Role of Collectors and Depressants in Mineral Flotation: A Theoretical Analysis Based on Extended DLVO Theory
Abstract
:1. Introduction
2. Theoretical Model
3. Results and Discussion
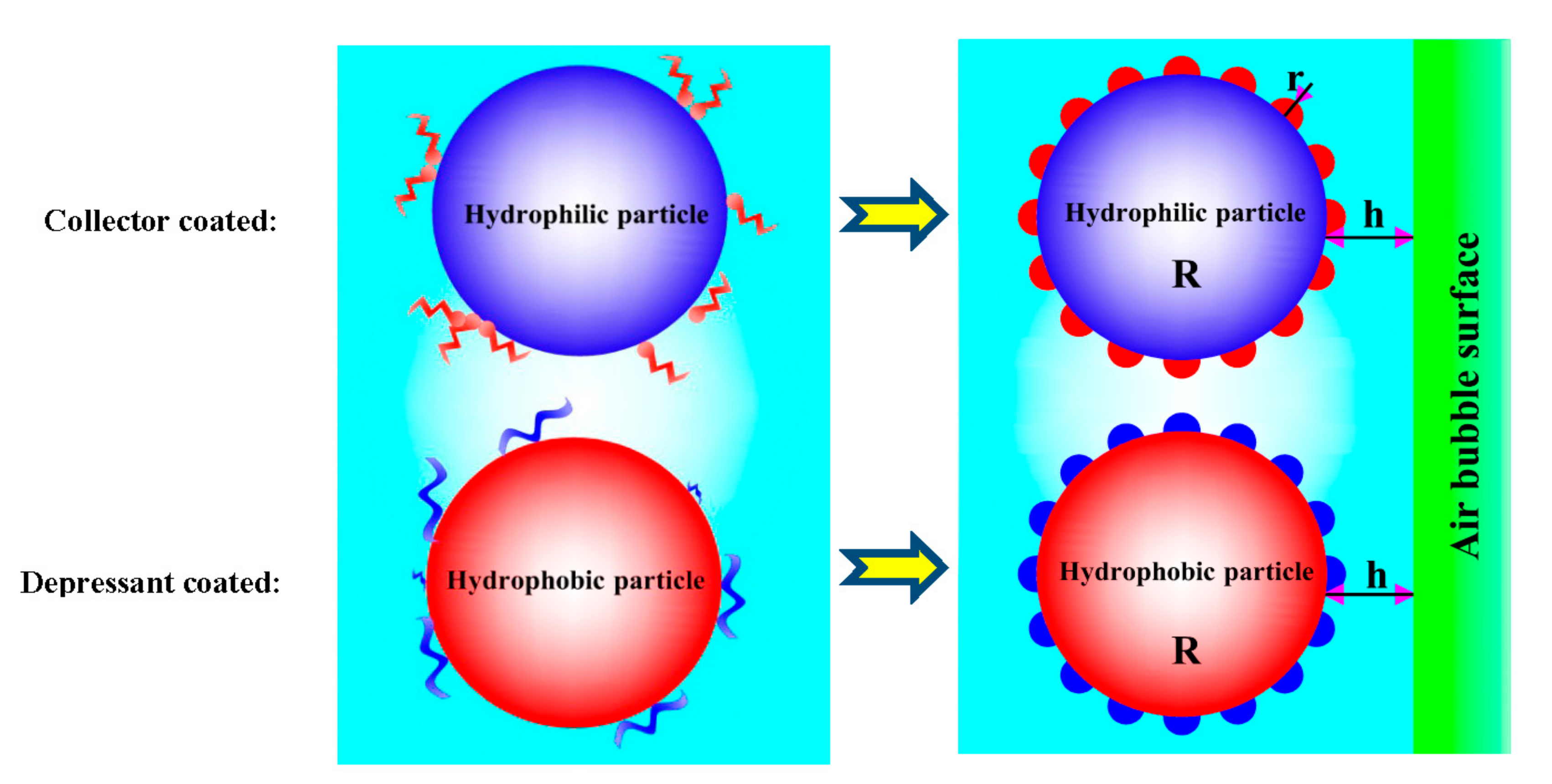
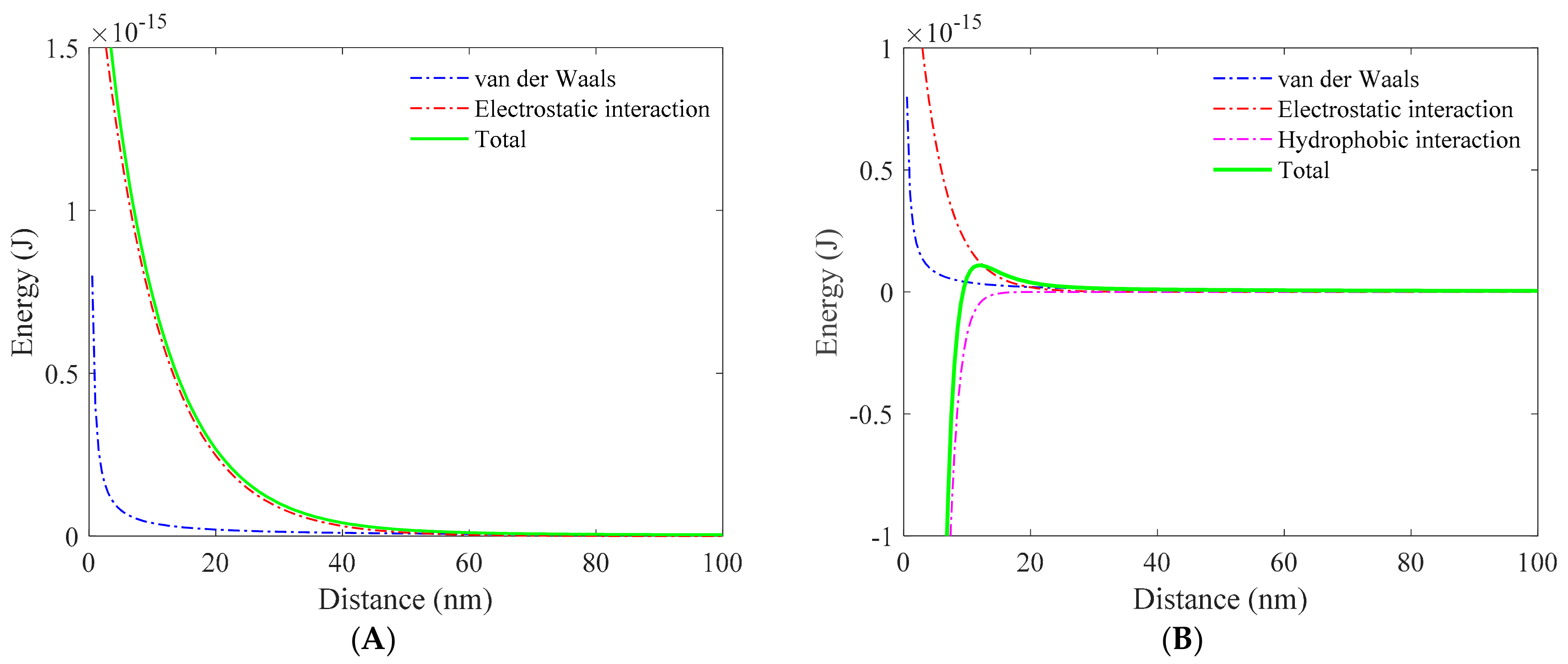
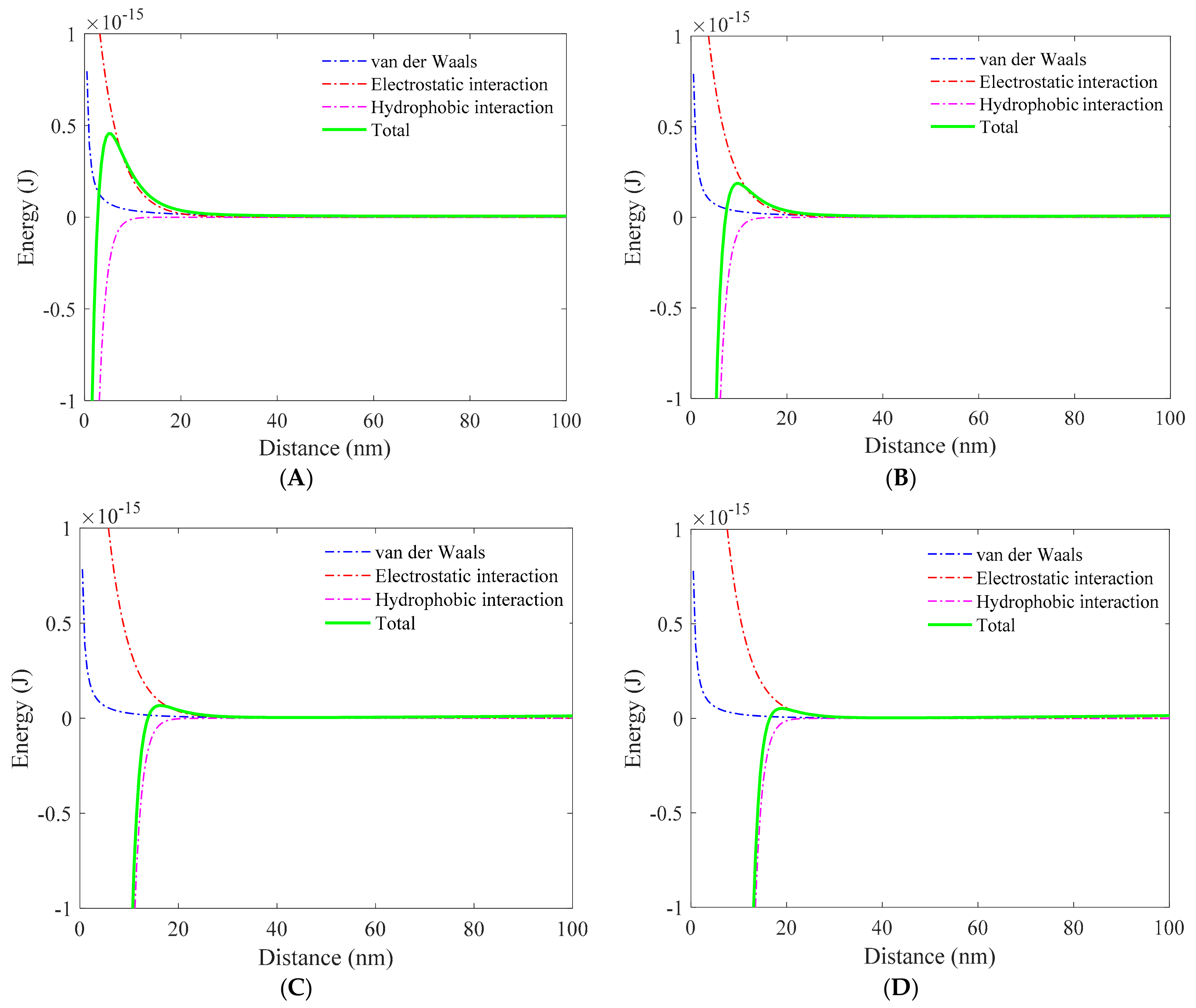
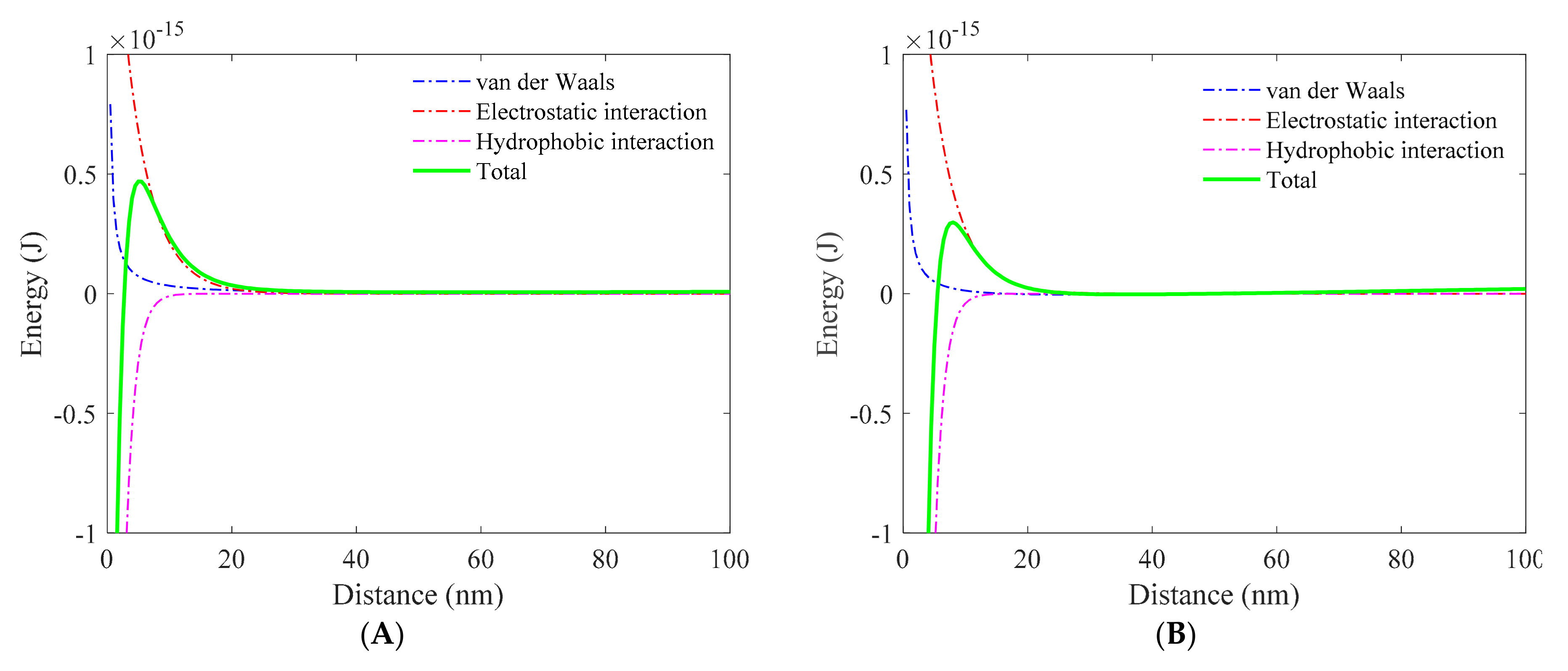
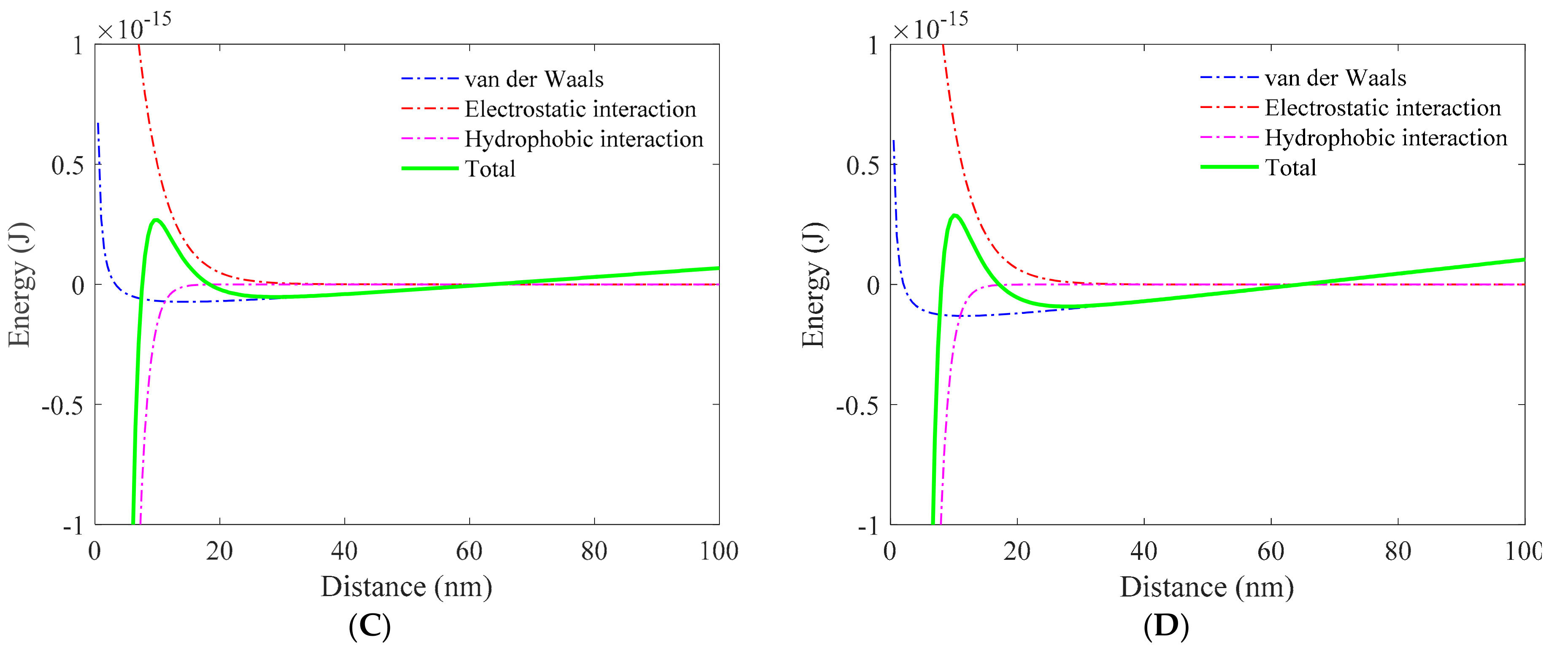
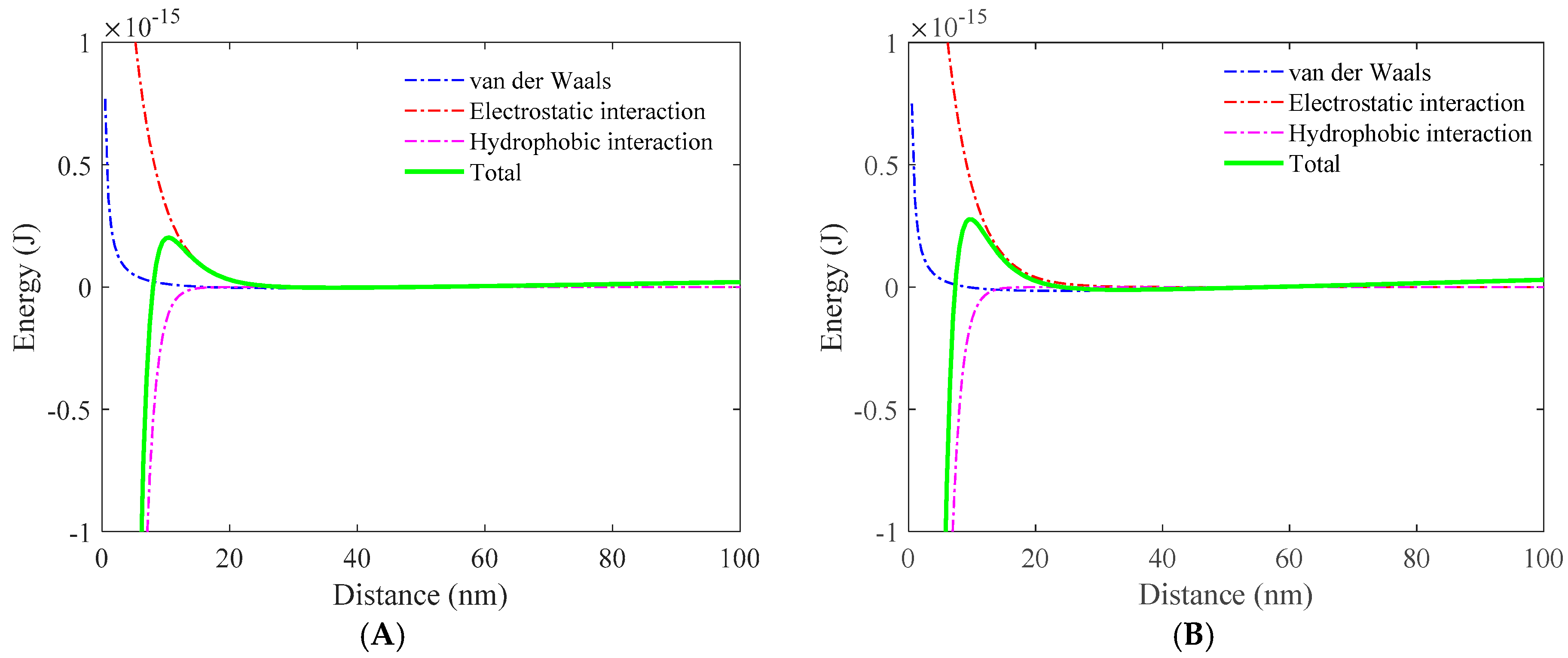
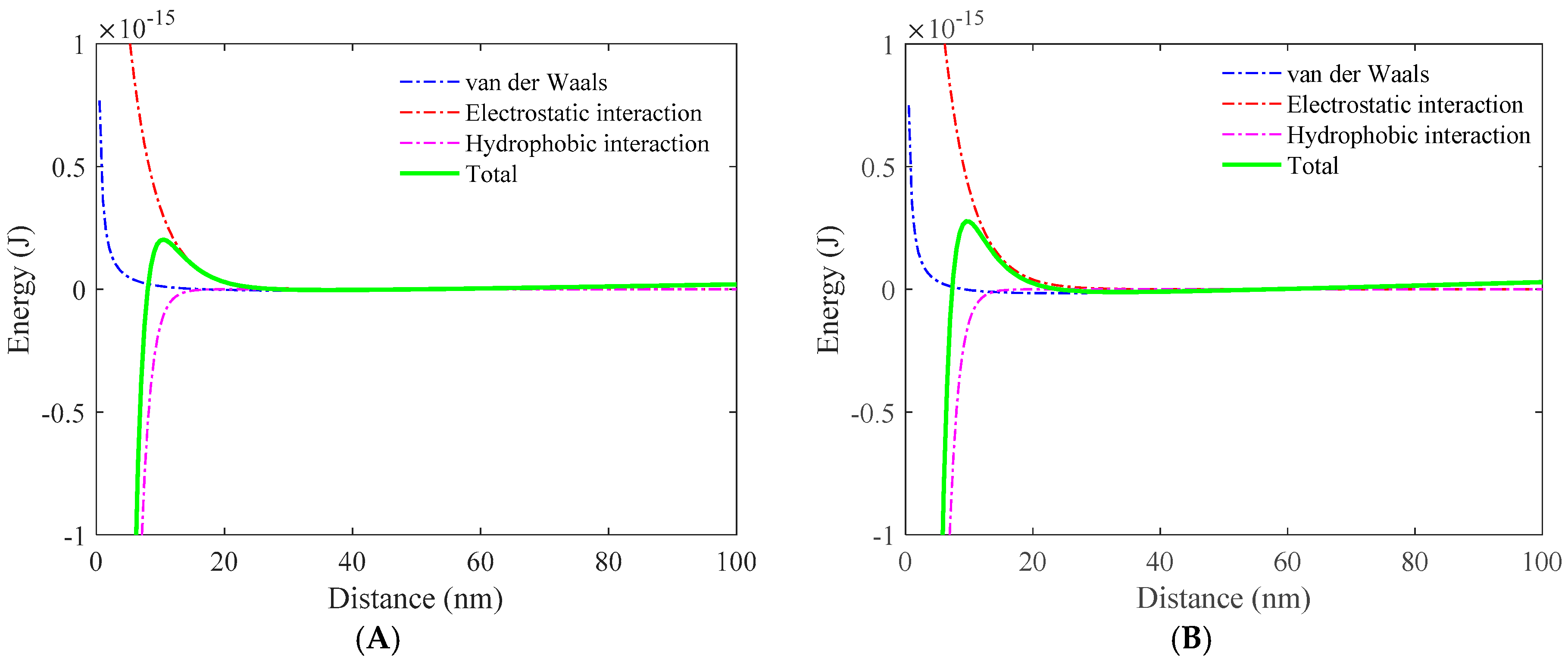
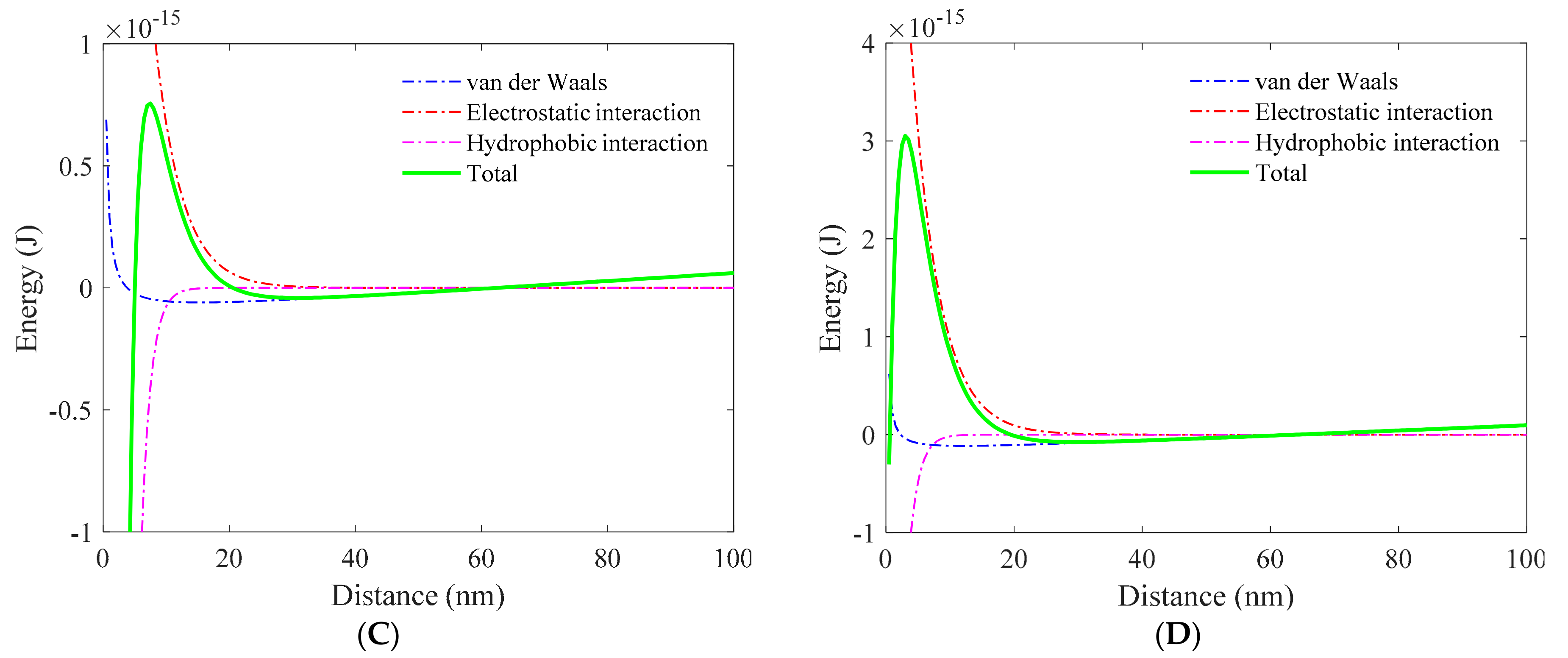
3.1. Role of Collector in Bubble–Particle Attachment
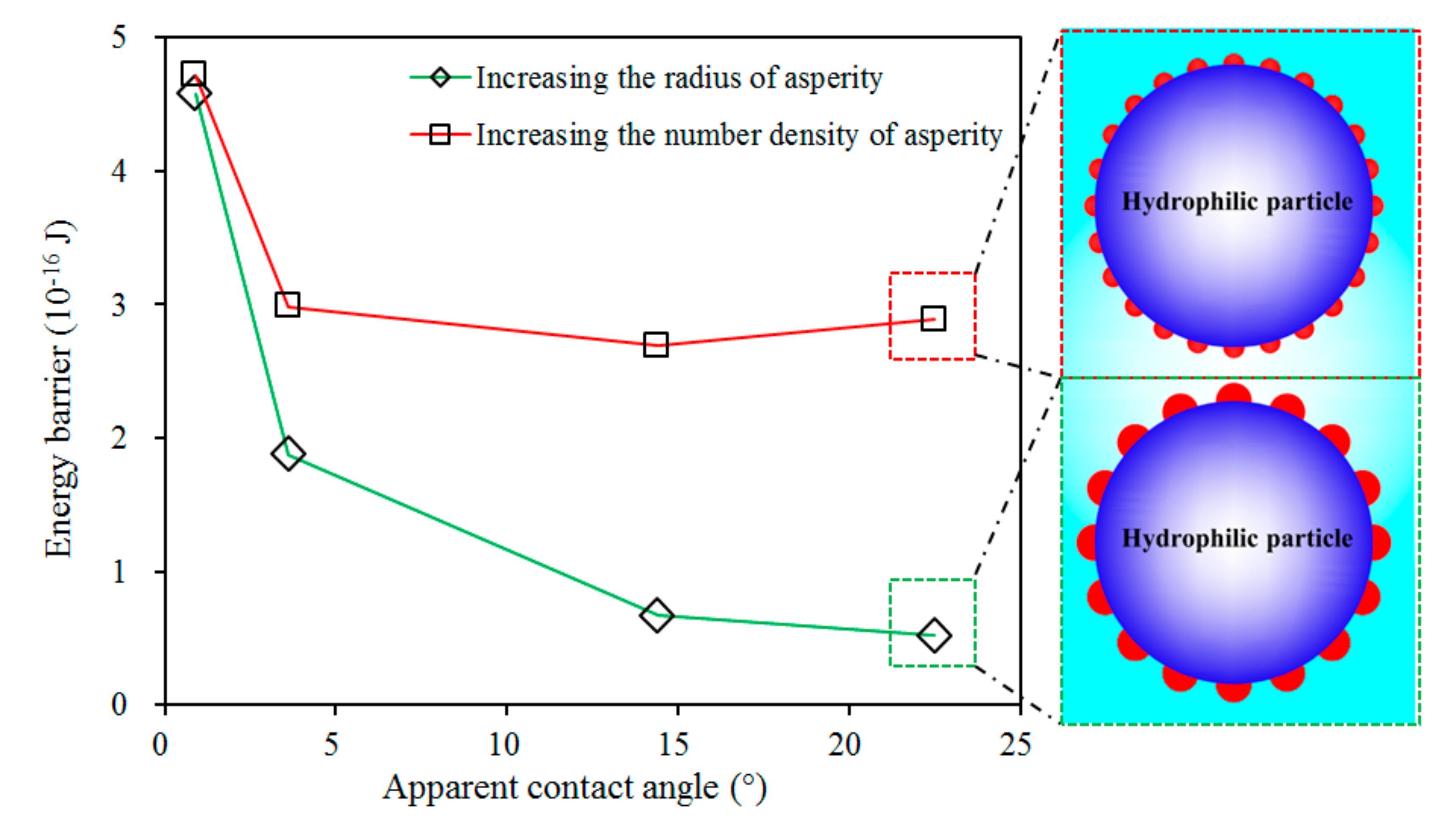
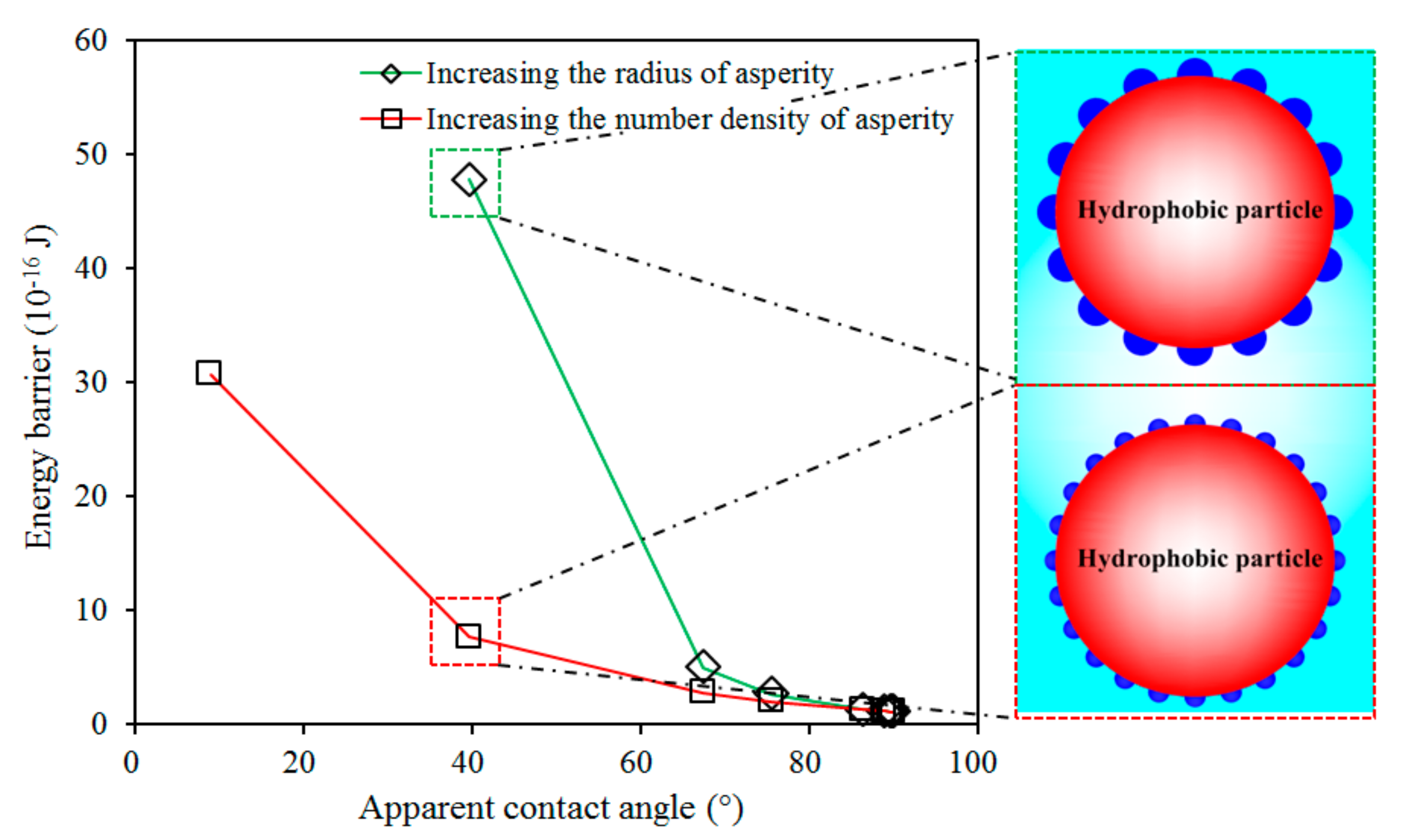
3.2. Role of Depressant in Bubble–Particle Attachment
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nguyen, A.V.; Schulze, H.J. Colloidal Science of Flotation; Marcel Dekker Inc.: New York, NY, USA, 2004. [Google Scholar]
- Fuerstenau, M.C.; Jameson, G.J.; Yoon, R.H. Froth Flotation: A Century of Innovation; SME: Dearborn, MI, USA, 2007. [Google Scholar]
