Froth Flotation of Lepidolite—A Review
Abstract
1. Introduction
2. Crystal Structure and Properties of Lepidolite and Major Gangue Minerals
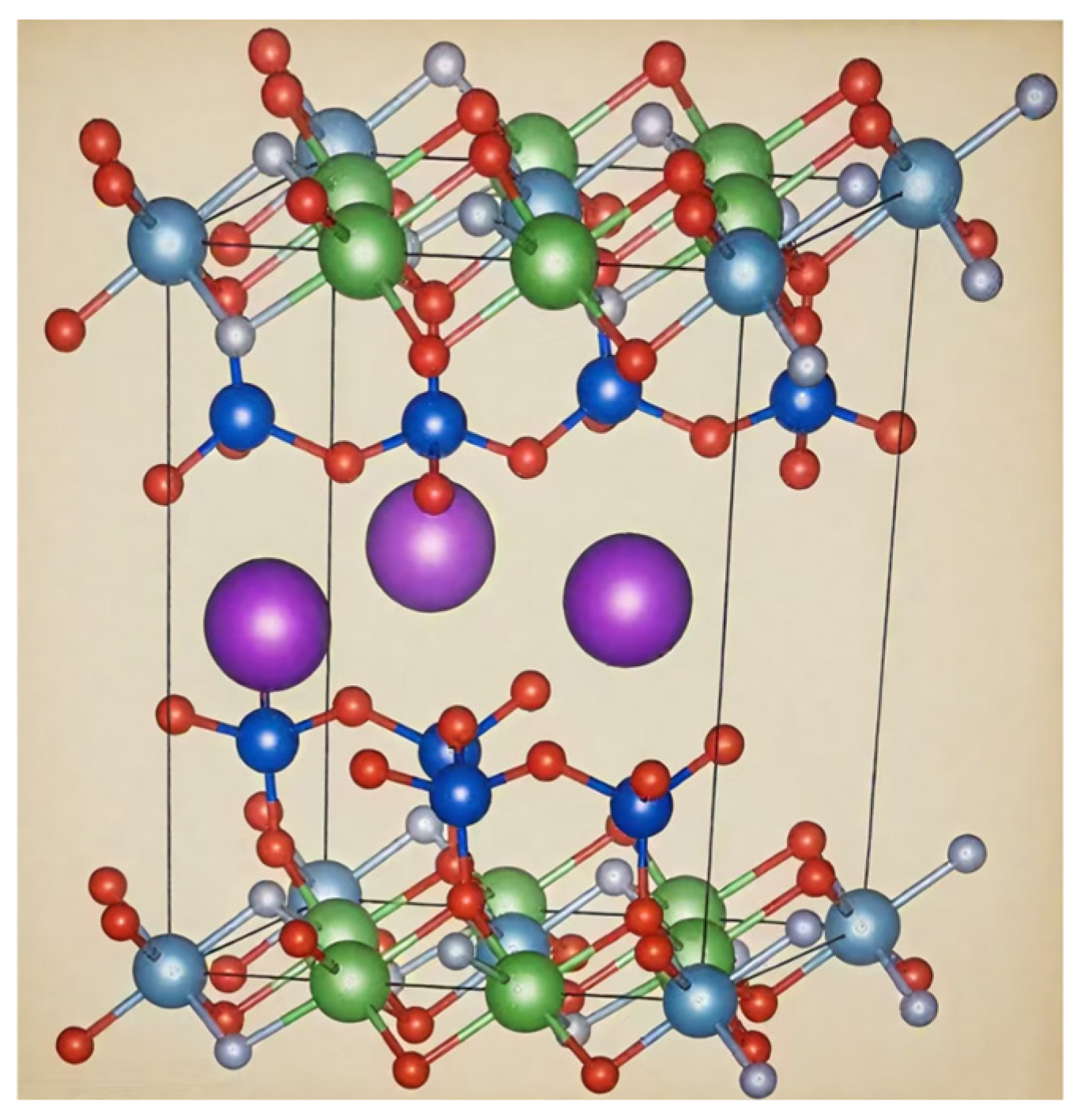
2.1. Crystal Structure and Properties of Lepidolite
2.2. Crystal Structure and Properties of Major Gangue Minerals
2.2.1. Crystal Structure and Properties of Muscovite
2.2.2. Crystal Structure and Properties of Feldspar
2.2.3. Crystal Structure and Properties of Quartz
3. Lepidolite Flotation Process
3.1. Changes in Lepidolite Flotation Systems
3.2. Innovations in New Flotation Technology for Lepidolite
3.3. Industrialization Challenges and Synergistic Sorting Strategies
4. Lepidolite Flotation Reagent
4.1. Collectors
4.1.1. Primary Amine
4.1.2. Secondary Amine
4.1.3. Ether Amine
4.1.4. Gemini Amine Collector
4.2. Combined Collector
4.3. Modifiers
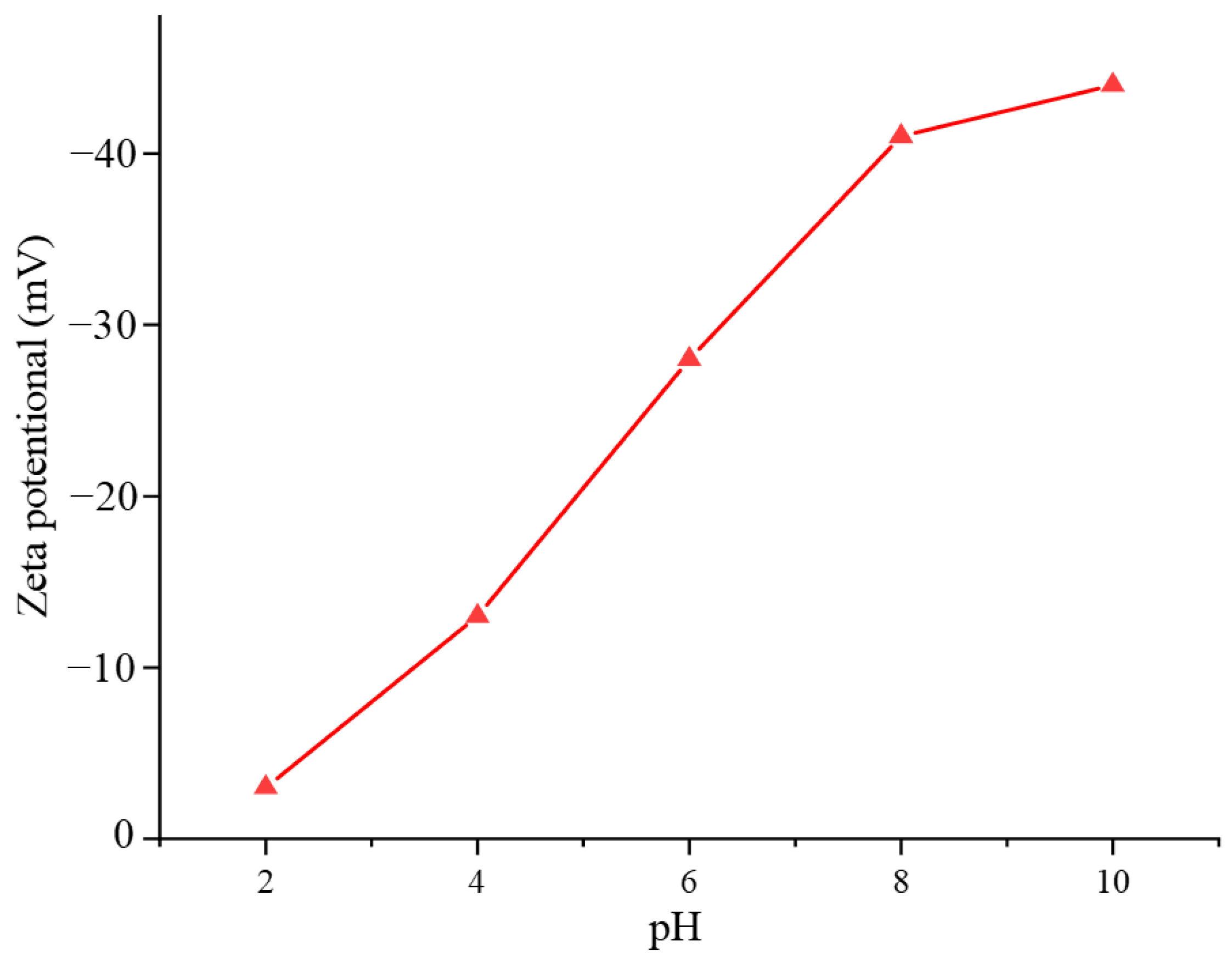
4.3.1. Activators
4.3.2. Depressants
Inorganic Depressants
Organic Depressants
Combined Depressants
5. Challenges in Lepidolite-Gangue Minerals Separation
5.1. Similar Physical Properties of Lepidolite and Gangue Minerals
5.2. Physical Limitations of Microfine Particle Embedding and Susceptibility to Sludging
5.3. Similarity Interference Between Mineral Crystal Chemistry and Surface Properties
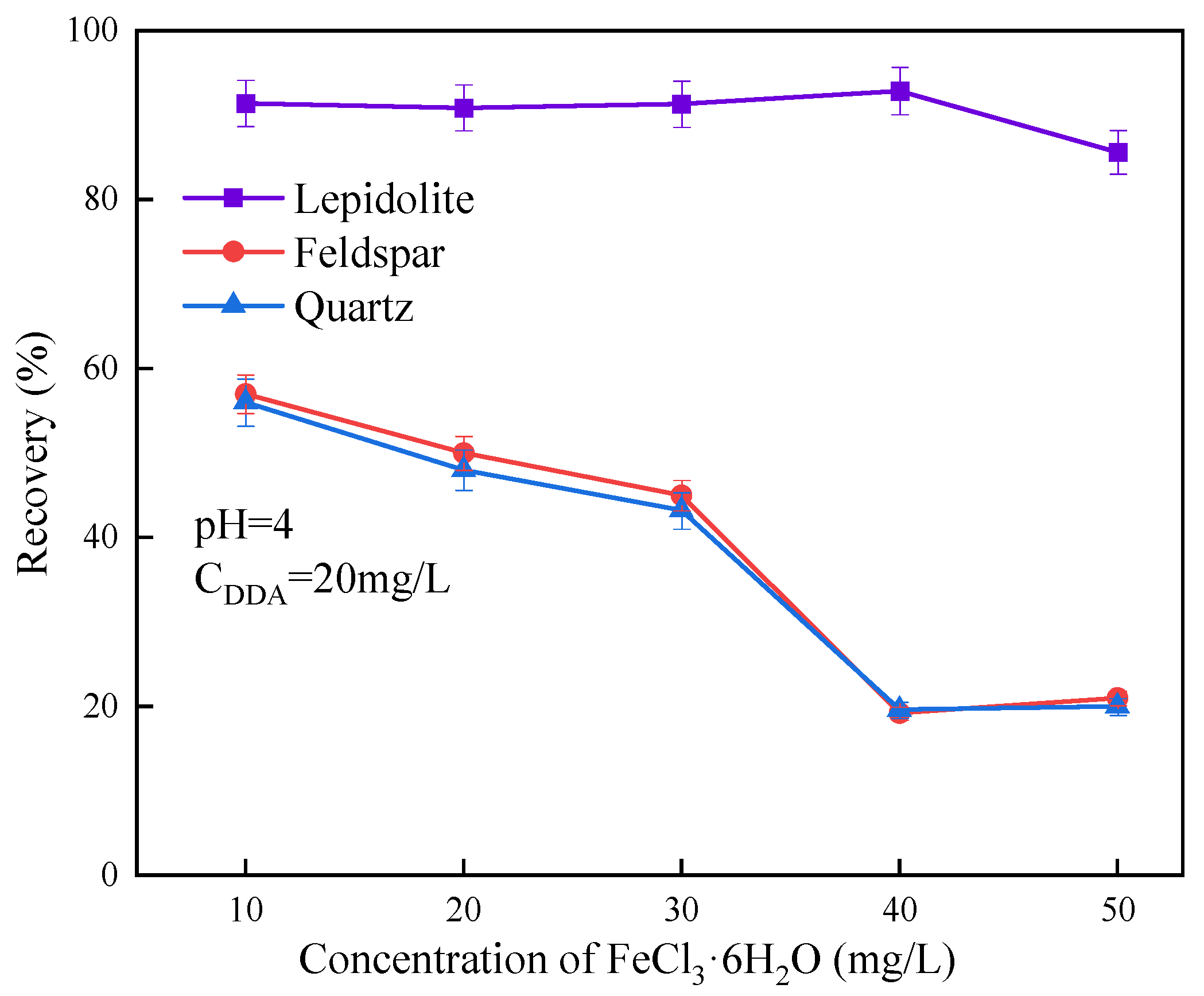
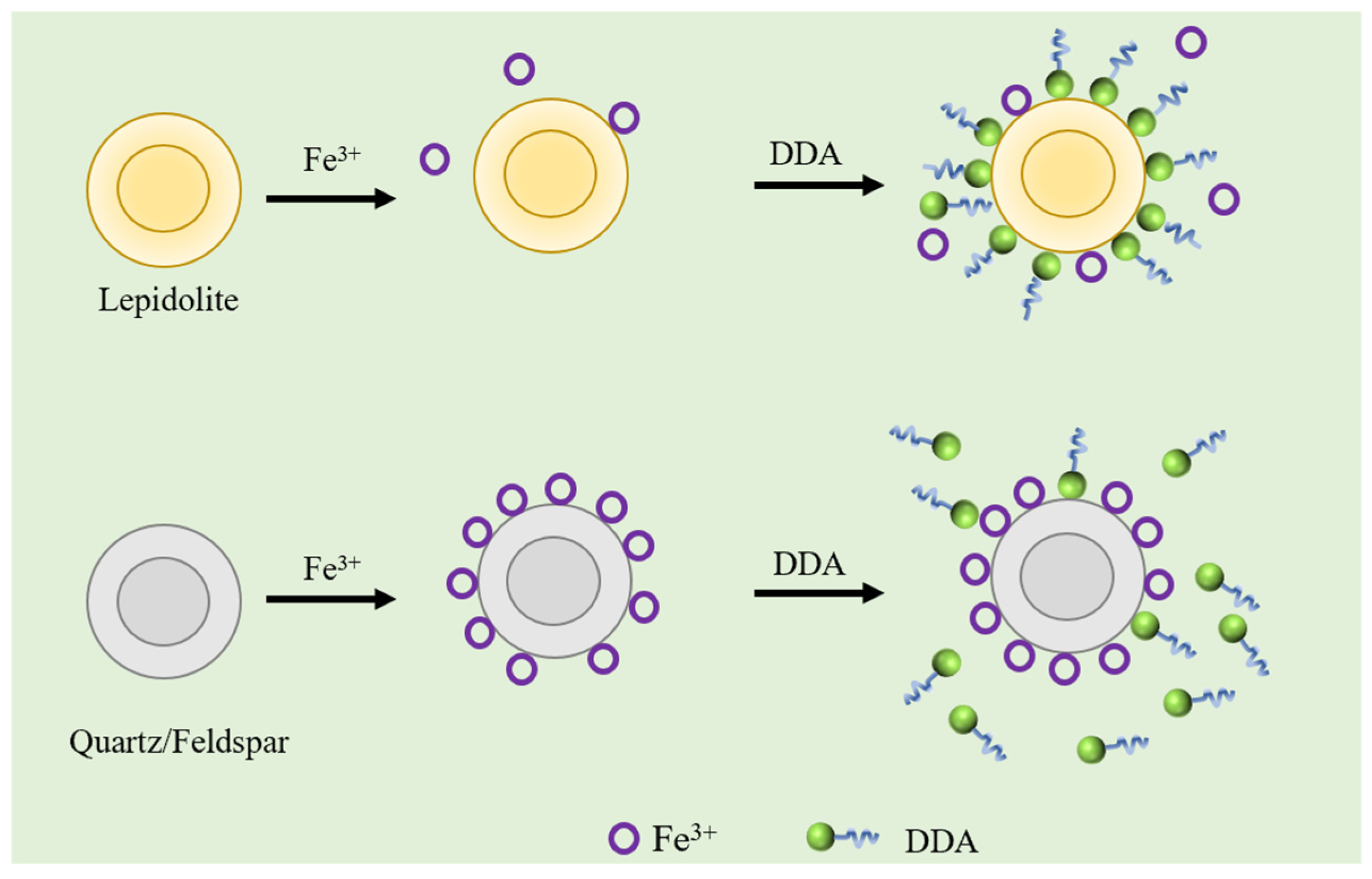
5.4. Interfacial Chemical Contamination Induced by Reactivity of Gangue Minerals
6. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef]
