Methylene Blue Adsorption Study on Microcline Particles in the Function of Particle Size Range and Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Materials
2.2. X-ray Diffraction
2.3. Determination of Particle Size Distributions in Aqueous Suspension
2.4. Methylene Blue Adsorption by Batch Technique
2.5. Effect of Adsorbent Dose on Adsorption
2.6. Analysis of Methylene Blue
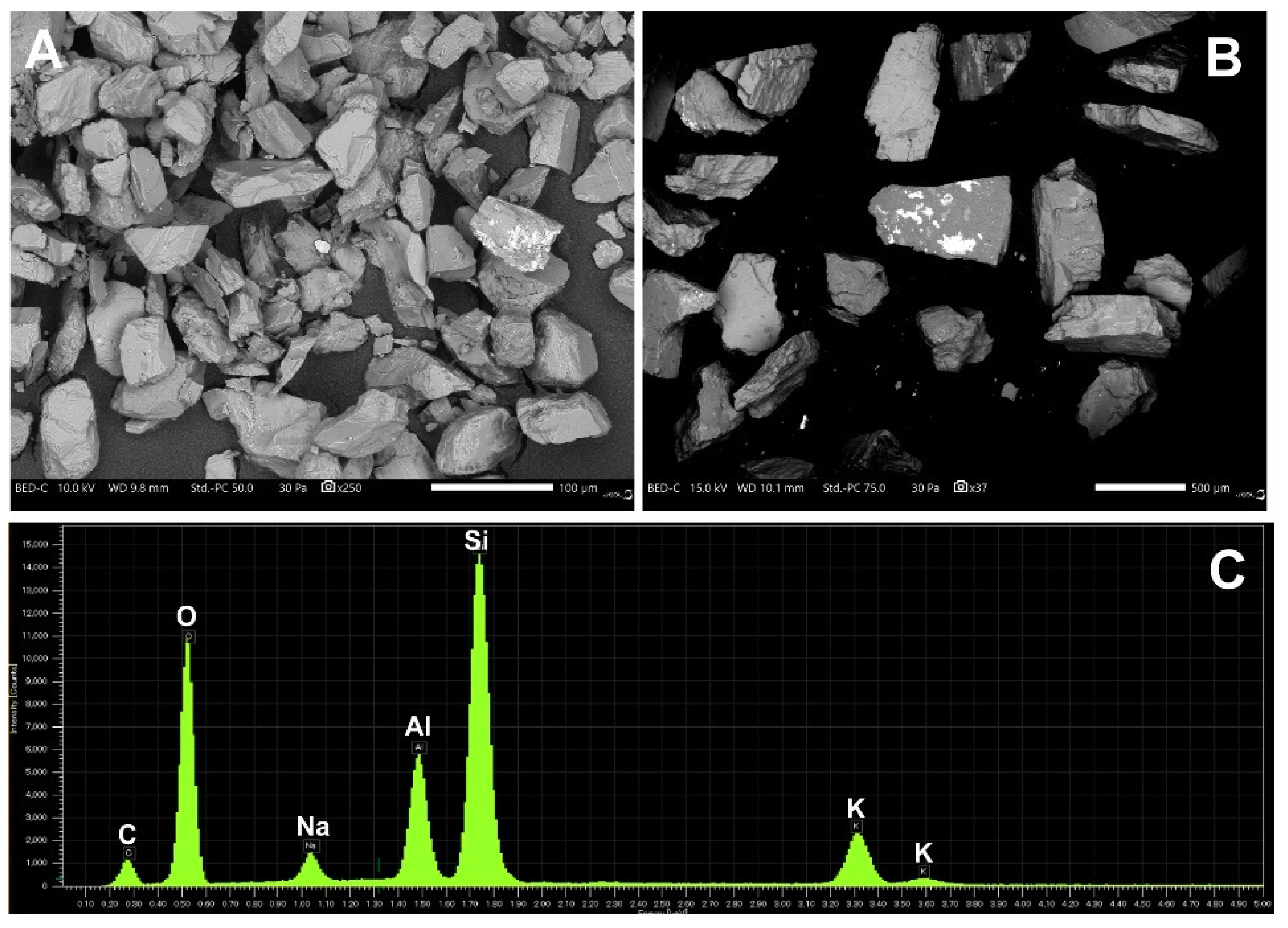
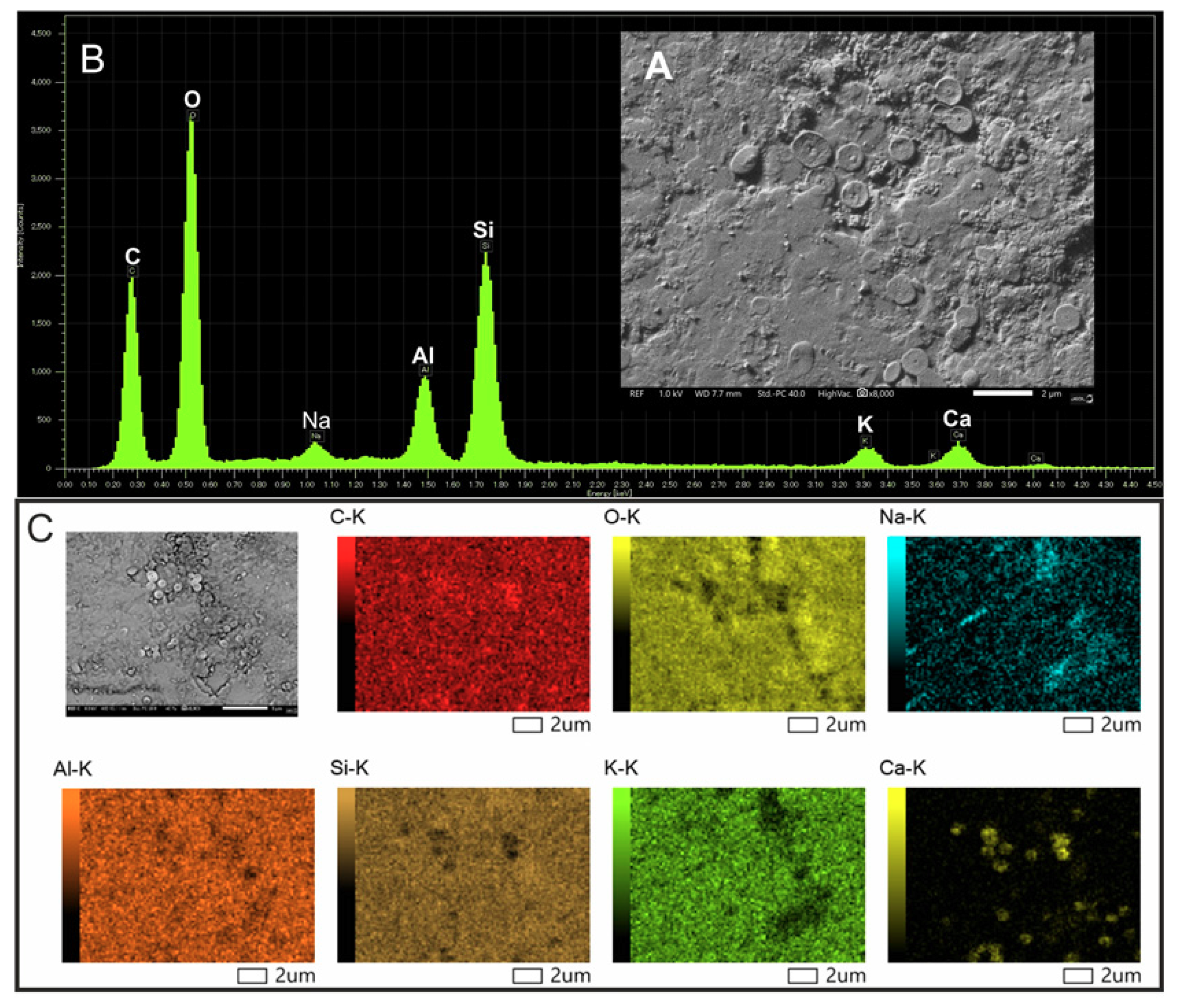
2.7. Scanning Electron Microscopy and Elemental Analysis
3. Results
3.1. X-ray Diffraction of Microcline Particles
3.2. The Particle Size Distribution of Microcline Particles in Aqueous Suspensions
3.3. Optimum Adsorbate/Adsorbent Dose in Methylene Blue Adsorption on Microcline Particles
3.4. Methylene Blue Adsorption Isotherms on Microcline Particles at Room and Elevated Temperatures
3.5. Structural and Elemental Analysis of Native and Methylene Blue Treated Microcline Particles by SEM-EDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brasswell, E. Evidence for trimerization in aqueous solutions of methylene blue. J. Phys. Chem. 1968, 72, 2477–2483. [Google Scholar] [CrossRef]
- Cenens, J.; Schoonheydt, R.A. Visible spectroscopy of methylene blue on hectorite, laponite B, and barasym in aqueous suspension. Clays Clay Miner. 1988, 36, 214–224. [Google Scholar] [CrossRef]
