Approaching Gravity as a Continuum Using the Rat Partial Weight-Bearing Model
Abstract
:1. Introduction
2. Establishing the Rat Partial Weight-Bearing (PWB) Model
2.1. Housing Environment
2.2. Suspension Apparatus
2.3. Using a Pelvic Harness during Suspension (HLS)
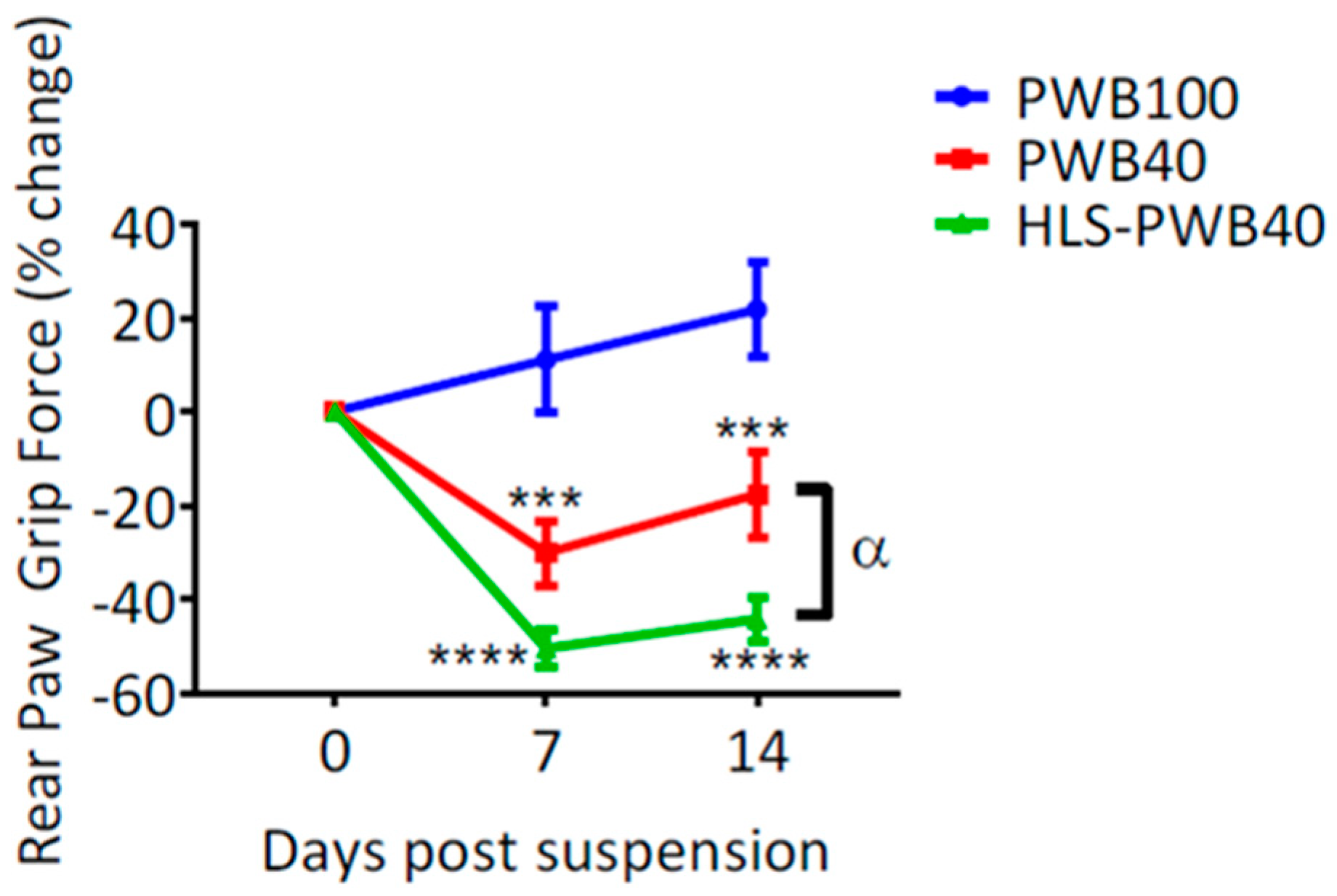
2.4. Reliability
3. A Global Model to Study Physiological Alterations
3.1. Behavior
3.2. Muscular System
3.3. Skeletal System
3.4. Cardiovascular System
4. Future Perspectives
4.1. Investigating Shifts Lower than 1g
4.2. Mechanisms Contributing to PWB Phenotypes
4.3. Sex-Based Differences
4.4. New Therapeutic Countermeasures
5. Outstanding Questions
5.1. Circadian Rhythm
5.2. Radiation
5.3. Biomarkers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.F.; Hargens, A.R. Spaceflight-induced intracranial hypertension and visual impairment: Pathophysiology and countermeasures. Physiol. Rev. 2018, 98, 59–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorhies, A.A.; Mark Ott, C.; Mehta, S.; Pierson, D.L.; Crucian, B.E.; Feiveson, A.; Oubre, C.M.; Torralba, M.; Moncera, K.; Zhang, Y.; et al. Study of the impact of long-duration space missions at the International Space Station on the astronaut microbiome. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Y.; Wu, X.; Liu, D.; Xu, D.; Wang, F. On-orbit sleep problems of astronauts and countermeasures. Mil. Med. Res. 2018, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA twins study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364. [Google Scholar] [CrossRef]
- Tuday, E.C.; Meck, J.V.; Nyhan, D.; Shoukas, A.A.; Berkowitz, D.E. Microgravity-induced changes in aortic stiffness and their role in orthostatic intolerance. J. Appl. Physiol. 2007, 102, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clément, G. Fundamentals of Space Medicine; Springer: New York, NY, USA, 2011. [Google Scholar]
- Narici, M.V.; de Boer, M.D. Disuse of the musculo-skeletal system in space and on earth. Eur. J. Appl. Physiol. 2011, 111, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, M.P.; Risin, D. The current state of bone loss research: Data from spaceflight and microgravity simulators. J. Cell. Biochem. 2013, 114, 1001–1008. [Google Scholar] [CrossRef]
- Yates, B.J.; Kerman, I.A. Post-spaceflight orthostatic intolerance: Possible relationship to microgravity-induced plasticity in the vestibular system. Brain Res. Rev. 1998, 28, 73–82. [Google Scholar] [CrossRef]
- Clément, G.; Ngo-Anh, J.T. Space physiology II: Adaptation of the central nervous system to space flight-past, current, and future studies. Eur. J. Appl. Physiol. 2012, 113, 1655–1672. [Google Scholar] [CrossRef]
- Denayer, T.; Stöhrn, T.; van Roy, M. Animal models in translational medicine: Validation and prediction. New Horiz. Transl. Med. 2014, 2, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Bonjour, J.P.; Ammann, P.; Rizzoli, R. Importance of preclinical studies in the development of drugs for treatment of osteoporosis: A review related to the 1998 WHO guidelines. Osteoporos. Int. 1999, 9, 379–393. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Historical aspects of the early Soviet/Russian manned space program. J. Appl. Physiol. 2001, 91, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Grigor’ev, A.I.; Il’in, E.A. Animals in Space: On the 50th Anniversary of Space Biology. In Herald of the Russian Academy of Sciences; 2007. Available online: https://history.nasa.gov/animals.html (accessed on 18 December 2019).