- Nguyen, A.V. Froth Flotation. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–26. [Google Scholar]
- Yoon, R.H. The role of hydrodynamic and surface forces in bubble-particle interaction. Int. J. Miner. Process. 2000, 58, 129–143. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Z.; Li, G.; Rowson, N.A. Attachment of solid particles to air bubbles in surfactant-free aqueous solutions. Chem. Eng. Sci. 2004, 59, 2639–2645. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Pan, L.; Pinchasik, B.E.; Cao, Y.; Liu, J.; Kappl, M.; Butt, H.J. Recent experimental advances for understanding bubble-particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Gui, X.; Cao, Y. The hydrophobic force for bubble-particle attachment in flotation-a brief review. Phys. Chem. Chem. Phys. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Jung, S.; Yoon, R.H. Effect of hydrophobicity on the stability of the wetting films of water formed on gold surfaces. J. Colloid Interface Sci. 2011, 361, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Jung, S.; Yoon, R.H. A fundamental study on the role of collector in the kinetics of bubble-particle interaction. Int. J. Miner. Process. 2012, 106, 37–41. [Google Scholar] [CrossRef]
- Kor, M.; Korczyk, P.M.; Addai-Mensah, J.; Krasowska, M.; Beattie, D.A. Carboxymethylcellulose adsorption on molybdenite: The effect of electrolyte composition on adsorption, bubble–surface collisions, and flotation. Langmuir 2014, 30, 11975–11984. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, J.; Yuan, D.; Shi, C.; Cui, X.; Zhang, H.; Liu, Q.; Zeng, H. Interaction mechanisms between air bubble and molybdenite surface: Impact of solution salinity and polymer adsorption. Langmuir 2017, 33, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pelton, R.; Raegen, A.; Montgomery, M.; Dalnoki-Veress, K. Nanoparticle flotation collectors: Mechanisms behind a new technology. Langmuir 2011, 27, 10438–10446. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pelton, R. Nanoparticle flotation collectors II: The role of nanoparticle hydrophobicity. Langmuir 2011, 27, 11409–11415. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pelton, R.; Montgomery, M.; Cui, Y. Nanoparticle flotation collectors III: The role of nanoparticle diameter. ACS Appl. Mater. Interfaces 2012, 4, 4882–4890. [Google Scholar] [CrossRef] [PubMed]
- Albijanic, B.; Ozdemir, O.; Nguyen, A.V.; Bradshaw, D. A review of induction and attachment times of wetting thin films between air bubbles and particles and its relevance in the separation of particles by flotation. Adv. Colloid Interface Sci. 2010, 159, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.; Bruckard, W.; Koh, P.; Nguyen, A. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Elsevier Pte Ltd.: Atlanta, GA, USA, 2012. [Google Scholar]
- Butt, H.J.; Kappl, M. Surface and Interfacial Forces; WILEY-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Ralston, J.; Dukhin, S. The interaction between particles and bubbles. Colloids Surf. A 1999, 151, 3–14. [Google Scholar] [CrossRef]
- Laskowski, J.K.; Kitchener, J.A. The hydrophilic-hydrophobic transition on silica. J. Colloid Interface Sci. 1969, 29, 670–679. [Google Scholar] [CrossRef]
- Blake, T.D.; Kitchener, J.A. Stability of aqueous films on hydrophobic methylated silica. J. Chem. Soc. Faraday Trans. 1 1972, 68, 1435–1442. [Google Scholar] [CrossRef]
- Israelachvili, J.; Pashley, R. The hydrophobic interaction is long range, decaying exponentially with distance. Nature 1982, 300, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Claesson, P.M.; Ederth, T.; Bergeron, V.; Rutland, M.W. Techniques for measuring surface forces. Adv. Colloid Interface Sci. 1996, 67, 119–183. [Google Scholar] [CrossRef]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent progress in understanding hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.W.; Bowen, P.K. Hydrophobic nano-asperities in control of energy barrier during particle–surface interactions. Surf. Innov. 2015, 3, 164–171. [Google Scholar] [CrossRef]