- Rápó, E.; Szép, R.; Keresztesi, Á.; Suciu, M.; Tonk, S. Adsorptive Removal of Cationic and Anionic Dyes from Aqueous Solutions by Using Eggshell Household Waste as Biosorbent. Acta Chim. Slov. 2018, 65, 709–717. [Google Scholar] [CrossRef]
- Ovchinnikov, O.V.; Evtukhova, A.V.; Kondratenko, T.S.; Smirnov, M.S.; Khokhlov, V.Y.; Erina, O.V. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Bergmann, K.; O’Konski, C.T. A Spectroscopic study of Methylene Blue Monomer, Dimer, and Complexes with Montmorillonite. J. Phys. Chem. 1963, 67, 2169–2177. [Google Scholar] [CrossRef]
- Spencer, W.; Sutter, J.R. Kinetics study of the monomer-dimer equilibrium of methylene blue in aqueous solution. J. Phys. Chem. 1979, 83, 1573–1576. [Google Scholar] [CrossRef]
- Mukerjee, P.; Ghosh, A.K. “Isoextraction” method and the study of the self-association of methylene blue in aqueous solutions. J. Am. Chem. Soc. 1970, 92, 6403–6407. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Mukerjee, P. Multiple association equilibria in the self-association of methylene blue and other dyes. J. Am. Chem. Soc. 1970, 92, 6408–6412. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Mukerjee, P. Ionic strength effects on the activity coefficient of methylene blue and its self-association. Study of the self-association of methylene blue from protonation equilibriums. J. Am. Chem. Soc. 1970, 92, 6413–6415. [Google Scholar] [CrossRef]
