Multiple Non-Species-Specific Pathogens Possibly Triggered the Mass Mortality in Pinna nobilis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Diagnostic Analysis
2.3. Statistical Analysis
3. Results
3.1. Protozoa
3.2. Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schultz, P.; Huber, M. Revision of the Worldwide Recent Pinnidae and some Remarks on Fossil European Pinnidae. Acta Conch. 2013, 13, 1–164. [Google Scholar]
- Rouanet, E.; Trigos, S.; Vicente, N. From youth to death of old age: The 50-year story of a Pinna nobilis fan mussel population at Port-Cros Island (Port-Cros National Park, Provence, Mediterranean Sea). Sci. Rep. Port-Cros Natl. Park 2015, 29, 209–222. [Google Scholar]
- Zavodnik, D.; Hrs-Brenko, M.; Legac, M. Synopsis of the fan shell Pinna nobilis L. in the eastern Adriatic Sea. In Les Espèces Marines à Protéger en Méditerranée; Boudouresque, C.F., Avon, M., Gravez, V., Eds.; GIS Posidonie: Marseille, France, 1991; pp. 169–178. [Google Scholar]
- Basso, L.; Vázquez-Luis, M.; García-March, J.R.; Deudero, S.; Alvarez, E.; Vicente, N.; Duarte, C.M.; Hendriks, I.E. The Pen Shell, Pinna nobilis. Adv. Mar. Biol. 2015, 71, 109–160. [Google Scholar] [CrossRef]
- Trigos, S.; Vicente, N.; Prado, P.; Espinós, F.J. Adult spawning and early larval development of the endangered bivalve Pinna nobilis. Aquaculture 2018, 483, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.; Vicente, N.; Gaulejac, B. Ecology of the pterioid bivalves Pinna bicolor Gmelin and Pinna nobilis L. Mar. Life 1993, 3, 37–45. [Google Scholar]
- Katsanevakis, S. Population ecology of the endangered fan mussel Pinna nobilis in a marine lake. Endanger. Species Res. 2004, 1, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Rabaoui, L.; Tlig-Zouari, S.; Katsanevakis, S.; Hassine, O.K.B. Modelling population density of Pinna nobilis (Bivalvia) on the eastern and southeastern coast of Tunisia. J. Molluscan Stud. 2010, 76, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Luis, M.; Borg, J.A.; Morell, C.; Banach-Esteve, G.; Deudero, S. Influence of boat anchoring on Pinna nobilis: A field experiment using mimic units. Mar. Freshw. Res. 2015, 66, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Sanna, D.; Cossu, P.; Dedola, G.L.; Scarpa, F.; Maltagliati, F.; Castelli, A.; Franzoi, P.; Lai, T.; Cristo, B.; Curini-Galletti, M.; et al. Mitochondrial DNA Reveals Genetic Structuring of Pinna nobilis across the Mediterranean Sea. PLoS ONE 2013, 8, e67372. [Google Scholar] [CrossRef] [Green Version]