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar] [PubMed]
- Morey, E.R.; Sabelman, E.E.; Turner, R.T.; Baylink, D.J. A new rat model simulating some aspects of space flight. Physiologist 1979, 22, S23–S24. [Google Scholar]
- NASA Transition Authorization Act. National Space Exploration Campaign Report Pursuant. 2018. Available online: https://www.nasa.gov/sites/default/files/atoms/files/nationalspaceexplorationcampaign.pdf (accessed on 24 October 2018).
- National Aeronautics and Space Admi, Mars Exploration Program. In Van Nostrand’s Scientific Encyclopedia; 2007. Available online: http://mars.jpl.nasa.gov/programmissions/overview/ (accessed on 24 July 2017).
- Wagner, E.B.; Granzella, N.P.; Saito, H.; Newman, D.J.; Young, L.R.; Bouxsein, M.L. Partial weight suspension: A novel murine model for investigating adaptation to reduced musculoskeletal loading. J. Appl. Physiol. 2010, 109, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Mortreux, M.; Nagy, J.A.; Ko, F.C.; Bouxsein, M.L.; Rutkove, S.B. A novel partial gravity ground-based analog for rats via quadrupedal unloading. J. Appl. Physiol. 2018, 125, 175–182. [Google Scholar] [CrossRef]
- Ellman, R.; Spatz, J.; Cloutier, A.; Palme, R.; Christiansen, B.A.; Bouxsein, M.L. Partial reductions in mechanical loading yield proportional changes in bone density, bone architecture, and muscle mass. J. Bone Miner. Res. 2013, 28, 875–885. [Google Scholar] [CrossRef]
- Wilson, J.M.; Krigsfeld, G.S.; Sanzari, J.K.; Wagner, E.B.; Mick, R.; Kennedy, A.R. Comparison of hindlimb unloading and partial weight suspension models for spaceflight-type condition induced effects on white blood cells. Adv. Space Res. 2012, 49, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Swift, J.M.; Lima, F.; Macias, B.R.; Allen, M.R.; Greene, E.S.; Shirazi-Fard, Y.; Kupke, J.S.; Hogan, H.A.; Bloomfield, S.A. Partial weight bearing does not prevent musculoskeletal losses associated with disuse. Med. Sci. Sports Exerc. 2013, 45, 2052–2060. [Google Scholar] [CrossRef]
- Spatz, J.M.; Ellman, R.; Cloutier, A.M.; Louis, L.; van Vliet, M.; Dwyer, D.; Stolina, M.; Ke, H.Z.; Bouxsein, M.L. Sclerostin antibody inhibits skeletal deterioration in mice exposed to partial weight-bearing. Life Sci. Space Res. 2017, 12, 32–38. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Martinez, D.A.; Boudreaux, R.D.; Mantri, A.V. Microgravity stress: Bone and connective tissue. Compr. Physiol. 2016, 6, 645–686. [Google Scholar] [CrossRef] [PubMed]
- Macias, B.R.; Lima, F.; Swift, J.M.; Shirazi-Fard, Y.; Greene, E.S.; Allen, M.R.; Fluckey, J.; Hogan, H.A.; Braby, L.; Wang, S.; et al. Simulating the Lunar Environment: Partial Weightbearing and High-LET Radiation-Induce Bone Loss and Increase Sclerostin-Positive Osteocytes. Radiat. Res. 2016, 186, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.L.; Costa, R.M.E.; Costa, M.F.O.E. Rats. Rodent Model Tools Ethical Biomed. Res. 2015, 2, 61–94. [Google Scholar] [CrossRef]
- Febo, M. Technical and conceptual considerations for performing and interpreting functional MRI studies in awake rats. Front. Psychiatry 2011, 2. [Google Scholar] [CrossRef] [Green Version]
- Young, J.W.; Jentsch, J.D.; Bussey, T.J.; Wallace, T.L.; Hutcheson, D.M. Consideration of species differences in developing novel molecules as cognition enhancers. Neurosci. Biobehav. Rev. 2013, 37, 2181–2193. [Google Scholar] [CrossRef] [Green Version]
- Blais, E.M.; Rawls, K.D.; Dougherty, B.V.; Li, Z.I.; Kolling, G.L.; Ye, P.; Wallqvist, A.; Papin, J.A. Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? DMM Dis. Model. Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, T.; Uchiyama, S. Absolute and relative growth of rat skeletal muscle. Physiol. Behav. 1995, 57, 913–919. [Google Scholar] [CrossRef]
- Stark, D.M. Wire-Bottom Versus Solid-Bottom Rodent Caging Issues Important to Scientists and Laboratory Animal Science Specialists. Contemp. Top. Lab. Anim. Sci. 2001, 40, 11–14. [Google Scholar]
- Gordon, C.J.; Fogelson, L. Metabolic and thermoregulatory responses of the rat maintained in acrylic or wire-screen cages: Implications for pharmacological studies. Physiol. Behav. 1994, 56, 73–79. [Google Scholar] [CrossRef]
- Mortreux, M.; Riveros, D.; Bouxsein, M.L.; Rutkove, S.B. Mimicking a space mission to mars using hindlimb unloading and partial weight bearing in rats. J. Vis. Exp. 2019, 2019, e59327. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.; Globus, R.K.; Kaplansky, A.; Durnova, G. The Hindlimb Unloading Rat Model: Literature Overview, Technique Update and Comparison with Space Flight Data. Adv. Space Biol. Med. 2005, 10, 7–40. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Globus, R.K.; Morey-Holton, E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. 2016, 120, 1196–1206. [Google Scholar] [CrossRef]
- Chowdhury, P.; Long, A.; Harris, G.; Soulsby, M.E.; Dobretsov, M. Animal model of simulated microgravity: A comparative study of hindlimb unloading via tail versus pelvic suspension. Physiol. Rep. 2013, 1, e00012. [Google Scholar] [CrossRef]
- Mortreux, M.; Ko, F.C.; Riveros, D.; Bouxsein, M.L.; Rutkove, S.B. Longitudinal time course of muscle impairments during partial weight-bearing in rats. NPJ Microgravity 2019, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Mortreux, M.; Riveros, D.; Bouxsein, M.L.; Rutkove, S.B. A moderate daily dose of resveratrol mitigates muscle deconditioning in a martian gravity analog. Front. Physiol. 2019, 10, 899. [Google Scholar] [CrossRef] [Green Version]
- Beery, A.K.; Kaufer, D. Stress, social behavior, and resilience: Insights from rodents. Neurobiol. Stress 2015, 1, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Tahimic, C.G.T.; Paul, A.M.; Schreurs, A.S.; Torres, S.M.; Rubinstein, L.; Steczina, S.; Lowe, M.; Bhattacharya, S.; Alwood, J.S.; Ronca, A.E.; et al. Influence of Social Isolation during Prolonged Simulated Weightlessness by Hindlimb Unloading. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Gaignier, F.; Schenten, V.; Bittencourt, M.d.; Gauquelin-Koch, G.; Frippiat, J.P.; Legrand-Frossi, C. Three weeks of murine hindlimb unloading induces shifts from B to T and from Th to Tc splenic lymphocytes in absence of stress and differentially reduces cell-specific mitogenic responses. PLoS ONE 2014, 9, e92664. [Google Scholar] [CrossRef] [Green Version]