- Ghosh, A.K. Study of the self-association of methylene blue from protonation equilibriums. J. Am. Chem. Soc. 1970, 92, 6415–6418. [Google Scholar] [CrossRef]
- Mukerjee, P.; Ghosh, A.K. Thermodynamic aspects of the self-association and hydrophobic bonding of methylene blue. Model system for stacking interactions. J. Am. Chem. Soc. 1970, 92, 6419–6424. [Google Scholar] [CrossRef]
- Allingham, M.M.; Cullen, J.M.; Giles, C.H.; Jain, S.K.; Woods, J.S. Adsorption at inorganic surfaces. II. Adsorption of dyes and related compounds by silica. J. Appl. Chem. 1958, 8, 108–116. [Google Scholar] [CrossRef]
- Bujdák, J.; Komadel, P. Interaction of methylene blue with reduced charge montmorillonite. J. Phys. Chem. 1997, 101, 9065–9068. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O.; Uysal, R. Electrokinetic and rheological behaviors of sepiolite suspensions in the presence of poly(acrylic acid sodium salt)s, polyacrylamides, and poly(ethylene glycol)s of different molecular weights. J. Appl. Polymer Sci. 2008, 109, 1850–1860. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O. Effects of Electrolytes on the Electrokinetic Properties of Pumice Suspensions. J. Dispers. Sci. Technol. 2009, 30, 548–555. [Google Scholar] [CrossRef]
- Awala, H.A.; El Jamal, M.M. Equilibrium and kinetics study of adsorption of some dyes onto feldspar. J. Univ. Chem. Tech. Metall. 2011, 46, 45–52. [Google Scholar]
