An Alternative Depressant of Chalcopyrite in Cu–Mo Differential Flotation and Its Interaction Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Micro-Flotation Experiment
2.3. Adsorption of CMC on Chalcopyrite and Molybdenite
2.4. Zeta Potential Measurements
2.5. Electrochemical Tests
2.6. Infrared Spectrum Measurements
2.7. X-ray Photoelectron Spectroscopy Measurements
3. Results and Discussion
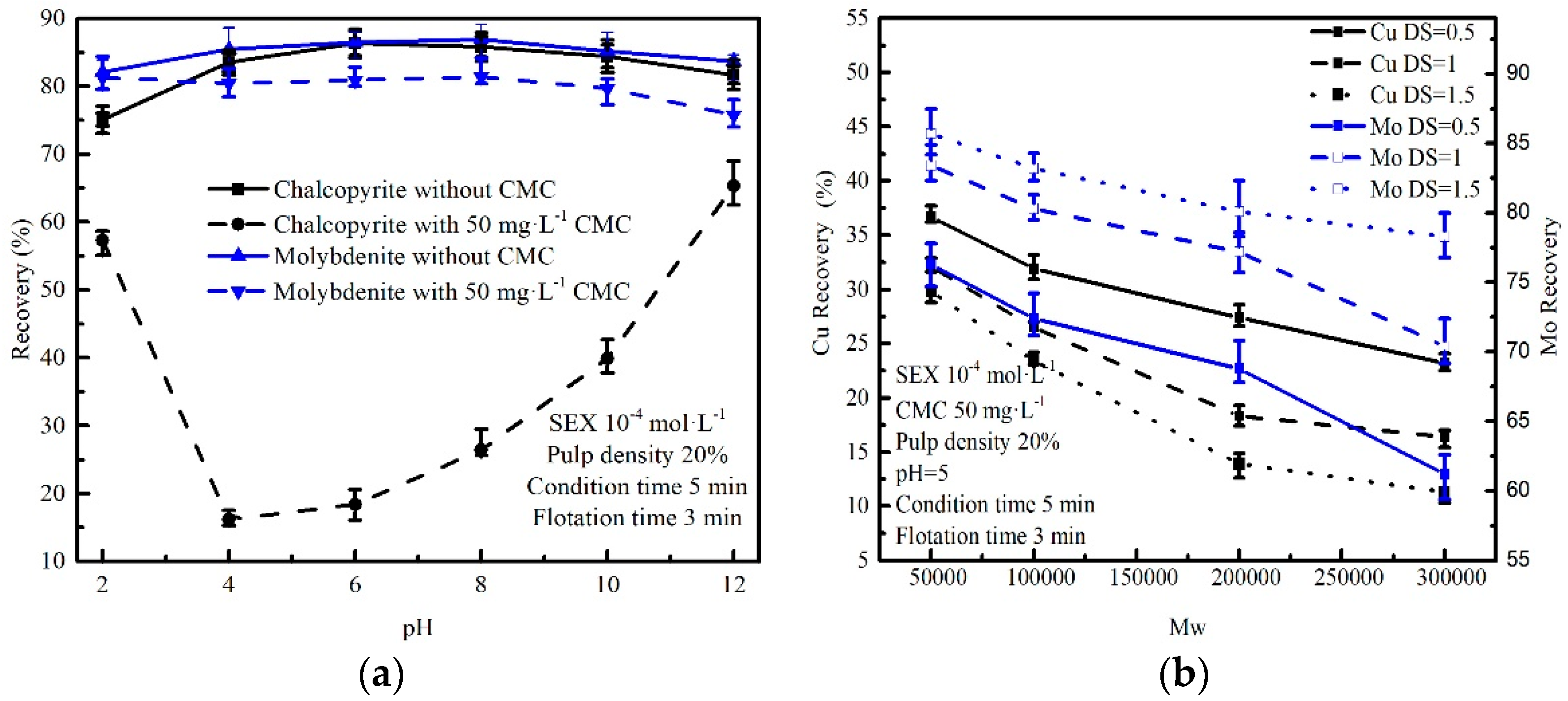
3.1. Micro-Flotation of Single Mineral Tests
3.2. The Effect of DS and Mw
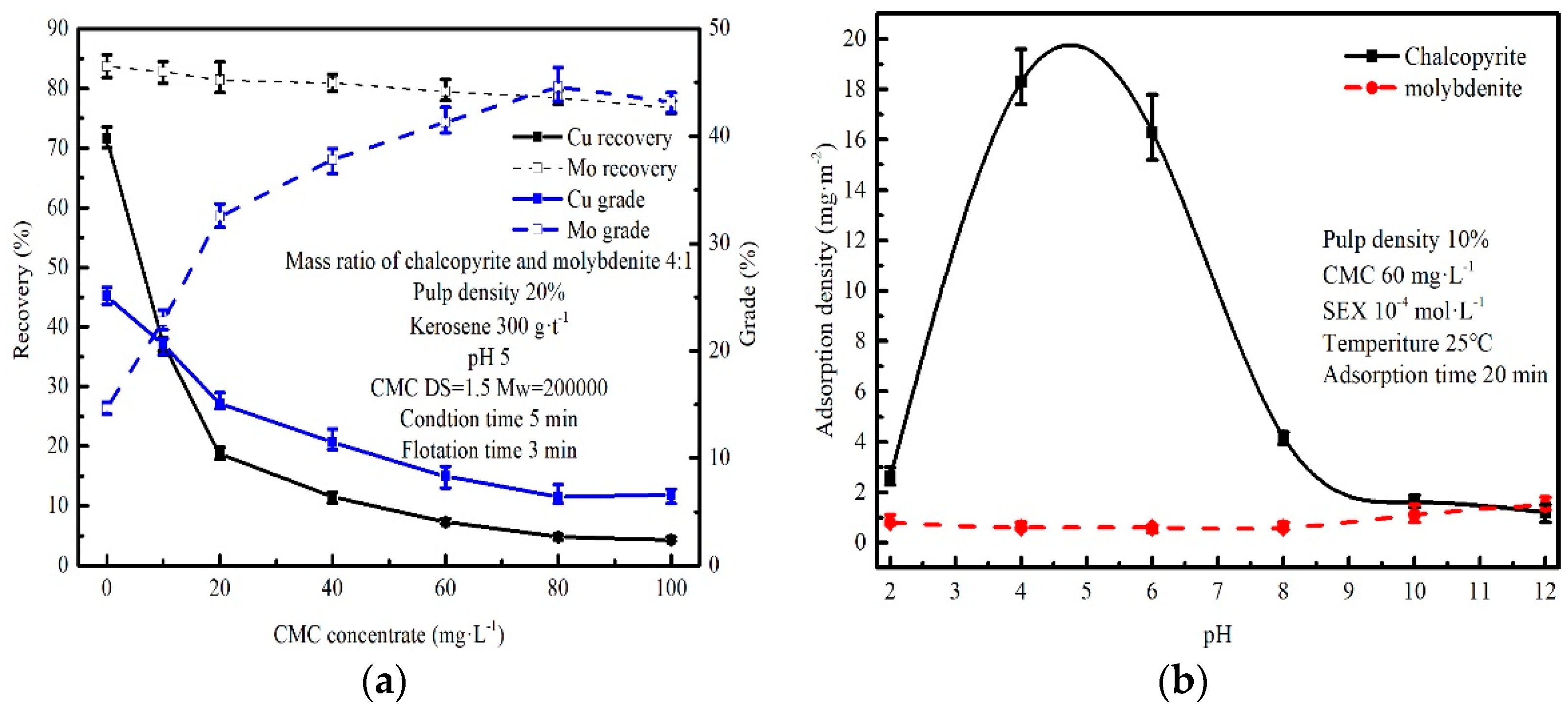
3.3. Flotation of Chalcopyrite–Molybdenite Mixed Minerals
3.4. Adsorption of CMC to Chalcopyrite and Molybdenite
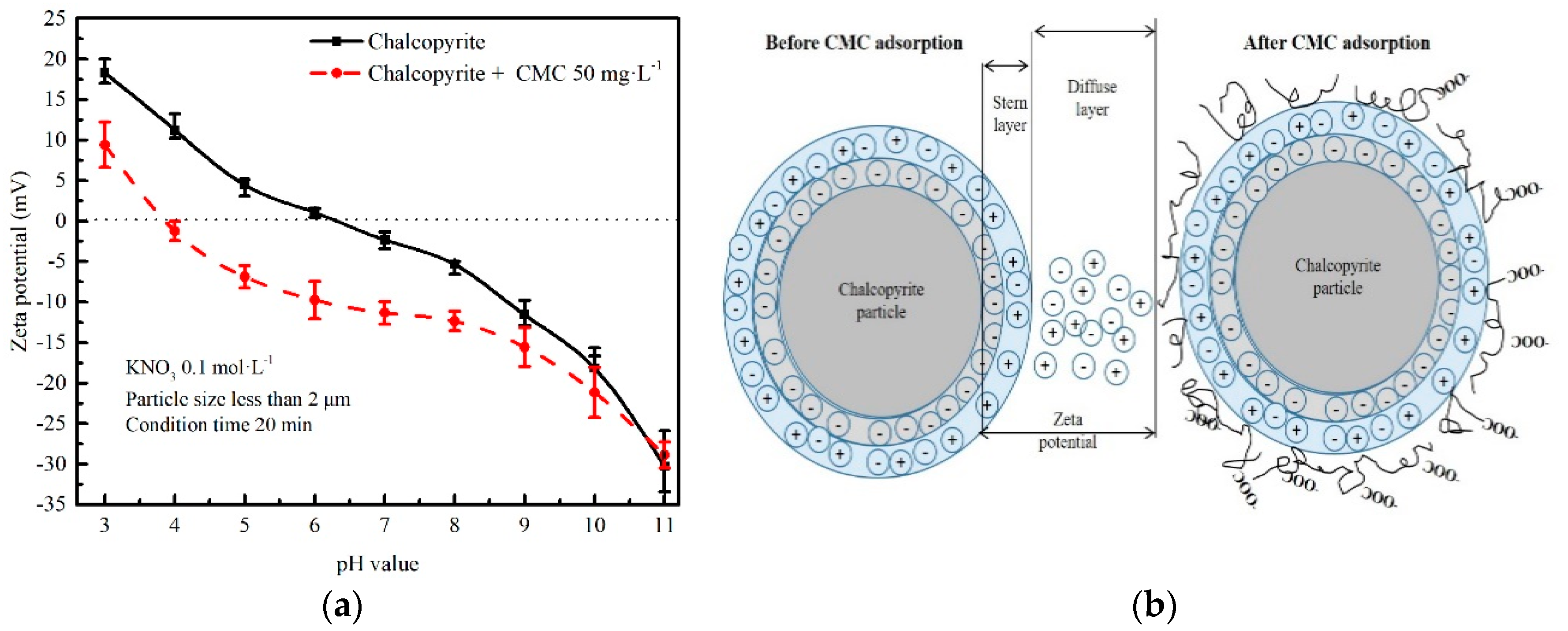
3.5. Zeta Potential Measurements
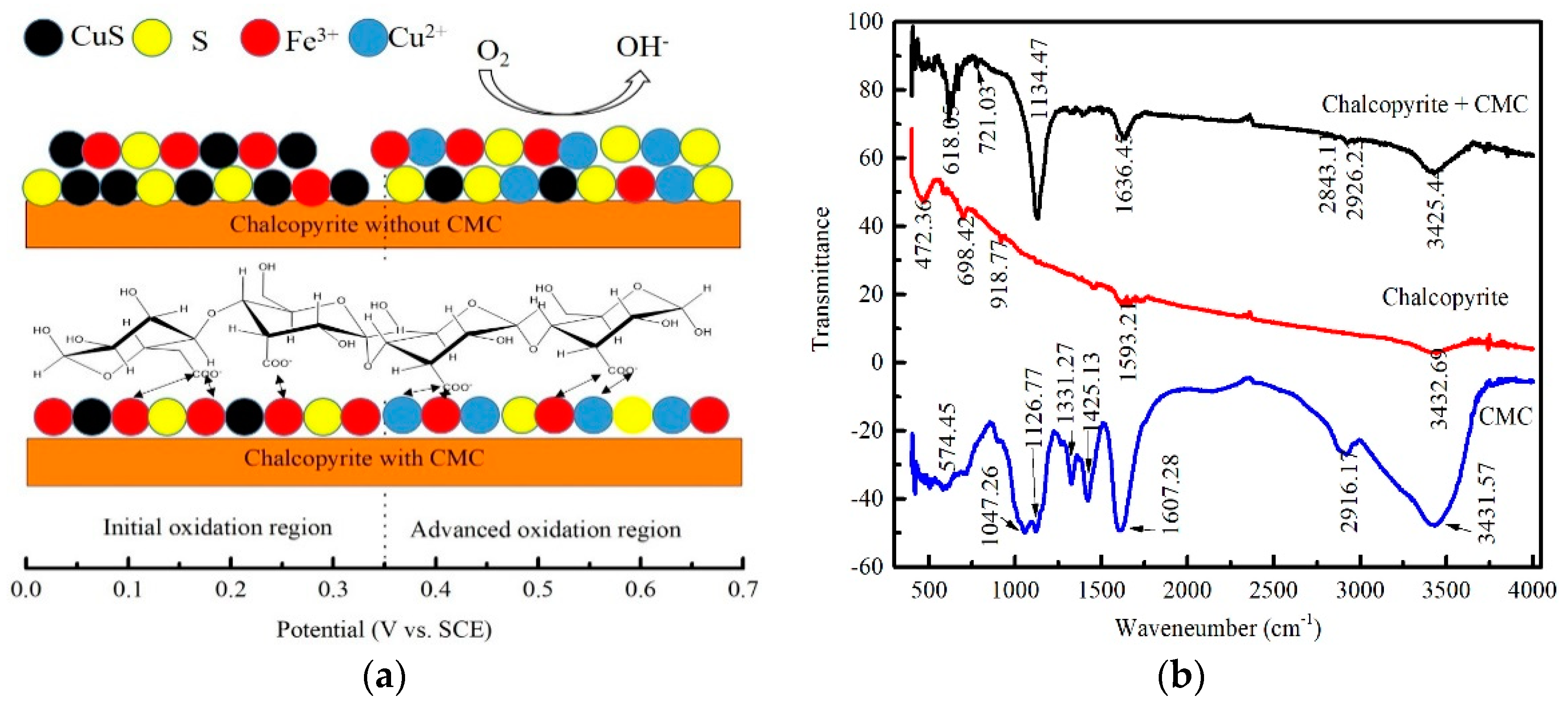
3.6. Electrochemical Study
3.7. FTIR Analysis
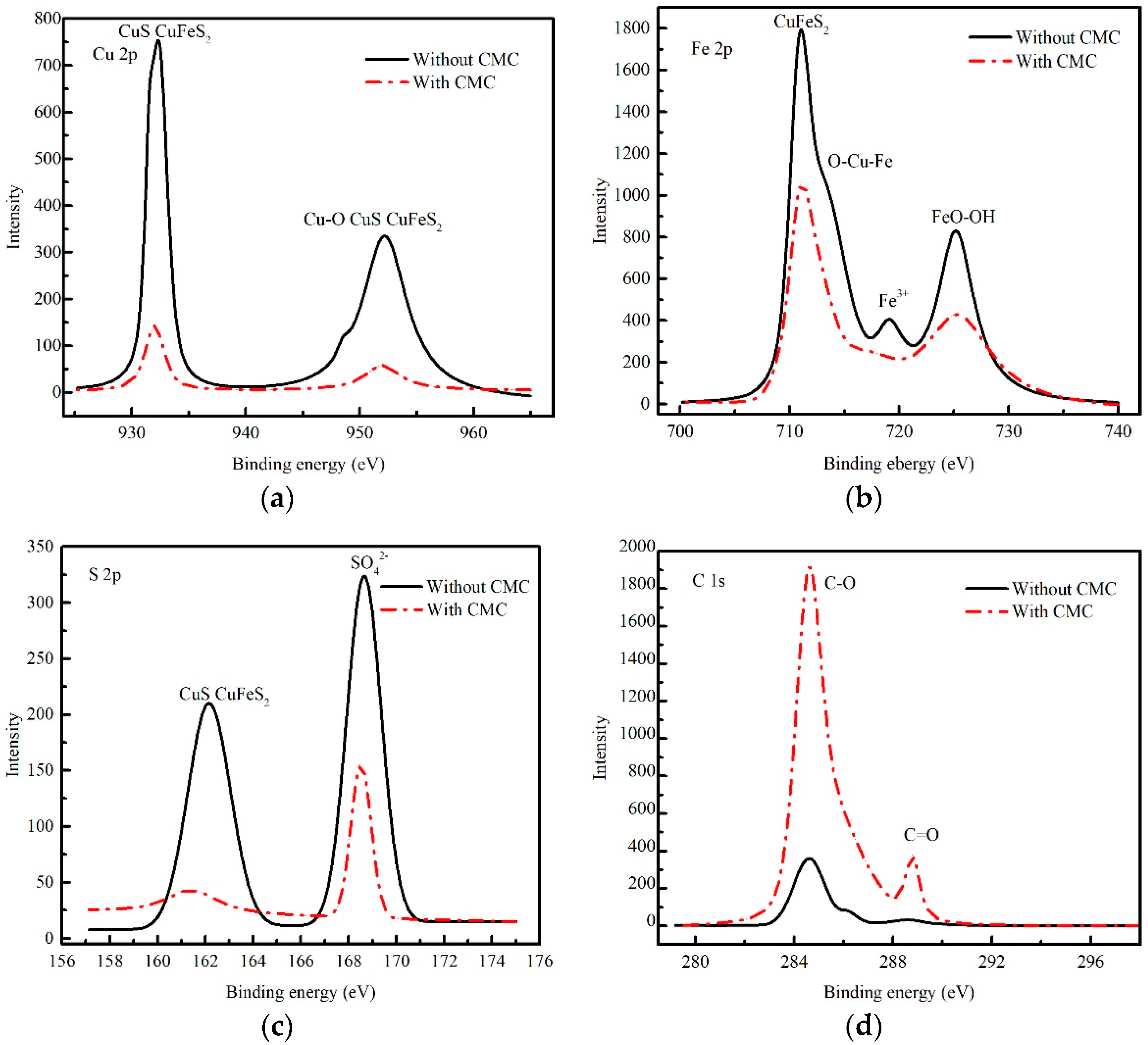
3.8. XPS Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H. Application and marketing research of molybdenum. China Molybdenum Ind. 2013, 37, 11–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Yu, J.; Cao, W.; Feng, P.; Jiao, S. Advances in Surface Modification of Molybdenum and Molybdenum Alloys at Elevated Temperature. Mater. Rev. 2017, 31, 83–87. [Google Scholar] [CrossRef]
- Ji, G.; Deng, H.; Wang, C.; Jiang, A.; Han, J. Current status of molybdenum-ore resources in China and screening of national molybdenum-ore geological material data. China Min. Mag. 2016, 25, 139–145. [Google Scholar] [CrossRef]