- Mortreux, M.; Riveros, D.; Semple, C.; Bouxsein, M.L.; Rutkove, S.B. The partial weight-bearing rat model using a pelvic harness does not impact stress or hindlimb blood flow. Acta Astronaut. 2020, 168, 249–255. [Google Scholar] [CrossRef]
- Hankenson, F.C.; Marx, J.O.; Gordon, C.J.; David, J.M. Effects of Rodent Thermoregulation on Animal Models in the Research Environment. Comp. Med. 2018, 68, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Calonne, J.; Arsenijevic, D.; Scerri, I.; Miles-Chan, J.L.; Montani, J.P.; Dulloo, A.G. Low 24-h core body temperature as a thrifty metabolic trait driving catch-up fat during weight regain after caloric restriction. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E699–E709. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.; Harvey, L.; van der Beek, E.M.; van Dijk, G. Home alone: A systematic review and meta-analysis on the effects of individual housing on body weight, food intake and visceral fat mass in rodents. Obes. Rev. 2018, 19, 614–637. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.F.; Koopmans, S.J.; Slangen, J.L.; van der Gugten, J. Effects of fasting on plasma catecholamine, corticosterone and glucose concentrations under basal and stress conditions in individual rats. Physiol. Behav. 1989, 45, 989–994. [Google Scholar] [CrossRef]
- McCowen, K.C.; Malhotra, A.; Bistrian, B.R. Stress-induced hyperglycemia. Crit. Care Clin. 2001, 17, 107–124. [Google Scholar] [CrossRef]
- Rafacho, A.; Ortsäter, H.; Nadal, A.; Quesada, I. Glucocorticoid treatment and endocrine pancreas function: Implications for glucose homeostasis, insulin resistance and diabetes. J. Endocr. 2014, 223, R49–R62. [Google Scholar] [CrossRef]
- Kalsbeek, A.; la Fleur, S.; Fliers, E. Circadian control of glucose metabolism. Mol. Metab. 2014, 3, 372–383. [Google Scholar] [CrossRef]
- Fluttert, M.; Dalm, S.; Oitzl, M.S. A refined method for sequential blood sampling by tail incision in rats. Lab. Anim. 2000, 34, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Ko, F.C.; Mortreux, M.; Riveros, D.; Nagy, J.A.; Rutkove, S.B.; Bouxsein, M.L. Dose-dependent skeletal deficits due to varied reductions in mechanical loading in rats. NPJ Microgravity 2020, 6, 15. [Google Scholar] [CrossRef]
- Tanaka, K.; Nishimura, N.; Kawai, Y. Adaptation to microgravity, deconditioning, and countermeasures. J. Physiol. Sci. 2017, 67, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Physiology of a microgravity environment invited review: Microgravity and skeletal muscle. J. Appl. Physiol. 2000, 89, 823–839. [Google Scholar] [CrossRef] [Green Version]
- Keyak, J.H.; Koyama, A.K.; LeBlanc, A.; Lu, Y.; Lang, T.F. Reduction in proximal femoral strength due to long-duration spaceflight. Bone 2009, 44, 449–453. [Google Scholar] [CrossRef]
- Bailey, J.F.; Miller, S.L.; Khieu, K.; O’Neill, C.W.; Healey, R.M.; Coughlin, D.G.; Sayson, J.V.; Chang, D.G.; Hargens, A.R.; Lotz, J.C. From the international space station to the clinic: How prolonged unloading may disrupt lumbar spine stability. Spine J. 2018, 18, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Desplanches, D. Structural and functional adaptations of skeletal muscle to weightlessness. Int. J. Sport. Med. Suppl. 1997, 18 (Suppl. 4), S259–S264. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Functional and structural adaptations of skeletal muscle to microgravity. J. Exp. Biol. 2001, 204, 3201–3208. [Google Scholar]
- Petersen, N.; Jaekel, P.; Rosenberger, A.; Weber, T.; Scott, J.; Castrucci, F.; Lambrecht, G.; Ploutz-Snyder, L.; Damann, V.; Kozlovskaya, I.; et al. Exercise in space: The European Space Agency approach to in-flight exercise countermeasures for long-duration missions on ISS. Extrem. Physiol. Med. 2016, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlovskaya, I.B.; Yarmanova, E.N.; Yegorov, A.D.; Stepantsov, V.I.; Fomina, E.V.; Tomilovaskaya, E.S.; Reeves, J.M. Russian countermeasure systems for adverse effects of microgravity on long-duration ISS flights. Aerosp. Med. Hum. Perform. 2015, 86, A24–A31. [Google Scholar] [CrossRef]
- Loehr, J.A.; Lee, S.M.C.; English, K.L.; Sibonga, J.; Smith, S.M.; Spiering, B.A.; Hagan, R.D. Musculoskeletal adaptations to training with the advanced resistive exercise device. Med. Sci. Sports Exerc. 2011, 43, 146–156. [Google Scholar] [CrossRef]
- Trappe, S.; Costill, D.; Gallagher, P.; Creer, A.; Peters, J.R.; Evans, H.; Riley, D.A.; Fitts, R.H. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. J. Appl. Physiol. 2009, 106, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, A.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Evans, H.; Spector, E.; Ploutz-Snyder, R.; et al. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos. Int. 2013, 24, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Heer, M.; Shackelford, L.C.; Sibonga, J.D.; Spatz, J.; Pietrzyk, R.A.; Hudson, E.K.; Zwart, S.R. Bone metabolism and renal stone risk during International Space Station missions. Bone 2015, 81, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, A.S.; Shirazi-Fard, Y.; Shahnazari, M.; Alwood, J.S.; Truong, T.A.; Tahimic, C.G.T.; Limoli, C.L.; Turner, N.D.; Halloran, B.; Globus, R.K. Dried plum diet protects from bone loss caused by ionizing radiation. Sci. Rep. 2016, 6, 21343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widrick, J.J.; Knuth, S.T.; Norenberg, K.M.; Romatowski, J.G.; Bain, J.L.W.; Riley, D.A.; Karhanek, M.; Trappe, S.W.; Trappe, T.A.; Costill, D.L.; et al. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J. Physiol. 1999, 516, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human pathophysiological adaptations to the space environment. Front. Physiol. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Oishi, Y.; Roy, R.R.; Edgerton, V.R. Influence of two weeks of non-weight bearing on rat soleus motoneurons and muscle fibers. Aviat. Space Environ. Med. 1997, 68, 421–425. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9143753 (accessed on 11 March 2019).