- Murakami, K. Thermodynamic and kinetic aspects of self-association of dyes in aqueous solution. Dye. Pigment. 2002, 53, 31–43. [Google Scholar] [CrossRef]
- Chirkst, D.E.; Krasotkin, I.S.; Cheremisina, O.V.; Streletskaya, M.I.; Ivanov, M.V. Determination of the Surface Area of Minerals by Sorption of Methylene Blue and Thermal Desorption of Argon. Russ. J. Appl. Chem. 2003, 76, 663–665. [Google Scholar] [CrossRef]
- Ayranci, E.; Duman, O. In-Situ UV-Visible Spectroscopic Study on the Adsorption of some Dyes onto Activated Carbon Cloth. Sep. Sci. Technol. 2009, 44, 3735–3752. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G. Adsorptive removal of triarylmethane dye (Basic Red 9) from aqueous solution by sepiolite as effective and low-cost adsorbent. Micropor. Mesopor. Mat. 2015, 210, 176–184. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G. Determination of adsorptive properties of expanded vermiculite for the removal of C. I. Basic Red 9 from aqueous solution: Kinetic, isotherm and thermodynamic studies. Appl. Clay Sci. 2015, 109–110, 22–32. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoğlan, B.K. Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoğlan, B.K. Removal of triphenilmethane and reactive azo dyes from aqueous solution by nanotube-κ-carrageenan-Fe3O4 nanocomposite. J. Alloys Compd. 2016, 687, 370–383. [Google Scholar] [CrossRef]