- Wang, J.; Zou, T.; Wang, Y.; Long, L.; Zhang, H.; Liao, Z.; Xie, H. Deposit associations, metallogenesis and metallogenic lineage of molybdenum-polymetallic deposits in China. Miner. Depos. 2014, 33, 447–470. [Google Scholar] [CrossRef]
- Meng, Q.; Cui, Yi.; Tong, X.; Zou, H.; Wang, K. Research Status of Copper-Molybdenum Separation. Min. Metall. 2014, 23, 19–22. [Google Scholar] [CrossRef]
- Castro, S.; Lopez-Valdivieso, A.; Laskowski, J.S. Review of the flotation of molybdenite. Part I: Surface properties and floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Yin, W.-Z.; Zhang, L.-R.; Xie, F. Flotation of Xinhua molybdenite using sodium sulfide as modifier. Trans. Nonferr. Met. Soc. China 2010, 20, 702–706. [Google Scholar] [CrossRef]
- Pearse, M.J. An overview of the use of chemical reagents in mineral processing. Miner. Eng. 2005, 18, 139–149. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Wei, D.; Wang, W.; Cui, B.; Liu, W. Effect of copper ions on the flotation separation of chalcopyrite and molybdenite using sodium sulfide as a depressant. Miner. Eng. 2018, 115, 44–52. [Google Scholar] [CrossRef]
- Miki, H.; Hirajima, T.; Muta, Y.; Suyantara, G.; Sasaki, K. Effect of Sodium Sulfite on Floatability of Chalcopyrite and Molybdenite. Minerals 2018, 8, 172. [Google Scholar] [CrossRef]
- Jiangang, F.; Hong, Z.; Leming, O. Application of Thioglycolic Acid in Molybdenite-copper Sulphide Separation. Min. Metall. Eng. 2002, 22, 36–38. [Google Scholar] [CrossRef]