- Riley, D.A.; Slocum, G.R.; Bain, J.L.W.; Sedlak, F.R.; Sowa, T.E.; Mellender, J.W. Rat hindlimb unloading: Soleus histochemistry, ultrastructure, and electromyography. J. Appl. Physiol. 1990, 69, 58–66. [Google Scholar] [CrossRef]
- Dupont, E.; Cieniewski-Bernard, C.; Bastide, B.; Stevens, L. Electrostimulation during hindlimb unloading modulates PI3K-AKT downstream targets without preventing soleus atrophy and restores slow phenotype through ERK. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R408–R417. [Google Scholar] [CrossRef]
- Momken, I.; Stevens, L.; Bergouignan, A.; Desplanches, D.; Rudwill, F.; Chery, I.; Zahariev, A.; Zahn, S.; Stein, T.P.; Sebedio, J.L.; et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011, 25, 3646–3660. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Staron, R.S.; Gordon, S.E.; Volek, J.S.; Koziris, L.P.; Duncan, N.D.; Nindl, B.C.; Gómez, A.L.; Marx, J.O.; Fry, A.C.; et al. The effects of 10 days of spaceflight on the shuttle endeavour on predominantly fast-twitch muscles in the rat. Histochem. Cell Biol. 2000, 114, 349–355. [Google Scholar] [CrossRef]
- Armstrong, R.B.; Phelps, R.O. Muscle fiber type composition of the rat hindlimb. Am. J. Anat. 1984, 171, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, P.D.; Sjödin, B.; Karlsson, J.; Jansson, E.; Saltin, B. Human soleus muscle: A comparison of fiber composition and enzyme activities with other leg muscles. Pflügers Arch. Eur. J. Physiol. 1974, 348, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Caiozzo, V.J.; Haddad, F.; Baker, M.J.; Herrick, R.E.; Prietto, N.; Baldwin, K.M. Microgravity-induced transformations of myosin isoforms and contractile properties of skeletal muscle. J. Appl. Physiol. 1996, 81, 123–132. [Google Scholar] [CrossRef]
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, G.E.; Haddad, F.; Roy, R.R.; Zhong, H.; Edgerton, V.R.; Baldwin, K.M. Transcriptional regulation of the myosin heavy chain IIB gene in inactive rat soleus. Muscle Nerve 2009, 40, 411–419. [Google Scholar] [CrossRef]
- Giger, J.M.; Haddad, F.; Qin, A.X.; Zeng, M.; Baldwin, K.M. Effect of unloading on type I myosin heavy chain gene regulation in rat soleus muscle. J. Appl. Physiol. 2005, 98, 1185–1194. [Google Scholar] [CrossRef]
- Ulanova, A.; Gritsyna, Y.; Vikhlyantsev, I.; Salmov, N.; Bobylev, A.; Abdusalamova, Z.; Rogachevsky, V.; Shenkman, B.; Podlubnaya, Z. Isoform composition and gene expression of thick and thin filament proteins in striated muscles of mice after 30-day space flight. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Shenkman, B.S. From Slow to Fast: Hypogravity-Induced Remodeling of Muscle Fiber Myosin Phenotype. Acta Nat. 2016, 8, 47–59. [Google Scholar] [CrossRef]
- Kim, J.H.; Thompson, L.D.V. Non-weight bearing-induced muscle weakness: The role of myosin quantity and quality in MHC type II fibers. Am. J. Physiol. Cell Physiol. 2014, 307, C190–C194. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.L.; Yasui, W.; Tanaka, T.; Ohira, Y.; Nagaoka, S.; Sekiguchi, C.; Hinds, W.E.; Roy, R.R.; Edgerton, V.R. Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J. Appl. Physiol. 1996, 81, 145–151. [Google Scholar] [CrossRef]
- Riley, D.A.; Ellis, S.; Slocom, G.R.; Satyanarayana, T.; Bain, J.L.W.; Sedlak, F.R. Hypogravity-induced atrophy of rat soleus and extensor digitorum longus muscles. Muscle Nerve 1987, 10, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.E.; Bhasin, S.; Lalani, R.; Datta, A.; Gonzalez-Cadavid, N.F. Alteration of gene expression profiles in skeletal muscle of rats exposed to microgravity during a spaceflight. J. Gravit. Physiol. 2002, 9, 61–70. [Google Scholar] [PubMed]
- Han, B.; Zhu, M.J.; Ma, C.; Du, M. Rat hindlimb unloading down-regulates insulin like growth factor-1 signaling and AMP-activated protein kinase, and leads to severe atrophy of the soleus muscle. Appl. Physiol. Nutr. Metab. 2007, 32, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Booth, F.W.; Gordon, S.E. Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277, R601–R606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vico, L.; van Rietbergen, B.; Vilayphiou, N.; Linossier, M.T.; Locrelle, H.; Normand, M.; Zouch, M.; Gerbaix, M.; Bonnet, N.; Novikov, V.; et al. Cortical and Trabecular Bone Microstructure Did Not Recover at Weight-Bearing Skeletal Sites and Progressively Deteriorated at Non-Weight-Bearing Sites During the Year Following International Space Station Missions. J. Bone Miner. Res. 2017, 32, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Takeda, T.; Sato, Y. Interventions to prevent bone loss in astronauts during space flight. Keio J. Med. 2005, 54, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, S.A.J.; Travis, N.D.; Lu, T.; Bateman, T.A. Development of a low-dose anti-resorptive drug regimen reveals synergistic suppression of bone formation when coupled with disuse. J. Appl. Physiol. 2008, 104, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Amblard, D.; Lafage-Proust, M.H.; Laib, A.; Thomas, T.; Rüegsegger, P.; Alexandre, C.; Vico, L. Tail suspension induces bone loss in skeletally mature mice in the C57Bl/6J strain but not in the C3H/HeJ strain. J. Bone Miner. Res. 2003, 18, 561–569. [Google Scholar] [CrossRef]
- Jia, B.; Xie, L.; Zheng, Q.; Yang, P.F.; Zhang, W.J.; Ding, C.; Qian, A.R.; Shang, P. A hypomagnetic field aggravates bone loss induced by hindlimb unloading in rat femurs. PLoS ONE 2014, 9, e105604. [Google Scholar] [CrossRef]
- Sakata, T.; Wang, Y.; Halloran, B.P.; Elalieh, H.Z.; Cao, J.; Bikle, D.D. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J. Bone Miner. Res. 2004, 19, 436–446. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Shi, W.G.; Li, H.; Hua, J.R.; Feng, X.; Wei, W.J.; Wang, J.F.; He, J.P.; Lei, S.W. Bone Loss Induced by Simulated Microgravity, Ionizing Radiation and/or Ultradian Rhythms in the Hindlimbs of Rats. Biomed. Environ. Sci. 2018, 31, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Delp, M.D.; Charvat, J.M.; Limoli, C.L.; Globus, R.K.; Ghosh, P. Apollo lunar astronauts show higher cardiovascular disease mortality: Possible deep space radiation effects on the vascular endothelium. Sci. Rep. 2016, 6, 1001–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckey, J.C.; Lane, L.D.; Levine, B.D.; Watenpaugh, D.E.; Wright, S.J.; Moore, W.E.; Gaffney, F.A.; Blomqvist, C.G. Orthostatic intolerance after spaceflight. J. Appl. Physiol. 1996, 81, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Meck, J.V.; Reyes, C.J.; Perez, S.A.; Goldberger, A.L.; Ziegler, M.G. Marked exacerbation of orthostatic intolerance after long-vs.-short-duration spaceflight in veteran astronauts. Psychosom. Med. 2001, 63, 865–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.C.; Feiveson, A.H.; Stein, S.; Stenger, M.B.; Platts, S.H.; Reeves, J.M. Orthostatic intolerance after iss and space shuttle missions. Aerosp. Med. Hum. Perform. 2015, 86, A54–A67. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.J.; South, D.A.; Meck, J.V. Fludrocortisone Does Not Prevent Orthostatic Hypotension in Astronauts after Spaceflight. Aviat. Space Environ. Med. 2004, 75, 235–239. Available online: https://pubmed.ncbi.nlm.nih.gov/15018291/ (accessed on 8 August 2020).