- Sanna, D.; Dedola, G.; Scarpa, F.; Lai, T.; Cossu, P.; Curini-Galletti, M.; Francalacci, P.; Casu, M. New mitochondrial and nuclear primers for the Mediterranean marine bivalve Pinna nobilis. Mediterr. Mar. Sci. 2014, 15, 416. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Luis, M.; Álvarez, E.; Barrajón, A.; García-March, J.R.; Grau, A.; Hendriks, I.E.; Jiménez, S.; Kersting, D.K.; Moreno, D.; Pérez, M.; et al. S.O.S. Pinna nobilis: A Mass Mortality Event in Western Mediterranean Sea. Front. Mar. Sci. 2017, 4, 220. [Google Scholar] [CrossRef] [Green Version]
- Catanese, G.; Grau, A.; Valencia, J.; García-March, J.R.; Vazquez-Luis, M.; Alvarez, E.; Deudero, S.; Darriba, S.; Carballal, M.J.; Villalba, A. Haplosporidium pinnae sp. nov., a haplosporidan parasite associated with mass mortalities of the fan mussel, Pinna nobilis, in the Western Mediterranean Sea. J. Invertebr. Pathol. 2018, 157, 9–24. [Google Scholar] [CrossRef]
- Panarese, R.; Tedesco, P.; Chimienti, G.; Latrofa, M.S.; Quaglio, F.; Passantino, G.; Buonavoglia, C.; Gustinelli, A.; Tursi, A.; Otranto, D. Haplosporidium pinnae associated with mass mortality in endangered Pinna nobilis (Linnaeus 1758) fan mussels. J. Invertebr. Pathol. 2019, 164, 32–37. [Google Scholar] [CrossRef]
- Katsanevakis, S. The cryptogenic parasite Haplosporidium pinnae invades the Aegean Sea and causes the collapse of Pinna nobilis populations. Aquat. Invasions 2019, 14, 150–164. [Google Scholar] [CrossRef]
- Carella, F.; Aceto, S.; Pollaro, F.; Miccio, A.; Iaria, C.; Carrasco, N.; Prado, P.; De Vico, G. A mycobacterial disease is associated with the silent mass mortality of the pen shell Pinna nobilis along the Tyrrhenian coastline of Italy. Sci. Rep. 2019, 9, 2725. [Google Scholar] [CrossRef] [PubMed]
- Prado, P.; Carrasco, N.; Catanese, G.; Grau, A.; Cabanes, P.; Carella, F.; García-March, J.R.; Tena, J.; Roque, A.; Bertomeu, E.; et al. Presence of Vibrio mediterranei associated to major mortality in stabled individuals of Pinna nobilis L. Aquaculture 2020, 519, 734899. [Google Scholar] [CrossRef]
- Andree, K.B.; Carrasco, N.; Carella, F.; Furones, D.; Prado, P. Vibrio mediterranei, a Potential Emerging Pathogen of Marine Fauna: Investigation of pathogenicity using a bacterial challenge in Pinna nobilis and development of a species-specific PCR. J. Appl. Microbiol. 2020. [Google Scholar] [CrossRef]
