 | Extrmt3 | |||||||
| General | Depressed | Non-Depressed | General | Depressed | Non-Depressed | General | Depressed | Non-Depressed | ||||
| 1. Anger/irritability | 1.409 | 1.519 | 2.010 | −2.266 | −2.296 | −1.064 | −0.795 | −0.879 | −0.290 | 0.636 | 0.599 | 0.391 |
| 2. Anxiety/tension | 1.477 | 1.284 | 1.909 | −1.373 | −1.645 | −0.546 | 0.094 | −0.087 | 0.954 | 1.310 | 1.479 | 1.950 |
| 3.Tearful/sensitivity to rejection | 1.681 | 1.429 | 1.655 | −1.876 | −2.380 | −1.006 | −0.806 | −1.033 | −0.046 | 0.168 | −0.012 | 0.804 |
| 4. Depressed mood | 2.042 | 1.898 | 2.521 | −1.265 | −2.120 | −0.253 | −0.286 | −0.664 | 0.511 | 0.672 | −0.476 | 1.502 |
| 5. Interest in work activities | 2.097 | 1.849 | 2.271 | −0.907 | −1.286 | −0.244 | 0.286 | −0.163 | 0.828 | 1.256 | 1.114 | 2.171 |
| 6. Interest in home activities | 2.361 | 2.132 | 2.779 | −1.014 | −1.300 | −0.397 | 0.078 | −0.127 | 0.585 | 0.975 | 0.982 | 1.506 |
| 7. Interest in social activities | 2.009 | 1.563 | 2.174 | −0.784 | −0.799 | −0.152 | 0.301 | 0.305 | 1.109 | 1.097 | 1.317 | 1.676 |
| 8. Concentration | 1.761 | 1.631 | 2.203 | −0.787 | −0.941 | −0.184 | 0.558 | 0.595 | 0.920 | 1.625 | 1.457 | 1.982 |
| 9. Fatigue/lack of energy | 2.353 | 2.599 | 3.392 | −1.580 | −1.721 | −0.578 | −0.357 | −0.388 | 0.348 | 0.735 | 0.889 | 1.084 |
| 10. Overeating/food cravings | 0.988 | 0.998 | 1.784 | −1.625 | −1.498 | −0.985 | −0.302 | −0.360 | −0.151 | 0.717 | 1.033 | −0.381 |
| 11. Insomnia | 0.616 | 0.821 | 0.256 | 0.905 | 0.814 | 4.703 | 3.198 | 2.600 | 10.116 | 6.184 | 4.161 | 15.782 |
| 12. Hypersomnia | 1.353 | 1.020 | 2.027 | −0.885 | −1.353 | 0.075 | 0.275 | 0.249 | 0.910 | 1.691 | 2.044 | 2.113 |
| 13. Feeling overwhelmed | 2.233 | 2.368 | 2.618 | −0.863 | −0.846 | 0.122 | 0.062 | −0.190 | 1.016 | 0.843 | 0.635 | 1.893 |
| 14. Physical symptoms | 1.318 | 1.195 | 1.818 | −2.076 | −2.037 | −1.284 | −0.794 | −0.948 | −0.130 | 0.457 | 0.352 | 0.538 |
| A. Interfere with work efficiency | 2.253 | 2.528 | 3.175 | −1.197 | −1.161 | −0.608 | 0.066 | −0.177 | 0.531 | 1.132 | 0.735 | 1.710 |
| B. Interfere with relationships with coworkers | 1.930 | 1.794 | 2.136 | −0.722 | −0.780 | −0.110 | 0.395 | 0.279 | 0.804 | 1.449 | 1.547 | 1.710 |
| C. Interfere with family relationships | 1.775 | 1.565 | 1.957 | −1.644 | −1.815 | −0.739 | −0.566 | −0.995 | 0.279 | 0.480 | 0.272 | 0.796 |
| D. Interfere with social life | 2.380 | 2.480 | 2.109 | −1.105 | −1.316 | −0.237 | 0.013 | −0.045 | 0.707 | 0.884 | 0.992 | 1.319 |
| E. Interfere with home responsibilities | 2.424 | 1.930 | 3.492 | −1.131 | −1.585 | −0.450 | 0.182 | −0.125 | 0.767 | 1.063 | 0.980 | 1.215 |
| PMS | PMDD | |||||
|---|---|---|---|---|---|---|
| Whole Sample | Depressed | Non-Depressed | Whole Sample | Depressed | Non-Depressed | |
| Specificity | 40% (30, 51) | 21% (10, 36) | 68% (46, 85) | 75% (68, 81) | 71% (59, 82) | 73% (58, 85) |
| Sensitivity | 75% (66, 82) | 79% (64, 90) | 67% (47, 83) | 51% (35, 67) | 65% (41, 85) | 14% (00, 58) |
| PPV | 63% (54, 70) | 50% (38, 62) | 71% (51, 87) | 32% (21, 45) | 41% (24, 59) | 0.07% (00, 34) |
| NPV | 54% (42, 67) | 50% (26, 74) | 63% (42, 81) | 87% (80, 92) | 87% (75, 95) | 85% (71, 94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliwerski, A.; Koszałkowska, K. The Influence of Depression on Biased Diagnosis of Premenstrual Syndrome and Premenstrual Dysphoric Disorder by the PSST Inventory. Life 2021, 11, 1278. https://doi.org/10.3390/life11111278
Śliwerski A, Koszałkowska K. The Influence of Depression on Biased Diagnosis of Premenstrual Syndrome and Premenstrual Dysphoric Disorder by the PSST Inventory. Life. 2021; 11(11):1278. https://doi.org/10.3390/life11111278
Chicago/Turabian StyleŚliwerski, Andrzej, and Karolina Koszałkowska. 2021. "The Influence of Depression on Biased Diagnosis of Premenstrual Syndrome and Premenstrual Dysphoric Disorder by the PSST Inventory" Life 11, no. 11: 1278. https://doi.org/10.3390/life11111278