- Király, E. Magmatic Evolution of the Mórágy Granite (SE Transdanubia, Hungary). Ann. Rep. Geol. Inst. Hung. 2009, 41–55. [Google Scholar]
- Yazdani, M.; Mahmoodi, N.M.; Arami, M.; Bahrami, H. Isotherm, kinetic and thermodynamic of cationic dye removal from binary system by feldspar. Sep. Sci. Technol. 2012, 47, 1660–1672. [Google Scholar] [CrossRef]
- Friedman, G.M.; Johnson, K.G. Exercises in Sedimentology; Wiley: New York, NY, USA, 1982; pp. 1–222. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; Pearson Education: Harlow, UK, 1992; pp. 1–696. [Google Scholar]
- Tsai, W.-T.; Hsu, H.C.; Su, T.Y.; Lin, K.Y.; Lin, C.M.; Dai, T.H. The adsorption of cationic dye from aqueous solution onto acid-activated andesite. J. Hazard. Mater. 2007, 147, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Karagozoglu, B.; Tasdemir, M.; Demirbas, E.; Kobya, M. The adsorption of basic dye (Astrazon Blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell activated carbon: Kinetic and equilibrium studies. J. Hazard. Mater. 2007, 147, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Mittal, A.; Krishnan, L.; Mittal, J. Adsorption treatment and recovery of the hazardous dye, Brilliant Blue FCF, over bottom ash and de-oiled soya. J. Colloid Interface Sci. 2006, 293, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.A.P.; Zanela, T.M.P.; Machado, C.; Flores, J.A.A.; Scheibe, L.F.; Hankins, N.P.; Debacher, N.A. Removal of methylene blue by adsorption on aluminosilicate waste: Equilibrium, kinetic and thermodynamic parameters. Water Sci. Technol. 