- Carella, F.; Antuofermo, E.; Farina, S.; Salati, F.; Mandas, D.; Prado, P.; Panarese, R.; Marino, F.; Fiocchi, E.; Pretto, T.; et al. In the wake of the ongoing mass mortality events: Co-occurrence of Mycobacterium, Haplosporidium and other pathogens in Pinna nobilis collected in Italy and Spain (Mediterranean Sea). Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, F.; Marcia, P.; Cossu, P.; Lai, T.; Sechi, S.; Curini-Galletti, M.; Sanna, D.; Azzena, I.; Zanello, A.; Gazale, V.; et al. Marine strategy as needful tool for the management of endangered species: The study case of Pinna nobilis (Mollusca: Bivalvia). Biol. Mar. Mediterr. in press.
- Garrabou, J.; Coma, R.; Bensoussan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; Diaz, D.; Harmelin, J.G.; Gambi, M.C.; Kersting, D.K.; et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Chang. Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Varello, K.; Prearo, M.; Burioli, E.A.V.; Pastorino, P.; Mugetti, D.; Meistro, S.; Righetti, M.; Bozzetta, E. Mycobacterium fortuitum infection in silver arowana (Osteoglossum bicirrhosum). Bull. Eur. Ass. Fish Pathol. 2019, 39, 83. [Google Scholar]
- Antuofermo, E.; Pais, A.; Polinas, M.; Cubeddu, T.; Righetti, M.; Sanna, M.A.; Prearo, M. Mycobacteriosis caused by Mycobacterium marinum in reared mullets: First evidence from Sardinia (Italy). J. Fish Dis. 2016, 40, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.A.; Strimmer, K.; Vingron, M.; Von Haeseler, A. Tree-puzzle: Maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 2002, 18, 502–504. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.A.; Von Haeseler, A. Phylogenetic inference using maximum likelihood methods. In The Phylogenetic Handbook; Cambridge University Press: Cambridge, UK, 2012; pp. 181–209. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, F.; Cossu, P.; Delogu, V.; Lai, T.; Sanna, D.; Leasi, F.; Norenburg, J.L.; Casu, M.; Curini-Galletti, M. Molecular support for morphology-based family-rank taxa: The contrasting cases of two families of Proseriata (Platyhelminthes). Zool. Scr. 2017, 46, 753–766. [Google Scholar] [CrossRef]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 457–472. [Google Scholar] [CrossRef]