- Stabley, J.N.; Prisby, R.D.; Behnke, B.J.; Delp, M.D. Chronic skeletal unloading of the rat femur: Mechanisms and functional consequences of vascular remodeling. Bone 2013, 57, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Andreev-Andrievskiy, A.A.; Popova, A.S.; Lagereva, E.A.; Vinogradova, O.L. Fluid shift versus body size: Changes of hematological parameters and body fluid volume in hindlimb-unloaded mice, rats and rabbits. J. Exp. Biol. 2018, 221, jeb182832. [Google Scholar] [CrossRef] [Green Version]
- Tarasova, O.; Borovik, A.; Tsvirkoun, D.; Lebedev, V.; Steeves, J.; Krassioukov, A. Orthostatic response in rats after hindlimb unloading: Effect of transcranial electrical stimulation. Aviat. Space Environ. Med. 2007, 78, 1023–1028. [Google Scholar] [CrossRef]
- Just, T.P.; Jendzjowsky, N.G.; Delorey, D.S. Hindlimb unweighting does not alter vasoconstrictor responsiveness and nitric oxide-mediated inhibition of sympathetic vasoconstriction. J. Physiol. 2015, 593, 2213–2224. [Google Scholar] [CrossRef] [Green Version]
- Mueller, P.J.; Foley, C.M.; Hasser, E.M. Hindlimb unloading alters nitric oxide and autonomic control of resting arterial pressure in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prisby, R.D.; Alwood, J.S.; Behnke, B.J.; Stabley, J.N.; Mccullough, D.J.; Ghosh, P.; Globus, R.K.; Delp, M.D. Effects of hindlimb unloading and ionizing radiation on skeletal muscle resistance artery vasodilation and its relation to cancellous bone in mice. J. Appl. Physiol. 2016, 120, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavei, G.; Biancardi, C.M.; Minetti, A.E.; Minetti, A.E. Chronic hindlimb suspension unloading markedly decreases turnover rates of skeletal and cardiac muscle proteins and adipose tissue triglycerides. J. Appl. Physiol. 2015, 119, 16–26. [Google Scholar] [CrossRef]
- Moffitt, J.A.; Heesch, C.M.; Hasser, E.M. Increased GABAA inhibition of the RVLM after hindlimb unloading in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Basso, N.; Jia, Y.; Bellows, C.G.; Heersche, J.N.M. The effect of reloading on bone volume, osteoblast number, and osteoprogenitor characteristics: Studies in hind limb unloaded rats. Bone 2005, 37, 370–378. [Google Scholar] [CrossRef]
- Kasper, C.E. Sarcolemmal disruption in reloaded atrophic skeletal muscle. J. Appl. Physiol. 1995, 79, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Mutin-Carnino, M.; Carnino, A.; Roffino, S.; Chopard, A. Effect of muscle unloading, reloading and exercise on inflammation during a head-down bed rest. Int. J. Sports Med. 2014, 35, 28–34. [Google Scholar] [CrossRef]
- Frenette, J.; St-Pierre, M.; Côté, C.H.; Mylona, E.; Pizza, F.X. Muscle impairment occurs rapidly and precedes inflammatory cell accumulation after mechanical loading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R351–R357. [Google Scholar] [CrossRef]
- Haddad, F.; Adams, G.R.; Bodell, P.W.; Baldwin, K.M. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J. Appl. Physiol. 2006, 100, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Mochalova, E.P.; Belova, S.P.; Mirzoev, T.M.; Shenkman, B.S.; Nemirovskaya, T.L. Atrogin-1/MAFbx mRNA expression is regulated by histone deacetylase 1 in rat soleus muscle under hindlimb unloading. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa-Caldwell, M.E.; Brown, J.L.; Perry, R.A.; Shimkus, K.L.; Shirazi-Fard, Y.; Brown, L.A.; Hogan, H.A.; Fluckey, J.D.; Washington, T.A.; Wiggs, M.P.; et al. Regulation of Mitochondrial Quality Following Repeated Bouts of Hindlimb Unloading. Appl. Physiol. Nutr. Metab. 2019, 45, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex Differ. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ploutz-Snyder, L.; Bloomfield, S.; Smith, S.M.; Hunter, S.K.; Templeton, K.; Bemben, D. Effects of sex and gender on adaptation to space: Musculoskeletal health. J. Womens Health 2014, 23, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Shufelt, C.L.; Pacheco, C.; Tweet, M.S.; Miller, V.M. Sex-specific physiology and cardiovascular disease. Adv. Exp. Med. Biol. 2018, 1065, 433–454. [Google Scholar] [CrossRef]
- Platts, S.H.; Bairey Merz, C.N.; Barr, Y.; Fu, Q.; Gulati, M.; Hughson, R.; Levine, B.D.; Mehran, R.; Stachenfeld, N.; Wenger, N.K. Effects of sex and gender on adaptation to space: Cardiovascular alterations. J. Womens Health 2014, 23, 950–955. [Google Scholar] [CrossRef] [Green Version]
- Semple, C.; Riveros, D.; Nagy, J.A.; Rutkove, S.B.; Mortreux, M. Partial Weight-Bearing in Female Rats: Proof of Concept in a Martian-Gravity Analog. Front. Physiol. 2020, 11, 302. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.L.; von Bergen, V.; Chyu, M.C.; Jenkins, M.R.; Mo, H.; Chen, C.H.; Kwun, I.S. Fruits and dietary phytochemicals in bone protection. Nutr. Res. 2012, 32, 897–910. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Sulera, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef]
- Tran, H.T.; Liong, S.; Lim, R.; Barker, G.; Lappas, M. Resveratrol ameliorates the chemical and microbial induction of inflammation and insulin resistance in human placenta, adipose tissue and skeletal muscle. PLoS ONE 2017, 12, e173373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, L.; Zhao, X.; Chen, X.; Wang, L.; Geng, Z. Effect of dietary resveratrol supplementation on meat quality, muscle antioxidative capacity and mitochondrial biogenesis of broilers. J. Sci. Food Agric. 2018, 98, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Yonamine, C.Y.; Pinheiro-Machado, E.; Michalani, M.L.; Alves-Wagner, A.B.; Esteves, J.V.; Freitas, H.S.; Machado, U.F. Resveratrol Improves Glycemic Control in Type 2 Diabetic Obese Mice by Regulating Glucose Transporter Expression in Skeletal Muscle and Liver. Molecules 2017, 22, 1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durbin, S.M.; Jackson, J.R.; Ryan, M.J.; Gigliotti, J.C.; Alway, S.E.; Tou, J.C. Resveratrol supplementation preserves long bone mass, microstructure, and strength in hindlimb-suspended old male rats. J. Bone Miner. Metab. 2014, 32, 38–47. [Google Scholar] [CrossRef]
- Habold, C.; Momken, I.; Ouadi, A.; Bekaert, V.; Brasse, D. Effect of prior treatment with resveratrol on density and structure of rat long bones under tail-suspension. J. Bone Miner. Metab. 2011, 29, 15–22. [Google Scholar] [CrossRef]
- Bennett, B.T.; Mohamed, J.S.; Alway, S.E. Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLoS ONE 2013, 8, e83518. [Google Scholar] [CrossRef] [Green Version]
- Bajotto, G.; Sato, Y.; Kitaura, Y.; Shimomura, Y. Effect of branched-chain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles. Eur. J. Appl. Physiol. 2011, 111, 1815–1828. [Google Scholar] [CrossRef]
- Stein, T.P.; Blanc, S. Does protein supplementation prevent muscle disuse atrophy and loss of strength? Crit. Rev. Food Sci. Nutr. 2011, 51, 828–834. [Google Scholar] [CrossRef]
- Powers, S.K. Can Antioxidants Protect Against Disuse Muscle Atrophy? Sports Med. 2014, 44 (Suppl. 2), 155–165. [Google Scholar] [CrossRef] [Green Version]