2016, 74, 2437–2445. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Dorkó, Z.; Szakolczai, A.; Tóth, B.; Horvai, G. Relationship between Commonly Used Adsorption Isotherm Equations Impedes Isotherm Selection. Period. Polytech. Chem. Eng. 2017, 61, 10–14. [Google Scholar] [CrossRef]
- Harrison, F.; Katti, S. Hazards of linearization of Langmuir’s model. Chemom. Intell. Lab. Syst. 1990, 9, 249–255. [Google Scholar] [CrossRef]
- Atun, G.; Tunçay, M.; Hisarli, G.; Talman, R.Y.; Hoşgömez, H. Adsorption equilibria between dye and surfactant in single and binary systems onto geological materials. Appl. Clay Sci. 2009, 15, 254–261. [Google Scholar] [CrossRef]
- Duman, O.; Özcan, C.; Polat, T.G.; Tunç, S. Carbon nanotube-based magnetic and non-magnetic adsorbents for the efficiency removal of diquat dibromide herbicide from water OMWCNT, OMWCNT-Fe3O4 and OMWCNT-κ-carrageenan-Fe3O4 nanocomposites. Environ. Pollut. 2019, 244, 723–732. [Google Scholar] [CrossRef]
- Mouzdahir, Y.E.; Elmchaouri, A.; Mahboub, R.; Gil, A.; Korili, S.A. Equilibrium modeling for the adsorption of methylene blue from aqueous solutions on activated clay minerals. Desalination 2010, 250, 335–338. [Google Scholar] [CrossRef]