- Scarpa, F.; Cossu, P.; Sanna, D.; Lai, T.; Casu, M.; Curini-Galletti, M. New insights on the genus Otoplana Du Plessis, 1889 (Platyhelminthes: Proseriata), with description of two new species from the Canary Islands. Mar. Biodivers. 2017, 49, 2075–2087. [Google Scholar] [CrossRef]
- Duchene, S.; Holt, K.E.; Weill, F.-X.; Le Hello, S.; Hawkey, J.; Edwards, D.J.; Fourment, M.; Holmes, E.C. Genome-scale rates of evolutionary change in bacteria. Microb. Genom. 2016, 2. [Google Scholar] [CrossRef]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Michaelidis, B. First detection of the invasive Haplosporidian and Mycobacteria parasites hosting the endangered bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Mar. Environ. Res. 2020, 155, 104889. [Google Scholar] [CrossRef]
- López-Sanmartín, M.; Catanese, G.; Grau, A.; Valencia, J.; García-March, J.R.; Navas, J.I. Real-Time PCR based test for the early diagnosis of Haplosporidium pinnae affecting fan mussel Pinna nobilis. PLoS ONE 2019, 14, e0212028. [Google Scholar] [CrossRef] [PubMed]
- Maeno, Y.; Yurimoto, T.; Nasu, H.; Ito, S.; Aishima, N.; Matsuyama, T.; Kamaishi, T.; Oseko, N.; Watanabe, Y. Virus-like particles associated with mass mortalities of the pen shell Atrina pectinata in Japan. Dis. Aquat. Org. 2006, 71, 169–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secci, M.; Biancacci, C.; Giglioli, A.; Loddo, D.; Pasquini, V.; Addis, P. Geostatistical approach to investigate spatial patterns of the endangered fan mussel Pinna nobilis (Linnaeus, 1758). Reg. Stud. Mar. Sci. 2019, 32, 100884. [Google Scholar] [CrossRef] [Green Version]
- Patarnello, T.; Volckaert, F.A.M.J.; Castilho, R. Pillars of Hercules: Is the Atlantic-Mediterranean transition a phylogeographical break? Mol. Ecol. 2007, 16, 4426–4444. [Google Scholar] [CrossRef]
- Addis, P.; Secci, M.; Brundu, G.; Manunza, A.; Corrias, S.; Cau, A. Density, size structure, shell orientation and epibiontic colonization of the fan mussel Pinna nobilis L. 1758 (Mollusca: Bivalvia) in three contrasting habitats in an estuarine area of Sardinia (W Mediterranean). Sci. Mar. 2009, 73, 143–152. [Google Scholar] [CrossRef]
- Rabaoui, L.; Tlig-Zouari, S.; Cosentino, A.; Ben Hassine, O.K. Associated fauna of the fan shell Pinna nobilis (Mollusca: Bivalvia) in the northern and eastern Tunisian coasts. Sci. Mar. 2009, 73, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Rabaoui, L.; Tlig-Zouari, S.; Ben Hassine, O.K. Distribution and habitat of the fan mussel Pinna nobilis Linnaeus, 1758 (Mollusc: Bivalvia) along the northern and eastern Tunisian coasts. Cah. Biol. Mar. 2008, 49, 67–78. [Google Scholar]