- Ferrando, B.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Puchades, C.; Derbré, F.; Gratas-Delamarche, A.; Laparre, L.; Olaso-Gonzalez, G.; Cerda, M.; Viosca, E.; et al. Allopurinol partially prevents disuse muscle atrophy in mice and humans. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Naito, H.; Powers, S.K.; Demirel, H.A.; Sugiura, T.; Dodd, S.L.; Aoki, J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J. Appl. Physiol. 2000, 88, 359–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, K.; Honda, M.; Kobayashi, T.; Uehara, K.; Kojima, A.; Akema, T.; Sugiura, T.; Yamada, S.; Ohira, Y.; Yoshioka, T. Heat stress facilitates the recovery of atrophied soleus muscle in rat. Jpn. J. Physiol. 2004, 54, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukai, R.; Horikawa, H.; Lin, P.Y.; Tsukumo, N.; Nikawa, T.; Kawamura, T.; Nemoto, H.; Terao, J. 8-Prenylnaringenin promotes recovery from immobilization-induced disuse muscle atrophy through activation of the Akt phosphorylation pathway in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1022–R1031. [Google Scholar] [CrossRef] [Green Version]
- Mukai, R.; Horikawa, H.; Fujikura, Y.; Kawamura, T.; Nemoto, H.; Nikawa, T.; Terao, J. Prevention of Disuse Muscle Atrophy by Dietary Ingestion of 8-Prenylnaringenin in Denervated Mice. PLoS ONE 2012, 7, e45048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Naeyer, H.; Lamon, S.; Russell, A.P.; Everaert, I.; De Spaey, A.; Jamart, C.; Vanheel, B.; Taes, Y.; Derave, W. Effects of tail suspension on serum testosterone and molecular targets regulating muscle mass. Muscle Nerve 2015, 52, 278–288. [Google Scholar] [CrossRef]
- Harjola, Jänkälä, and Härkönen, Myosin heavy chain mRNA and protein distribution in immobilized rat skeletal muscle are not affected by testosterone status. Acta Physiol. Scand. 2000, 169, 277–282. [CrossRef]
- Patton, D.F.; Mistlberger, R.E. Circadian adaptations to meal timing: Neuroendocrine mechanisms. Front. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef] [Green Version]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [Green Version]
- Mortreux, M.; Foppen, E.; Denis, R.G.; Montaner, M.; Kassis, N.; Denom, J.; Vincent, M.; Fumeron, F.; Kujawski-Lafourcade, M.; Andréelli, F.; et al. New roles for prokineticin 2 in feeding behavior, insulin resistance and type 2 diabetes: Studies in mice and humans. Mol. Metab. 2019, 29, 182–196. [Google Scholar] [CrossRef]
- Fink, A.M. Measuring the effects of night-shift work on cardiac autonomic modulation: An appraisal of heart rate variability metrics. Int. J. Occup. Med. Environ. Health 2020, 33, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Fonseca, D.A. Cardiovascular diseases: A therapeutic perspective around the clock. Drug Discov. Today 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Orihara, K.; Haraguchi, A.; Shibata, S. Crosstalk among circadian rhythm, obesity and allergy. Int. J. Mol. Sci. 2020, 21, 1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawley, J.A.; Sassone-Corsi, P.; Zierath, J.R. Chrono-nutrition for the prevention and treatment of obesity and type 2 diabetes: From mice to men. Diabetologia 2020, 63, 2253–2259. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Yao, K.; Su, W.; Tan, B.; Wu, X.; Huang, X.; Li, T.; Yin, Y.; Tosini, G.; et al. Circadian Rhythms and Obesity: Timekeeping Governs Lipid Metabolism. J. Pineal Res. 2020. [Google Scholar] [CrossRef]
- Sciarra, F.; Franceschini, E.; Campolo, F.; Gianfrilli, D.; Pallotti, F.; Paoli, D.; Isidori, A.M.; Venneri, M.A. Disruption of circadian rhythms: A crucial factor in the etiology of infertility. Int. J. Mol. Sci. 2020, 21, 3943. [Google Scholar] [CrossRef]
- Brainard, G.C.; Barger, L.K.; Soler, R.R.; Hanifin, J.P. The development of lighting countermeasures for sleep disruption and circadian misalignment during spaceflight. Curr. Opin. Pulm. Med. 2016, 22, 535–544. [Google Scholar] [CrossRef]
- Monk, T.H.; Buysse, D.J.; Billy, B.D.; Kennedy, K.S.; Willrich, L.M. Sleep and Circadian Rhythms in Four Orbiting Astronauts. J. Biol. Rhythm. 1998, 13, 188–201. [Google Scholar] [CrossRef]
- Dijk, D.J.; Neri, D.F.; Wyatt, J.K.; Ronda, J.M.; Riel, E.; Cecco, A.R.D.; Hughes, R.J.; Elliott, A.R.; Prisk, G.K.; West, J.B.; et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, 1647–1664. [Google Scholar] [CrossRef] [Green Version]
- Gundel, A.; Polyakov, V.V.; Zulley, J. The alteration of human sleep and circadian rhythms during spaceflight. J. Sleep Res. 1997, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.-H.; Qu, W.-M.; Chen, S.-G.; Chen, X.-P.; Lv, K.; Huang, Z.-L.; Wu, Y.-L. Keeping the right time in space: Importance of circadian clock and sleep for physiology and performance of astronauts. Mil. Med. Res. 2014, 1, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, N.; Otsuka, K.; Kubo, Y.; Hayashi, M.; Mizuno, K.; Ohshima, H.; Mukai, C. Effects of long-term microgravity exposure in space on circadian rhythms of heart rate variability. Chronobiol. Int. 2015, 32, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wan, Y.; Zhang, L.; Tian, Y.; Lv, K.; Li, Y.; Wang, C.; Chen, X.; Chen, S.; Guo, J. Alterations in the heart rate and activity rhythms of three orbital astronauts on a space mission. Life Sci. Sp. Res. 2015, 4, 62–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karemaker, J.M.; Berecki-Gisolf, J. 24-h blood pressure in space: The dark side of being an astronaut. Respir. Physiol. Neurobiol. 2009, 169. [Google Scholar] [CrossRef]
- Verheyden, B.; Beckers, F.; Couckuyt, K.; Liu, J.; Aubert, A.E. Respiratory modulation of cardiovascular rhythms before and after short-duration human spaceflight. Acta Physiol. 2007, 191, 297–308. [Google Scholar] [CrossRef]
- Centini, C.; Pompeiano, O. Sleep research in space: Expression of immediate early genes in forebrain structures of rats during the NASA Neurolab Mission (STS-90). Arch. Ital. Biol. 2007, 145, 117–150. [Google Scholar] [CrossRef]
- Agarwal, R. Regulation of circadian blood pressure: From mice to astronauts. Curr. Opin. Nephrol. Hypertens. 2010, 19, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, B.; Yang, L.; Bai, Y.G.; Song, J.B.; Ge, Y.L.; Ma, H.Z.; Cheng, J.H.; Ma, J.; Xie, M.J. BMAL1 disrupted intrinsic diurnal oscillation in rat cerebrovascular contractility of simulated microgravity rats by altering circadian regulation of mir-103/cav1.2 signal pathway. Int. J. Mol. Sci. 2019, 20, 3947. [Google Scholar] [CrossRef] [Green Version]
- Basner, M.; Dinges, D.F.; Mollicone, D.; Ecker, A.; Jones, C.W.; Hyder, E.C.; Di Antonio, A.; Savelev, I.; Kan, K.; Goel, N.; et al. Mars 520-d mission simulation reveals protracted crew hypokinesis and alterations of sleep duration and timing. Proc. Natl. Acad. Sci. USA 2013, 110, 2635–2640. [Google Scholar] [CrossRef] [Green Version]
- Goel, N.; Bale, T.L.; Epperson, C.N.; Kornstein, S.G.; Leon, G.R.; Palinkas, L.A.; Stuster, J.W.; Dinges, D.F. Effects of sex and gender on adaptation to space: Behavioral health. J. Womens Health 2014, 23, 975–986. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Groh, M. Studying the Van Allen Belts 60 Years after America’s First Spacecraft. NASA.gov; 31 January 2018. Available online: http://www.nasa.gov/feature/goddard/2018/studying-the-van-allen-belts-60-years-after-america-s-first-spacecraft (accessed on 12 August 2020).