- He, D.; Huang, H.; Xu, W.; Qin, F.; Liu, S. Adsorption properties and mechanism of purified palygorskite on methylene blue. Arab. J. Geosci. 2018, 11, 658–671. [Google Scholar] [CrossRef]
- Basaleh, A.A.; Al-Malack, M.H.; Saleh, T.A. Methylene blue removal using polyamide–vermiculite nanocomposites: Kinetics, equilibrium and thermodynamic study. J. Environ. Chem. Eng. 2019, 7, 103107–103118. [Google Scholar] [CrossRef]
- Youcef, L.D.; Belaroui, L.S.; López-Galindo, A. Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl. Clay Sci. 2019, 179, 105145–105155. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Patra, D.; Gopalan, B.; Ganesan, R. Direct solid-state synthesis of maghemite as a magnetically recoverable adsorbent for the abatement of methylene blue. J. Environ. Chem. Eng. 2019, 7, 103384–103398. [Google Scholar] [CrossRef]
- Szép, R.; Keresztes, R.; Korodi, A.; Tonk, S. Dew Point-indirect Particulate Matter Pollution Indicator in the Ciuc Basin–Harghita, Romania. Rev. Chim. 2016, 67, 1914–1921. [Google Scholar]
- Anghel, A.M.; Marinescu, F.; Ilie, M.; Ghiță, G.; Ionescu, P.; Mărcuș, I.; Tociu, C.; Moncea, A.; Mîțiu, M.; Popescu, I.; et al. Advanced Processing of Environmental Data for Establishing the Ecological Status of the Lower Danube Water in Terms of Nutrients. J. Environ. Prot. Ecol. 2017, 18, 853–861. [Google Scholar]
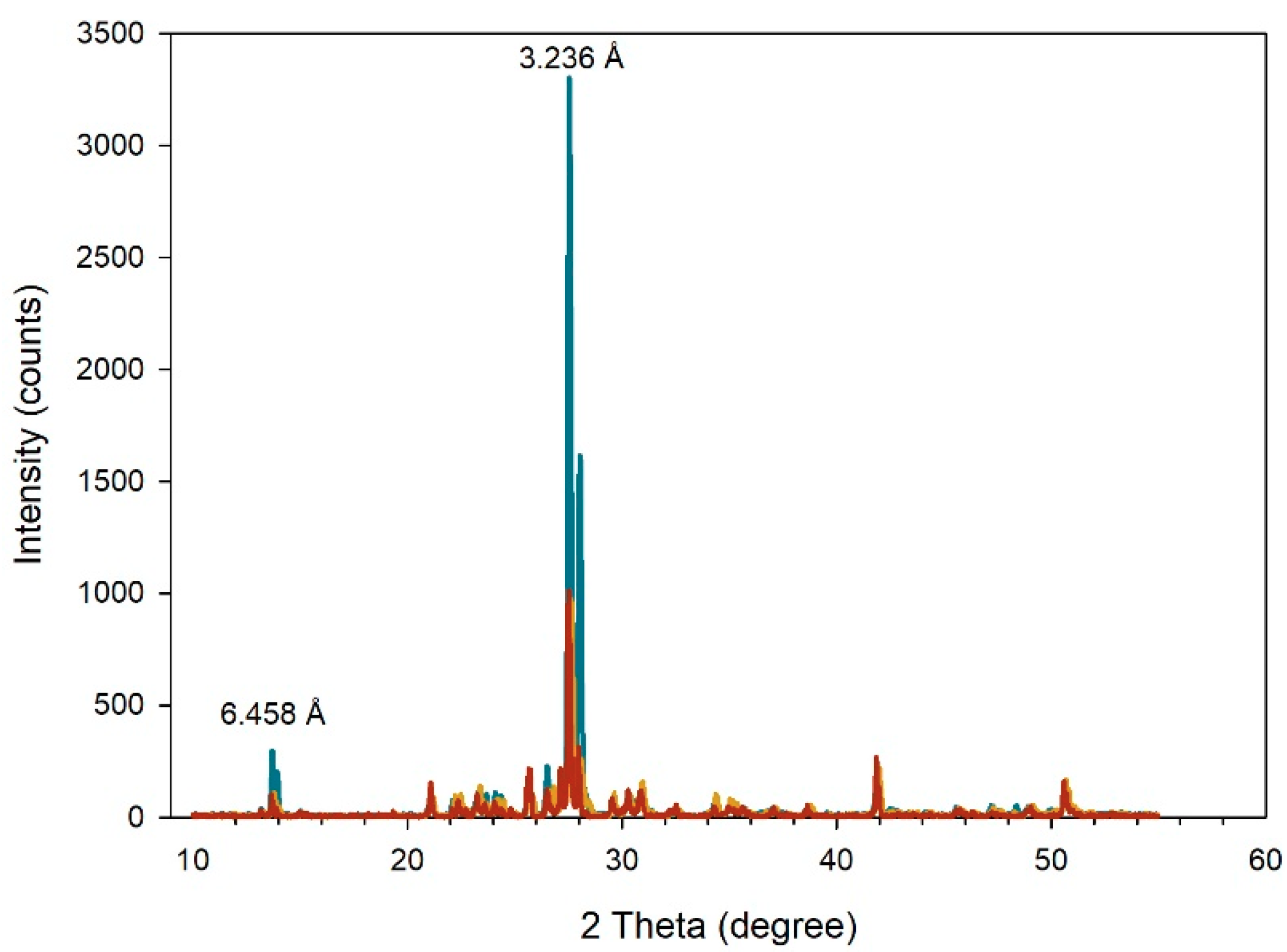