- García-March, J.R.; García-Carrascosa, A.M.; Cantero, A.L.P.; Wang, Y.-G. Population structure, mortality and growth of Pinna nobilis Linnaeus, 1758 (Mollusca, Bivalvia) at different depths in Moraira bay (Alicante, Western Mediterranean). Mar. Biol. 2006, 150, 861–871. [Google Scholar] [CrossRef]
- Nikula, R. Phylogeography of Cerastoderma glaucum (Bivalvia: Cardiidae) across Europe: A major break in the Eastern Mediterranean. Mar. Biol. 2003, 143, 339–350. [Google Scholar] [CrossRef]
- Calvo, M.; Templado, J.; Oliverio, M.; Machordom, A. Hidden Mediterranean biodiversity: Molecular evidence for a cryptic species complex within the reef building vermetid gastropod Dendropoma petraeum (Mollusca: Caenogastropoda). Biol. J. Linn. Soc. 2009, 96, 898–912. [Google Scholar] [CrossRef] [Green Version]
- Cossu, P.; Dedola, G.L.; Scarpa, F.; Sanna, D.; Lai, T.; Maltagliati, F.; Curini-Galletti, M.; Casu, M. Patterns of spatial genetic variation in Patella ulyssiponensis: Insights from the western Mediterranean marine ecoregion. Hydrobiologia 2015, 755, 39–55. [Google Scholar] [CrossRef]
- Cabanellas-Reboredo, M.; Vázquez-Luis, M.; Mourre, B.; Álvarez, E.; Deudero, S.; Amores, Á.; Addis, P.; Ballesteros, E.; Barrajón, A.; Coppa, S.; et al. Tracking a mass mortality outbreak of pen shell Pinna nobilis populations: A collaborative effort of scientists and citizens. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ford, S.E.; Haskin, H.H. History and epizootiology of Haplosporidium nelsoni (MSX), an oyster pathogen in Delaware Bay, 1957–1980. J. Invertebr. Pathol. 1982, 40, 118–141. [Google Scholar] [CrossRef]
- Haskin, H.H.; Andrews, J.D. Uncertainties and speculations about the life cycle of the eastern oyster pathogen Haplosporidium nelsoni (MSX). Dis. Process. Mar. Bivalve Molluscs 1988, 18, 5–22. [Google Scholar]
- Hudson, E.; Hill, B. Impact and spread of bonamiasis in the UK. Aquaculture 1991, 93, 279–285. [Google Scholar] [CrossRef]
- Hofmann, E.; Ford, S.; Powell, E.; Klinck, J.M. Modeling studies of the effect of climate variability on MSX disease in eastern oyster (Crassostrea virginica) populations. Ecol. Etiol. New. Emerg. Mar. Dis. 2001, 17, 195–212. [Google Scholar] [CrossRef]