- Liu, R.; Sun, W.; Hu, Y.; Xiong, D. Depression mechanism of small molecular mercapto organic depressants on flotation behavior of complex sulfides. Chin. J. Nonferr. Met. 2006, 16, 192–197. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice Flotation of Sulfide Ores; Elsevier Science: Amsterdam, The Netherlands, 2007; p. 685. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Liu, Q.; Bolin, N.J. Polysaccharides in flotation of sulphides. Part I. Adsorption of polysaccharides onto mineral surfaces. Int. J. Miner. Process. 1991, 33, 223–234. [Google Scholar] [CrossRef]
- Beattie, D.A.; Le, H.; Kaggwa, G.B.N.; Ralston, J. The effect of polysaccharides and polyacrylamides on the depression of talc and the flotation of sulphide minerals. Miner. Eng. 2006, 19, 598–608. [Google Scholar] [CrossRef]
- Huang, P.; Cao, M.; Liu, Q. Using chitosan as a selective depressant in the differential flotation of Cu–Pb sulfides. Int. J. Miner. Process. 2011, 93, 8–15. [Google Scholar] [CrossRef]
- Qiu, X.H. A Non-Toxic Depressant for Galena in Differential Flotation of Cu-Pb Sulfides. Adv. Mater. Res. 2013, 734–737, 1018–1021. [Google Scholar] [CrossRef]
- Wang, L. The Use of Polyacrylamide as a Selective Depressant in the Separation of Chalcopyrite and Galena. Bound. Value Probl. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Ahmadi, M.; Gharabaghi, M.; Abdollahi, H. Effects of type and dosages of organic depressants on pyrite floatability in microflotation system. Adv. Powder Technol. 2018. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of pyrite in selective flotation by different reagent systems—A Literature review. Miner. Eng. 2016, 96–97, 143–156. [Google Scholar] [CrossRef]