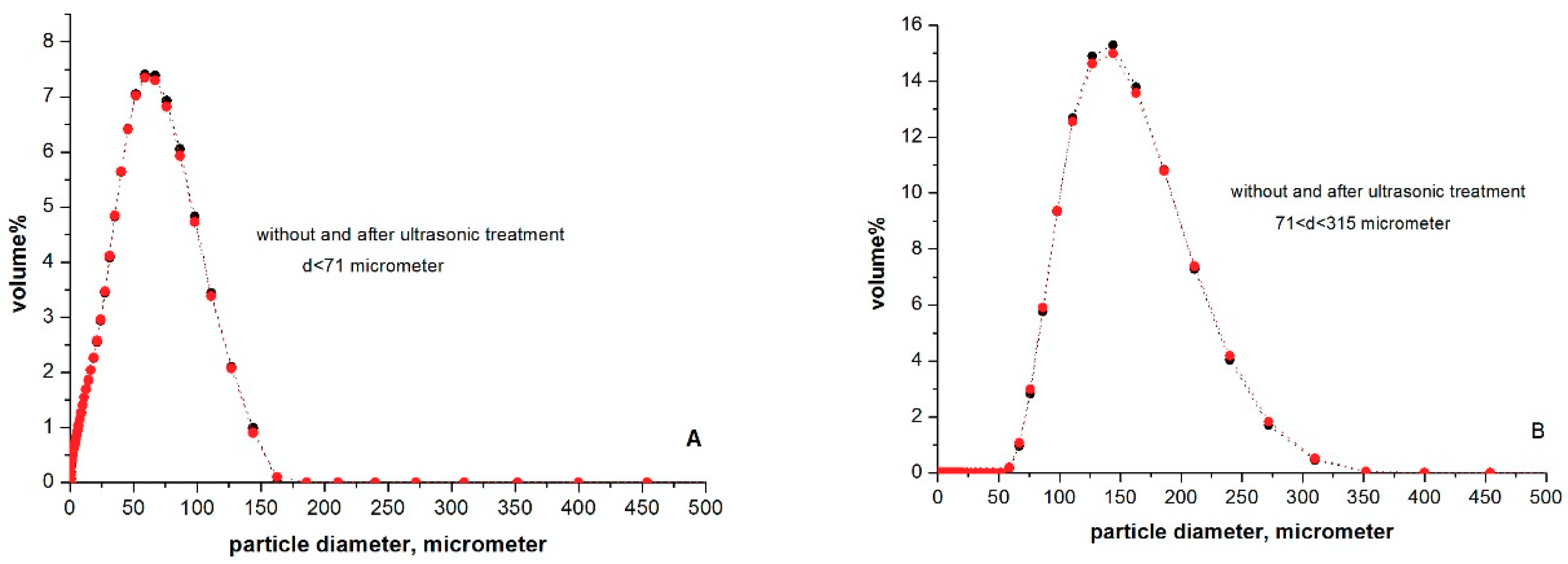
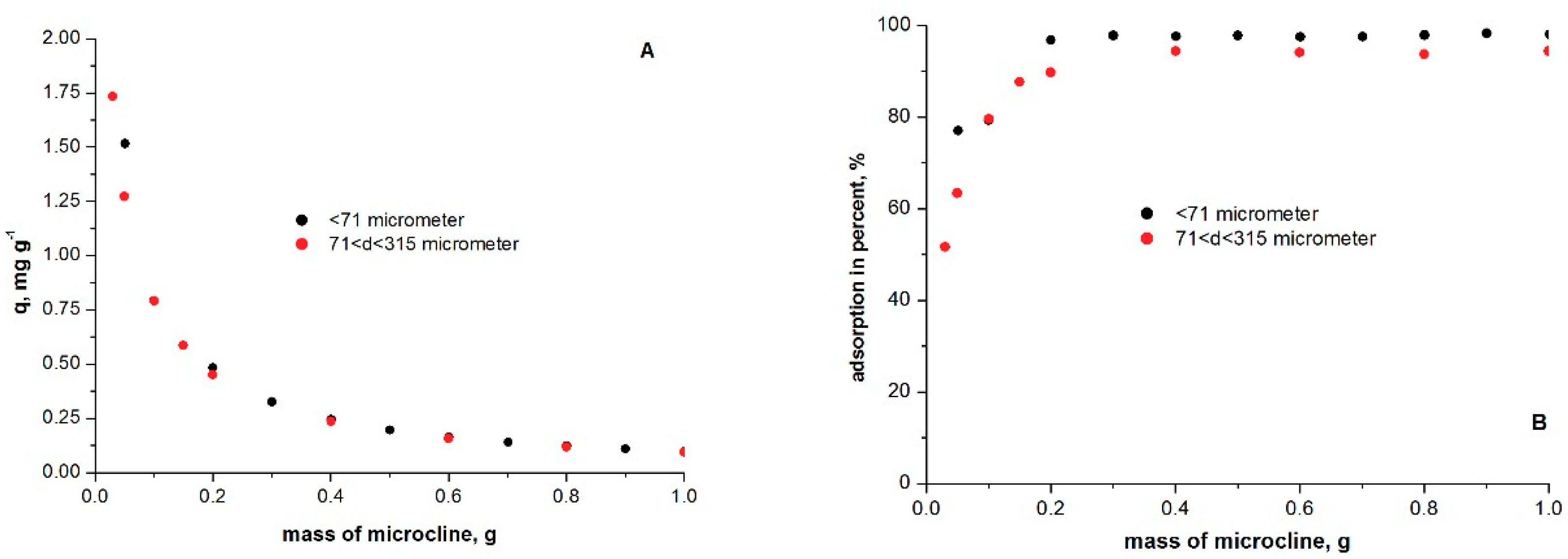
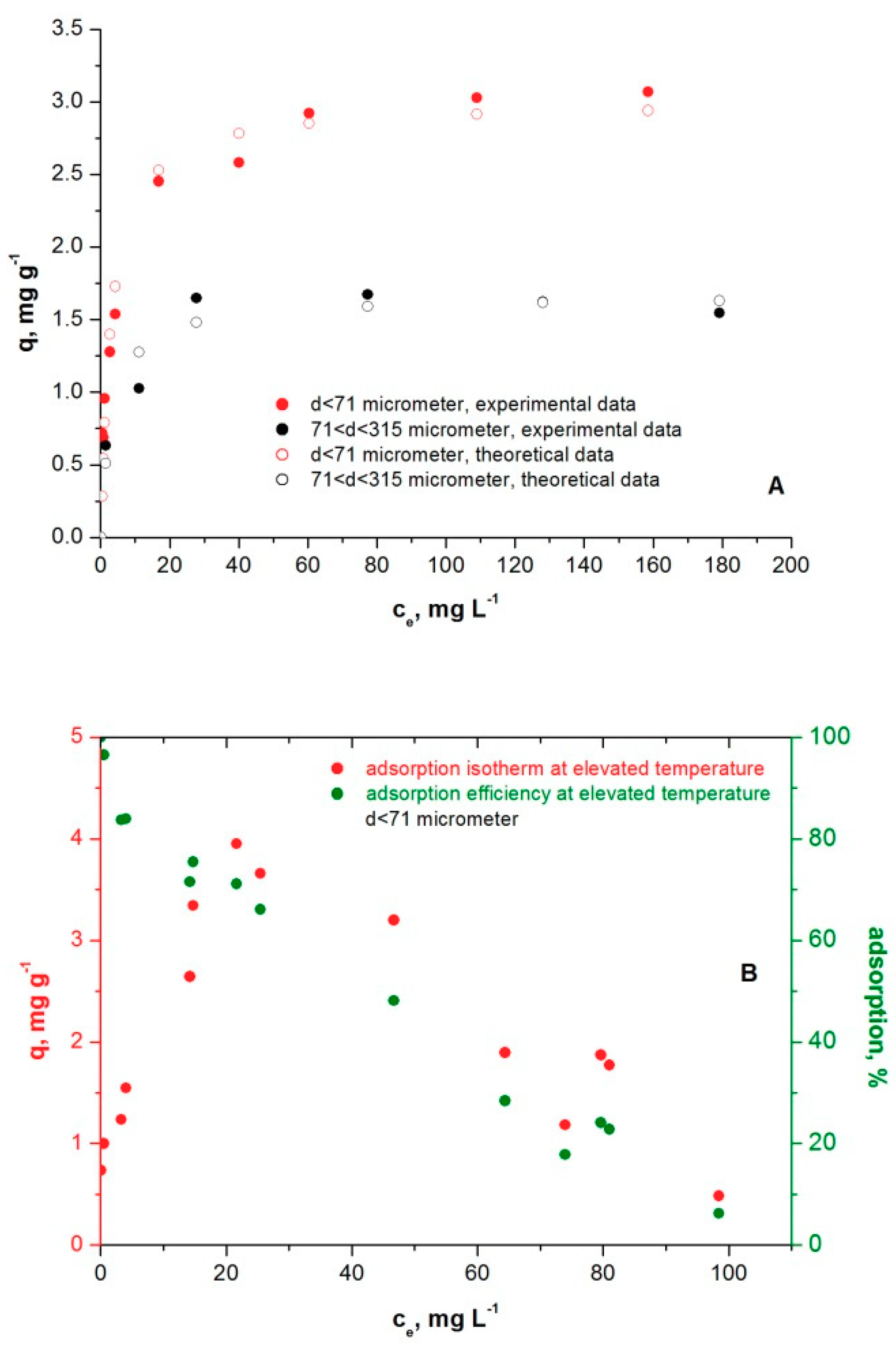

| Methods: | Statistics | d < 71 μm | 71 < d < 315 μm |
|---|---|---|---|
| Method of Moments Arithmetic (µm) | Mean | 51.870 | 155.7 |
| Sorting | 35.540 | 49.220 | |
| Skewness (Ska) | 0.559 | 0.769 | |
| Kurtosis (Ka) | 2.694 | 3.468 | |
| Method of Moments Geometric (µm) | Mean | 34.950 | 148.100 |
| Sorting | 3.003 | 1.366 | |
| Skewness (Skg) | −1.360 | 0.013 | |
| Kurtosis (Kg) | 4.537 | 2.581 |
| Microcline Particle Size Fraction | Q0, mg g−1 | b, L mg−1 | R2 |
|---|---|---|---|
| d < 71 µm | 3.00 | 0.32 | 0.97 |
| 71< d <315 µm | 1.66 | 0.30 | 0.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pernyeszi, T.; Farkas, R.; Kovács, J. Methylene Blue Adsorption Study on Microcline Particles in the Function of Particle Size Range and Temperature. Minerals 2019, 9, 555. https://doi.org/10.3390/min9090555
Pernyeszi T, Farkas R, Kovács J. Methylene Blue Adsorption Study on Microcline Particles in the Function of Particle Size Range and Temperature. Minerals. 2019; 9(9):555. https://doi.org/10.3390/min9090555
Chicago/Turabian StylePernyeszi, Tímea, Roland Farkas, and János Kovács. 2019. "Methylene Blue Adsorption Study on Microcline Particles in the Function of Particle Size Range and Temperature" Minerals 9, no. 9: 555. https://doi.org/10.3390/min9090555