- Speirs, J.; Contestabile, M.; Houari, Y.; Gross, R. The future of lithium availability for electric vehicle batteries. Renew. Sustain. Energy Rev. 2014, 35, 183–193. [Google Scholar] [CrossRef]
- Martins, L.S.; Guimarães, L.F.; Junior, A.B.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric car battery: An overview on global demand, recycling and future approaches towards sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef] [PubMed]
- Bruce, G.C.; Marcoux, L. Large lithium ion batteries for aerospace and aircraft applications. In Proceedings of the Sixteenth Annual Battery Conference on Applications and Advances, Long Beach, CA, USA, 12 January 2001; IEEE: New York, NY, USA; pp. 147–151. [Google Scholar]
- Labbé, J.-F.; Daw, G. Panorama 2011 du marché du lithium. In Bureau de Recherches Géologiques et Minières; Centre D’économie de la Sorbonne: Paris, France, 2012. [Google Scholar]
- Christmann, P.; Gloaguen, E.; Labbé, J.-F.; Melleton, J.; Piantone, P. Global lithium resources and sustainability issues. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–40. [Google Scholar]
- Garrett, D.E. Handbook of Lithium and Natural Calcium Chloride; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Delgado, M.; Valencia, C.; Sánchez, M.; Franco, J.; Gallegos, C. Thermorheological behaviour of a lithium lubricating grease. Tribol. Lett. 2006, 23, 47–54. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Li, G.; Luo, D.; Liu, J.; Zhang, Y.; Wang, X.; Shui, L.; Chen, Z. Aligned sulfur-deficient ZnS1−x nanotube arrays as efficient catalyzer for high-performance lithium/sulfur batteries. Nano Energy 2021, 84, 105891. [Google Scholar] [CrossRef]