- Suresh, L.; Walz, J.Y. Effect of surface roughness on the interaction energy between a colloidal sphere and a flat plate. J. Colloid Interface Sci. 1996, 183, 199–213. [Google Scholar] [CrossRef]
- Walz, J.Y. The effect of surface heterogeneities on colloidal forces. Adv. Colloid Interface Sci. 1998, 74, 119–168. [Google Scholar] [CrossRef]
- Tabor, R.F.; Manica, R.; Chan, D.Y.; Grieser, F.; Dagastine, R.R. Repulsive van der Waals forces in soft matter: Why bubbles do not stick to walls. Phys. Rev. Lett. 2011, 106, 064501. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. ζ potential of microbubbles in aqueous solutions: Electrical properties of the gas−water interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef] [PubMed]
- Elmahdy, A.; Mirnezami, M.; Finch, J. Zeta potential of air bubbles in presence of frothers. Int. J. Miner. Process. 2008, 89, 40–43. [Google Scholar] [CrossRef]
- Yang, C.; Dabros, T.; Li, D.; Czarnecki, J.; Masliyah, J.H. Measurement of the zeta potential of gas bubbles in aqueous solutions by microelectrophoresis method. J. Colloid Interface Sci. 2001, 243, 128–135. [Google Scholar] [CrossRef]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Measurement of forces in liquids using a force microscope. Langmuir 1992, 8, 1831–1836. [Google Scholar] [CrossRef]
- Gu, Y.; Li, D. The ζ-potential of glass surface in contact with aqueous solutions. J. Colloid Interface Sci. 2000, 226, 328–339. [Google Scholar] [CrossRef]
- Shi, C.; Cui, X.; Xie, L.; Liu, Q.X.; Chan, D.Y.C.; Israelachvili, J.N.; Zeng, H.B. Measuring forces and spatiotemporal evolution of thin water films between an air bubble and solid surfaces of different hydrophobicity. ACS Nano 2015, 9, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Shi, C.; Xie, L.; Liu, J.; Zeng, H. Probing interactions between air bubble and hydrophobic polymer surface: Impact of solution salinity and interfacial nanobubbles. Langmuir 2016, 32, 11236–11244. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.H.; Royne, A.; Kristiansen, K.; Rapp, M.V.; Das, S.; Gebbie, M.A.; Lee, D.W.; Stock, P.; Valtiner, M.; Israelachvili, J. Developing a general interaction potential for hydrophobic and hydrophilic interactions. Langmuir 2015, 31, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Gui, X.; Karakas, F.; Cao, Y. Role of Collectors and Depressants in Mineral Flotation: A Theoretical Analysis Based on Extended DLVO Theory. Minerals 2017, 7, 223. https://doi.org/10.3390/min7110223
Xing Y, Gui X, Karakas F, Cao Y. Role of Collectors and Depressants in Mineral Flotation: A Theoretical Analysis Based on Extended DLVO Theory. Minerals. 2017; 7(11):223. https://doi.org/10.3390/min7110223
Chicago/Turabian StyleXing, Yaowen, Xiahui Gui, Fırat Karakas, and Yijun Cao. 2017. "Role of Collectors and Depressants in Mineral Flotation: A Theoretical Analysis Based on Extended DLVO Theory" Minerals 7, no. 11: 223. https://doi.org/10.3390/min7110223