- Yu, K.; Doherty, A.H.; Genik, P.C.; Gookin, S.E.; Roteliuk, D.M.; Wojda, S.J.; Jiang, Z.S.; McGee-Lawrence, M.E.; Weil, M.M.; Donahue, S.W. Mimicking the effects of spaceflight on bone: Combined effects of disuse and chronic low-dose rate radiation exposure on bone mass in mice. Life Sci. Space Res. 2017, 15, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; Hargens, A. Skeletal changes during and after spaceflight. Nat. Rev. Rheumatol. 2018, 14, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.D.; Lang, T.F.; Bloomfield, S.A.; Bloomberg, J.J.; Judex, S.; Keyak, J.H.; Midura, R.J.; Pajevic, P.D.; Spatz, J.M. Effects of long-duration spaceflight, microgravity and radiation on the neuromuscular, sensorimotor and skeletal systems. J. Cosmol. 2010, 12, 3778–3780. [Google Scholar]
- Bellone, J.A.; Gifford, P.S.; Nishiyama, N.C.; Hartman, R.E.; Mao, X.W. Long-term effects of simulated microgravity and/or chronic exposure to low-dose gamma radiation on behavior and blood–brain barrier integrity. NPJ Microgravity 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanzari, J.K.; Romero-Weaver, A.L.; James, G.; Krigsfeld, G.; Lin, L.; Diffenderfer, E.S.; Kennedy, A.R. Leukocyte Activity Is Altered in a Ground Based Murine Model of Microgravity and Proton Radiation Exposure. PLoS ONE 2013, 8, e71757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Frishman, W.H. Effects of Spaceflight on Cardiovascular Physiology and Health. Cardiol. Rev. 2019, 27, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Malkani, S.; Cekanaviciute, E.; Mortreux, M.; Okunola, H.; Tarbier, M.; Schreurs, A.; Shirazi-fard, Y.; Tahimic, C.G.T.; Cheng-, M.; Costes, S.V.; et al. Circulating miRNA Signature Predicts and Rescues Spaceflight Associated Health Risks. Cell 2020. [Google Scholar] [CrossRef]
- Beheshti, A.; Shirazi-Fard, Y.; Choi, S.; Berrios, D.; Gebre, S.G.; Galazka, J.M.; Costes, S.V. Exploring the effects of spaceflight on mouse physiology using the open access NASA genelab platform. J. Vis. Exp. 2019, 2019. [Google Scholar] [CrossRef]
- Rzeszutek, I.; Singh, A. Small RNAs, Big Diseases. Int. J. Mol. Sci. 2020, 21, 5699. [Google Scholar] [CrossRef]
- Beheshti, A.; Vanderburg, C.; McDonald, J.T.; Ramkumar, C.; Kadungure, T.; Zhang, H.; Gartenhaus, R.B.; Evens, A.M. A circulating microRNA signature predicts age-based development of lymphoma. PLoS ONE 2017, 12, e170521. [Google Scholar] [CrossRef]
- Almog, N.; Ma, L.; Schwager, C.; Brinkmann, B.G.; Beheshti, A.; Vajkoczy, P.; Folkman, J.; Hlatky, L.; Abdollahi, A. Consensus Micro RNAs Governing the Switch of Dormant Tumors to the Fast-Growing Angiogenic Phenotype. PLoS ONE 2012, 7, e44001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almog, N.; Briggs, C.; Beheshti, A.; Ma, L.; Wilkie, K.P.; Rietman, E.; Hlatky, L. Transcriptional changes induced by the tumor dormancy-associated microRNA-190. Transcription 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelghaffar, S.; Shora, H.; Abdelatty, S.; Elmougy, F.; El Sayed, R.; Abdelrahman, H.; Soliman, H.; Algebaly, H.; Ahmed, S.; Alfy, P.; et al. Micrornas and risk factors for diabetic nephropathy in egyptian children and adolescents with type 1 diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Eghtedarian, R.; Dinger, M.E.; Ghafouri-Fard, S. Emerging roles of non-coding RNAs in the pathogenesis of type 1 diabetes mellitus. Biomed. Pharmacother. 2020, 129. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Kiaie, N.; Sathyapalan, T.; Al-Rasadi, K.; Jamialahmadi, T.; Sahebkar, A. The Role of MicroRNAs in Regulating Cytokines and Growth Factors in Coronary Artery Disease: The Ins and Outs. J. Immunol. Res. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Mishra, P.K.; Tandon, R.; Byrareddy, S.N. Diabetes and COVID-19 risk: A miRNA perspective. Am. J. Physiol. Circ. Physiol. 2020, 2020, ajpheart.00489. [Google Scholar] [CrossRef]