- Phark, J.H.; Duarte, S., Jr. Microstructural considerations for novel lithium disilicate glass ceramics: A review. J. Esthet. Restor. Dent. 2022, 34, 92–103. [Google Scholar] [CrossRef]
- Gloaguen, E.; Melleton, J.; Lefebvre, G.; Tourlière, B.; Yart, S.; Gourcerol, B. Ressources Métropolitaines en Lithium et Analyse du Potentiel par Méthodes de Prédictivité; BRGM: Orleans, France, 2018; p. 126. [Google Scholar]
- Sousa, R.; Ramos, V.; Guedes, A.; de Sousa, A.B.; Noronha, F.; Leite, M.M. Flotation of lithium ores to obtain high-grade Li2O concentrates. Are there any mineralogical limitations? Int. J. Min. Mater. Metall. Eng. 2019, 5, 7–18. [Google Scholar]
- Singer, D.A. Future copper resources. Ore Geol. Rev. 2017, 86, 271–279. [Google Scholar] [CrossRef]
- Kavanagh, L.; Keohane, J.; Garcia Cabellos, G.; Lloyd, A.; Cleary, J. Global lithium sources—Industrial use and future in the electric vehicle industry: A review. Resources 2018, 7, 57. [Google Scholar] [CrossRef]
- Helvaci, C.; Mordogan, H.; Çolak, M.; Gündogan, I. Presence and distribution of lithium in borate deposits and some recent lake waters of west-central Turkey. Int. Geol. Rev. 2004, 46, 177–190. [Google Scholar] [CrossRef]
- Friedman-Rudovsky, J. Dreams of a Lithium Empire; American Association for the Advancement of Science: New York, NY, USA, 2011. [Google Scholar]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Ebensperger, A.; Maxwell, P.; Moscoso, C. The lithium industry: Its recent evolution and future prospects. Resour. Policy 2005, 30, 218–231. [Google