- Zhao, K.; Yan, W.; Wang, X.; Hui, B.; Gu, G.; Wang, H. The flotation separation of pyrite from pyrophyllite using oxidized guar gum as depressant. Int. J. Miner. Process. 2017, 161, 78–82. [Google Scholar] [CrossRef]
- Rath, R.K.; Subramanian, S.; Pradeep, T. Surface Chemical Studies on Pyrite in the Presence of Polysaccharide-Based Flotation Depressants. J. Colloid Interface Sci. 2000, 229, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Zhang, C. Separation of Molybdenite from Chalcopyrite in the Presence of Novel Depressant 4-Amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one. Minerals 2017, 7, 146. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y. Evaluation of the replacement of NaCN with depressant mixtures in the separation of copper–molybdenum sulphide ore by flotation. Sep. Purif. Technol. 2017, 173, 9–16. [Google Scholar] [CrossRef]
- Sarquís, P.E.; Menéndez-Aguado, J.M.; Mahamud, M.M. Tannins: The organic depressants alternative in selective flotation of sulfides. J. Clean. Prod. 2014, 84, 723–726. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, Q. Flotation separation of copper–molybdenum sulfides using chitosan as a selective depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Khraisheh, M.; Holland, C.; Creany, C. Effect of molecular weight and concentration on the adsorption of CMC onto talc at different ionic strengths. Int. J. Miner. Process. 2005, 75, 197–206. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Beattie, D.A. Adsorption of tailored carboxymethyl cellulose polymers on talc and chalcopyrite: Correlation between coverage, wettability, and flotation. Miner. Eng. 2010, 23, 985–993. [Google Scholar] [CrossRef]
- Beaussart, A.; Mierczynska-Vasilev, A.; Beattie, D.A. Evolution of carboxymethyl cellulose layer morphology on hydrophobic mineral surfaces: Variation of polymer concentration and ionic strength. J. Colloid Interface Sci. 2010, 346, 303–310. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, G.; Li, S.; Wang, C.; Zhu, R. The Effect of Seaweed Glue in the Separation of Copper–Molybdenum Sulphide Ore by Flotation. Minerals 2018, 8, 41. [Google Scholar] [CrossRef]
- López-Valdivieso, A.; Lozano-Ledesma, L.A.; Robledo-Cabrera, A.; Orozco-Navarro, O.A. Carboxymethylcellulose (CMC) as PbS depressant in the processing of Pb-Cu bulk concentrates. Adsorption and floatability studies. Miner. Eng. 2017, 112, 77–83. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173. [Google Scholar] [CrossRef]
- Wuestenberg, T. Cellulose and Cellulose Derivatives in the Food Industry: Fundamentals and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2014; pp. 404–406. ISBN 978-3-527-68296-6. [Google Scholar]
- Fullston, D.; Fornasiero, D.; Ralston, J. Zeta potential study of the oxidation of copper sulfide minerals. Colloids Surf. A Physicochem. Eng. Asp. 1999, 146, 113–121. [Google Scholar] [CrossRef]