- Burreson, E.M.; Ford, S.E. A review of recent information on the Haplosporidia, with special reference to Haplosporidium nelsoni (MSX disease). Aquat. Living Resour. 2004, 17, 499–517. [Google Scholar] [CrossRef]
- Laing, I.; Dunn, P.; Peeler, E.; Feist, S.; Longshaw, M. Epidemiology of Bonamia in the UK, 1982 to 2012. Dis. Aquat. Org. 2014, 110, 101–111. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, C.C.C.R.; Da Fonseca, M.M.R. The remarkable Rhodococcus erythropolis. Appl. Microbiol. Biotechnol. 2005, 67, 715–726. [Google Scholar] [CrossRef]
- Ward, A.L.; Reddyvari, P.; Borisova, R.; Shilabin, A.G.; Lampson, B.C. An inhibitory compound produced by a soil isolate of Rhodococcus has strong activity against the veterinary pathogen R. equi. PLoS ONE 2018, 13, e0209275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, W.; Tamura, T. Three Types of Antibiotics Produced from Rhodococcus erythropolis Strains. Microbes Environ. 2008, 23, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, A.B.; Birkbeck, T.; Nilsen, H.; MacPherson, H.; Wangel, C.; Myklebust, C.; Laidler, L.; Aarflot, L.; Thoen, E.; Nygard, S.; et al. Vaccine-associated systemic Rhodococcus erythropolis infection in farmed Atlantic salmon Salmo salar. Dis. Aquat. Org. 2006, 72, 9–17. [Google Scholar] [CrossRef] [PubMed]

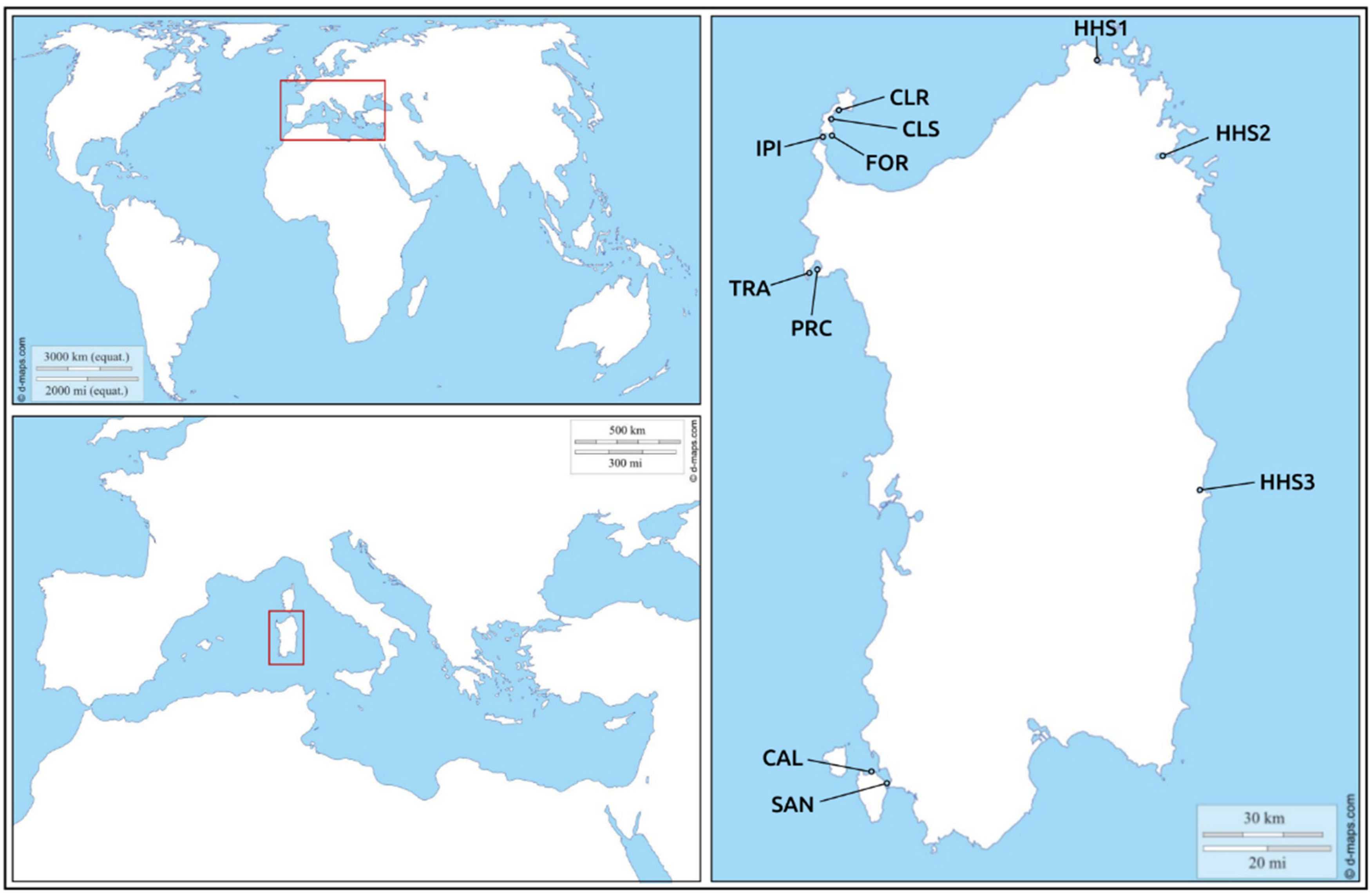

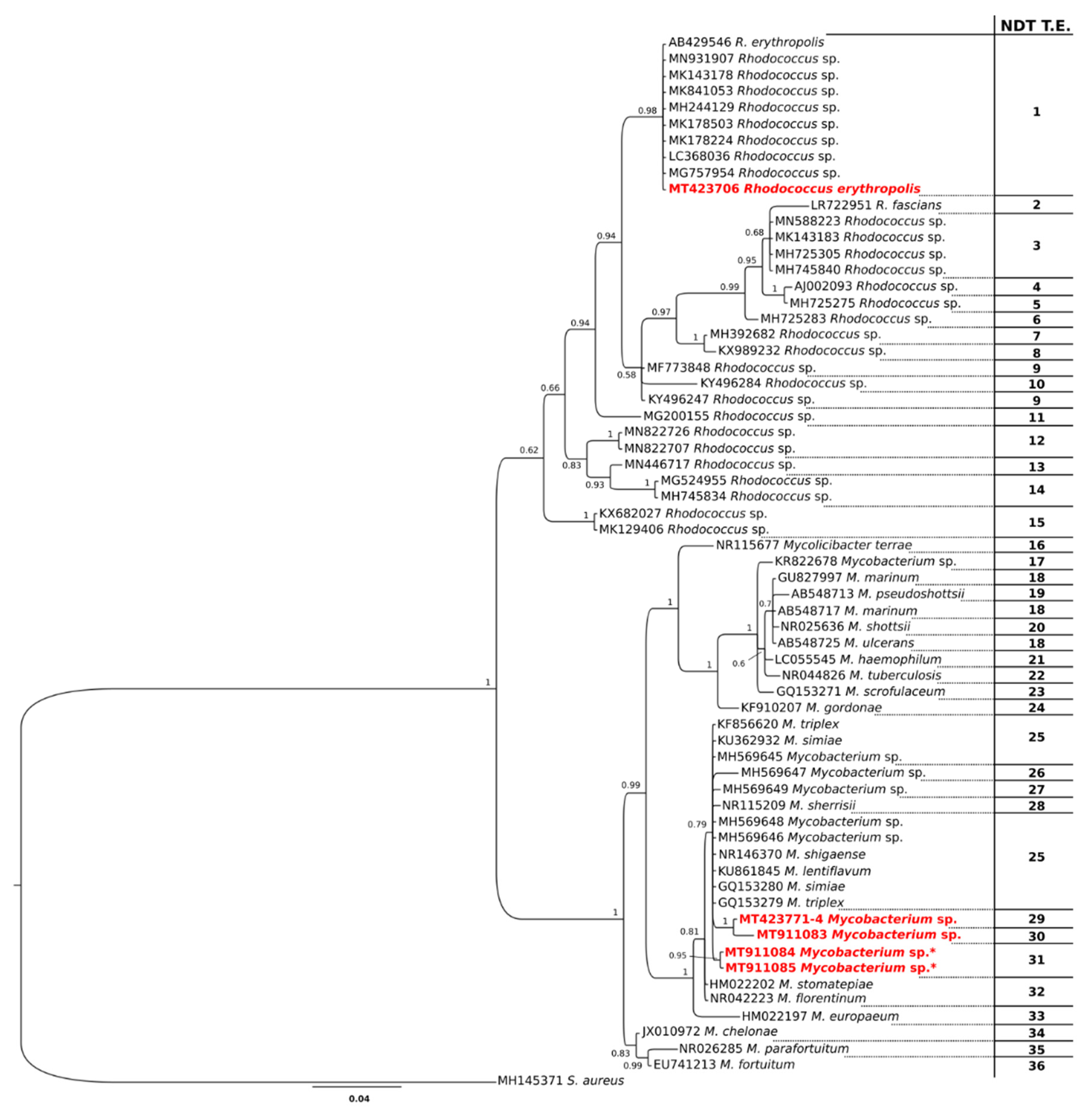
| Sample_ID | Locality | Code | Coordinates | SD | FS | H. pinnae | Mycobacterium spp. | Vibrio spp. |
|---|---|---|---|---|---|---|---|---|
| PN1 | Sant’Antioco | SAN | 39°03′49.2″ N 8°27′47.6″ E | NO | D | YES | NO | NO |
| PN2 | NO | D | YES | NO | NO | |||
| PN3 | NO | D | NO | NO | NO | |||
| PN4 | NO | D | NO | NO | NO | |||
| PN5 | Calasetta-Cussorgia | CAL | 39°06′27.7″ N 8°23′54.8″ E | NO | D | YES | NO | NO |
| PN6 | YES | D | YES | NO | NO | |||
| PN7 | NO | D | NO | NO | NO | |||
| PN8 | NO | D | NO | NO | NO | |||
| PN9 | NO | D | NO | NO | NO | |||
| PN10 | YES | D | NO | NO | NO | |||
| PN11 | YES | D | NO | NO | NO | |||
| PN12 | NO | D | NO | NO | NO | |||
| PN13 | NO | D | NO | NO | NO | |||
| PN14 | NO | D | NO | NO | NO | |||
| PN15 | NO | D | NO | NO | NO | |||
| PN16 | NO | D | NO | NO | NO | |||
| PN17 | NO | D | YES | NO | NO | |||
| PN18 | Tramariglio-MPA Capo Caccia Isola Piana | TRA | 40°35′23.6″ N 8°10′12.4″ E | NO | D | YES | NO | NO |
| PN19 | NO | D | NO | YES | NO | |||
| PN20 | Porto Conte-MPA Capo Caccia Isola Piana | PRC | 40°35′46.7″ N 8°12′58.1″ E | NO | A | NO | NO | NO |