Scholar] [CrossRef]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global lithium availability: A constraint for electric vehicles? J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- Tkachev, A.; Rundqvist, D.; Vishnevskaya, N. Metallogeny of lithium through geological time. Russ. J. Earth Sci. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, S.; Mishra, P.K. A critical review of recent progress on lithium ion batteries: Challenges, applications, and future prospects. Microchem. J. 2025, 212, 113494. [Google Scholar] [CrossRef]
- Bowell, R.J.; Lagos, L.; de los Hoyos, C.R.; Declercq, J. Classification and characteristics of natural lithium resources. Elements 2020, 16, 259–264. [Google Scholar] [CrossRef]
- Naumov, A.; Naumova, M. Modern state of the world lithium market. Russ. J. Non-Ferr. Met. 2010, 51, 324–330. [Google Scholar] [CrossRef]
- Dorn, F.M.; Peyré, F.R. Lithium as a Strategic Resource. J. Lat. Am. Geogr. 2020, 19, 68–90. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Kuang, G. Recovery of lithium from mineral resources: State-of-the-art and perspectives—A review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Zhuo, W.; Ranxiao, H.; Datian, W.; Fengming, X.; Wei, S.; Dehui, Z.; Yuandong, Z. The basic characteristics and development potential evaluation of salt lake brine-type lithium deposits. Geol. China 2023, 50, 102–117. [Google Scholar]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Reichel, S.; Aubel, T.; Patzig, A.; Janneck, E.; Martin, M. Lithium recovery from lithium-containing micas using sulfur oxidizing microorganisms. Miner. Eng. 2017, 106, 18–21. [Google Scholar] [CrossRef]