- Mhlanga, S.S.; O’Connor, C.T.; Mcfadzean, B. A study of the relative adsorption of guar onto pure minerals. Miner. Eng. 2012, 36–38, 172–178. [Google Scholar] [CrossRef]
- Bard, A.J.; Inzelt, G.; Scholz, F. Electrochemical Dictionary, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 173–175. ISBN 978-3-642-29551-5. [Google Scholar]
- Li, S.-K.; Gu, G.-H.; Qiu, G.-Z.; Chen, Z.-X. Flotation and electrochemical behaviors of chalcopyrite and pyrite in the presence of N-propyl-N′-ethoxycarbonyl thiourea. Trans. Nonferr. Met. Soc. China 2018, 28, 1241–1247. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The galvanic interaction between chalcopyrite and pyrite in the presence of lignosulfonate-based biopolymers and its effects on flotation performance. Miner. Eng. 2018, 122, 91–98. [Google Scholar] [CrossRef]
- Williams, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry, 6th ed.; McGraw-Hill Education: New York, NY, USA, 2007; pp. 120–125. ISBN 978-0077118129. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH: New York, NY, USA, 2006; pp. 431–436. ISBN 978-3-527-60644-3. [Google Scholar]
- Borer, P.; Hug, S.J.; Sulzberger, B.; Kraemer, S.M.; Kretzschmar, R. ATR-FTIR spectroscopic study of the adsorption of desferrioxamine B and aerobactin to the surface of lepidocrocite (y-FeOOH). Geochim. Cosmochim. Acta 2009, 73, 4661–4672. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Lepkova, K.; Eksteen, J.J.; Oraby, E.A. Electrochemical behaviour and surface analysis of chalcopyrite in alkaline glycine solutions. Hydrometallurgy 2018, 182, 32–43. [Google Scholar] [CrossRef]