- Beheshti, A.; Ray, S.; Fogle, H.; Berrios, D.; Costes, S.V. A microRNA signature and TGF-β1 response were identified as the key master regulators for spaceflight response. PLoS ONE 2018, 13, e199621. [Google Scholar] [CrossRef]
- Wang, L.L.; Spieker, A.J.; Li, J.; Rutkove, S.B. Electrical impedance myography for monitoring motor neuron loss in the SOD1 G93A amyotrophic lateral sclerosis rat. Clin. Neurophysiol. 2011, 122, 2505–2511. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Spieker, A.J.; Rosen, G.D.; Rutkove, S.B. Electrical impedance alterations in the rat hind limb with unloading. J. Musculoskelet. Neuronal Interact. 2019, 13, 37–44. [Google Scholar]
- Nagy, J.A.; Kapur, K.; Taylor, R.S.; Sanchez, B.; Rutkove, S.B. Electrical impedance myography as a biomarker of myostatin inhibition with ActRIIB-mFc: A study in wild-type mice. Futur. Sci. OA 2018, 4, FSO308. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Staats, W.L.; Spieker, A.; Sung, M.; Rutkove, S.B. A Technique for Performing Electrical Impedance Myography in the Mouse Hind Limb: Data in Normal and ALS SOD1 G93A Animals. PLoS ONE 2012, 7, e45004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shefner, J.M.; Rutkove, S.B.; Caress, J.B.; Benatar, M.; David, W.S.; Cartwright, M.S.; Macklin, E.A.; Bohorquez, J.L. Reducing sample size requirements for future ALS clinical trials with a dedicated electrical impedance myography system. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Rutkove, S.B. Present Uses, Future Applications, and Technical Underpinnings of Electrical Impedance Myography. Curr. Neurol. Neurosci. Rep. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.K.; Skinner, J.; Martucci, M.; Rutkove, S.B.; Halter, R.J. Toward Electrical Impedance Tomography Coupled Ultrasound Imaging for Assessing Muscle Health. IEEE Trans. Med. Imaging 2019, 38, 1409–1419. [Google Scholar] [CrossRef]
- Roy, B.; Darras, B.T.; Zaidman, C.M.; Wu, J.S.; Kapur, K.; Rutkove, S.B. Exploring the relationship between electrical impedance myography and quantitative ultrasound parameters in Duchenne muscular dystrophy. Clin. Neurophysiol. 2019, 130, 515–520. [Google Scholar] [CrossRef]
- Rutkove, S.B.; Shefner, J.M.; Gregas, M.; Butler, H.; Caracciolo, J.; Lin, C.; Fogerson, P.M.; Mongiovi, P.; Darras, B.T. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle Nerve 2010, 42, 915–921. [Google Scholar] [CrossRef]
- Semple, C.; Riveros, D.; Sung, D.-M.; Nagy, J.A.; Rutkove, S.B.; Mortreux, M. Using electrical impedance myography as a biomarker of muscle deconditioning in rats exposed to micro- and partial-gravity analogs. Front. Physiol. 2020, 11, 1181. [Google Scholar] [CrossRef]

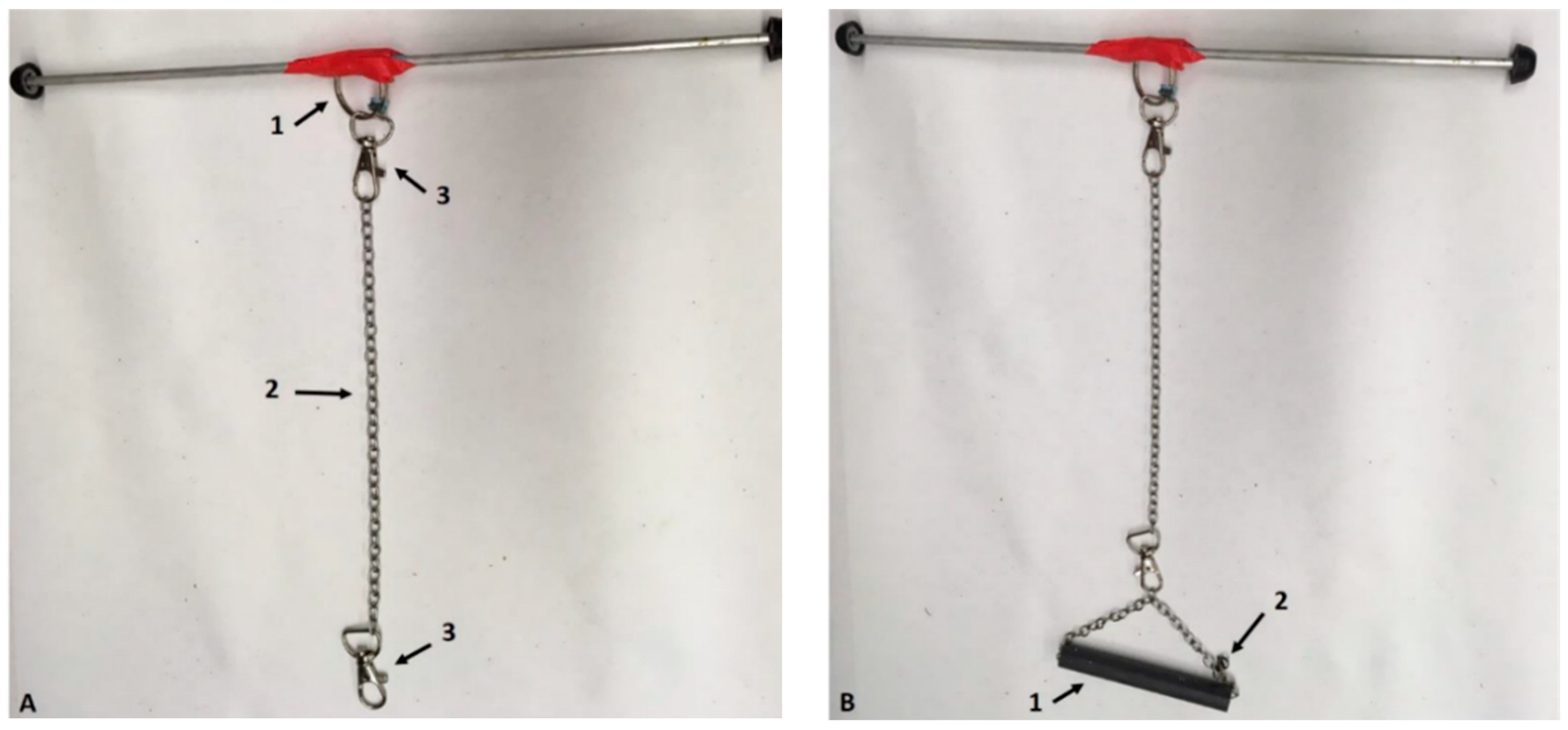


| Visual Assessments (Hands-Off) | Clinical Assessments (Hand-On) |
|---|---|
| Integrity of the harness, jacket, suspension apparatus | Body weight, unloaded weight, achieved PWB |
| Posture | Porphyric staining |
| Alertness and ability to walk | Fur coat appearance around jacket and harness |
| Food intake | Presence of skin irritation, redness or abrasion |
| Water intake | Efficient grooming of the genital area (evidence of sperm plugs, vaginal secretions) |
| Presence and consistence of feces | Appearance of the teeth |
| Evidence of grooming | Edema |
| Use of all limbs to balance | Evidence of broken nails/bleeding |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortreux, M.; Rosa-Caldwell, M.E. Approaching Gravity as a Continuum Using the Rat Partial Weight-Bearing Model. Life 2020, 10, 235. https://doi.org/10.3390/life10100235
Mortreux M, Rosa-Caldwell ME. Approaching Gravity as a Continuum Using the Rat Partial Weight-Bearing Model. Life. 2020; 10(10):235. https://doi.org/10.3390/life10100235
Chicago/Turabian StyleMortreux, Marie, and Megan E. Rosa-Caldwell. 2020. "Approaching Gravity as a Continuum Using the Rat Partial Weight-Bearing Model" Life 10, no. 10: 235. https://doi.org/10.3390/life10100235