- Liu, K. Research Progress in flotation collectors for lepidolite mineral: An overview. Miner. Process. Extr. Metall. Rev. 2024, 45, 728–742. [Google Scholar] [CrossRef]
- Brown, B. The crystal structure of a 3lepidolite. Am. Mineral. 1978, 63, 332–336. [Google Scholar]
- Tian, J.; Xu, L.; Wu, H.; Fang, S.; Deng, W.; Peng, T.; Sun, W.; Hu, Y. A novel approach for flotation recovery of spodumene, mica and feldspar from a lithium pegmatite ore. J. Clean. Prod. 2018, 174, 625–633. [Google Scholar] [CrossRef]
- Choi, J.; Kim, W.; Chae, W.; Kim, S.B.; Kim, H. Electrostatically controlled enrichment of lepidolite via flotation. Mater. Trans. 2012, 53, 2191–2194. [Google Scholar] [CrossRef]
- Choubey, P.K.; Kim, M.-s.; Srivastava, R.R.; Lee, J.-c.; Lee, J.-Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Wang, J.; Guo, H.; Hu, Q.; Peng, W. Extraction of lithium from lepidolite by sulfation roasting and water leaching. Int. J. Miner. Process. 2012, 110, 1–5. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Crumiere, G.; Sousa, R.; Leite, M.M.; de Sousa, A.B.; Korbel, C.; Tripathy, S.K. Separation of lepidolite from hard-rock pegmatite ore via dry processing and flotation. Miner. Eng. 2022, 187, 107768. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Q.; Yang, W.; Miao, Y.; Feng, Q. A novel combined collector with superior selectivity for flotation separation of lepidolite from feldspar: Experimental insight and MD simulation. Sep. Purif. Technol. 2024, 347, 127627. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, Z.; Wei, Q.; Qin, W. Key technologies and development trends for efficient flotation recovery of lepidolite. Green Smart Min. Eng. 2024, 1, 273–288. [Google Scholar] [CrossRef]