- Wang, J.; Gan, X.; Zhao, H.; Hu, M.; Li, K.; Qin, W.; Qiu, G. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis. Miner. Eng. 2016, 98, 264–278. [Google Scholar] [CrossRef]
- Ramachandran, T.; Vishista, K. Structural, optical and magnetic properties of nanocrystalline zinc ferrite particles from glycine assisted combustion: Effect of Sr 2+ dopant. Int. J. Mater. Res. 2015, 106, 127–136. [Google Scholar] [CrossRef]
- Tang, R.; Jiang, C.; Qian, W.; Jian, J.; Zhang, X.; Wang, H.; Yang, H. Dielectric relaxation, resonance and scaling behaviors in Sr3Co2Fe24O41 hexaferrite. Sci. Rep. 2015, 5, 13645. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Element | Cu | Fe | S | Mo | Si | O |
|---|---|---|---|---|---|---|
| Chalcopyrite | 33.7 | 32.3 | 32.2 | 0.6 | 1.2 | |
| Molybdenite | 40.2 | 58.8 | 0.5 | 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Yang, H.; Chen, G.; Luo, W. An Alternative Depressant of Chalcopyrite in Cu–Mo Differential Flotation and Its Interaction Mechanism. Minerals 2019, 9, 1. https://doi.org/10.3390/min9010001
Qiu X, Yang H, Chen G, Luo W. An Alternative Depressant of Chalcopyrite in Cu–Mo Differential Flotation and Its Interaction Mechanism. Minerals. 2019; 9(1):1. https://doi.org/10.3390/min9010001
Chicago/Turabian StyleQiu, Xuemin, Hongying Yang, Guobao Chen, and Wenjie Luo. 2019. "An Alternative Depressant of Chalcopyrite in Cu–Mo Differential Flotation and Its Interaction Mechanism" Minerals 9, no. 1: 1. https://doi.org/10.3390/min9010001