| PN21 | Cala Reale-MPA Isola dell’Asinara | CLR | 41°03′47.6″ N 8°16′59.5″ E | NO | D | NO | NO | NO |
| PN22 | YES | D | YES | NO | NO | |||
| PN23 | NO | D | YES | NO | NO | |||
| PN24 | YES | D | YES | NO | NO | |||
| PN25 | NO | D | YES | NO | NO | |||
| PN26 | NO | D | YES | NO | NO | |||
| PN27 | YES | D | YES | NO | NO | |||
| PN28 | YES | D | YES | NO | NO | |||
| PN29 | YES | D | YES | NO | NO | |||
| PN30 | YES | D | YES | NO | NO | |||
| PN31 | Fornelli-MPA Isola dell’Asinara | FOR | 40°59′04.1″ N 8°15′06.3″ E | YES | D | NO | NO | NO |
| PN32 | YES | D | NO | NO | NO | |||
| PN33 | NO | D | NO | NO | NO | |||
| PN34 | NO | D | YES | NO | NO | |||
| PN35 | YES | D | NO | NO | NO | |||
| PN36 | YES | D | YES | NO | NO | |||
| PN37 | YES | D | YES | NO | NO | |||
| PN38 | Cala Scombro di dentro-MPA Isola dell’Asinara | CLS | 41°01′43.5″ N 8°14′44.6″ E | YES | D | YES | NO | NO |
| PN39 | YES | D | YES | NO | NO | |||
| PN40 | NO | D | YES | NO | NO | |||
| PN41 | YES | D | YES | NO | NO | |||
| PN42 | YES | D | YES | NO | NO | |||
| PN43 | YES | D | NO | NO | NO | |||
| PN44 | NO | D | YES | NO | NO | |||
| PN45 | NO | D | YES | NO | NO | |||
| PN46 | YES | D | YES | NO | NO | |||
| PN47 | YES | D | YES | NO | NO | |||
| PN48 | Isola Piana-MPA Isola dell’Asinara | IPI | 40°58′42.9″ N 8°13′22.6″ E | NO | A | NO | YES | NO |
| PR1 | NO | A | NO | YES | NO | |||
| PR2 | NO | A | NO | YES | NO |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpa, F.; Sanna, D.; Azzena, I.; Mugetti, D.; Cerruti, F.; Hosseini, S.; Cossu, P.; Pinna, S.; Grech, D.; Cabana, D.; et al. Multiple Non-Species-Specific Pathogens Possibly Triggered the Mass Mortality in Pinna nobilis. Life 2020, 10, 238. https://doi.org/10.3390/life10100238
Scarpa F, Sanna D, Azzena I, Mugetti D, Cerruti F, Hosseini S, Cossu P, Pinna S, Grech D, Cabana D, et al. Multiple Non-Species-Specific Pathogens Possibly Triggered the Mass Mortality in Pinna nobilis. Life. 2020; 10(10):238. https://doi.org/10.3390/life10100238
Chicago/Turabian StyleScarpa, Fabio, Daria Sanna, Ilenia Azzena, Davide Mugetti, Francesco Cerruti, Sepideh Hosseini, Piero Cossu, Stefania Pinna, Daniele Grech, David Cabana, and et al. 2020. "Multiple Non-Species-Specific Pathogens Possibly Triggered the Mass Mortality in Pinna nobilis" Life 10, no. 10: 238. https://doi.org/10.3390/life10100238