- Wei, Q.; Feng, L.; Dong, L.; Jiao, F.; Qin, W. Selective co-adsorption mechanism of a new mixed collector on the flotation separation of lepidolite from quartz. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125973. [Google Scholar] [CrossRef]
- Overhauser, A. Crystal structure of lithium at 4.2 K. Phys. Rev. Lett. 1984, 53, 64. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Wan, H.; Yang, Y.; Yu, H.-z.; Wang, H.-d. Enhanced acid treatment to extract lithium from lepidolite with a fluorine-based chemical method. Hydrometallurgy 2019, 183, 9–19. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, S.; Sun, W.; Hu, L.; Zhong, H.; He, Z. Eco-friendly leaching of rubidium from biotite-containing minerals with oxalic acid and effective removal of Hg2+ from aqueous solution using the leaching residues. J. Clean. Prod. 2021, 306, 127167. [Google Scholar] [CrossRef]
- Mulwanda, J.; Senanayake, G.; Oskierski, H.; Altarawneh, M.; Dlugogorski, B.Z. Leaching of lepidolite and recovery of lithium hydroxide from purified alkaline pressure leach liquor by phosphate precipitation and lime addition. Hydrometallurgy 2021, 201, 105538. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Li, C.; Yi, Y.; Liu, W.; Zhang, L. Energy-efficient and simultaneous extraction of lithium, rubidium and cesium from lepidolite concentrate via sulfuric acid baking and water leaching. Hydrometallurgy 2019, 185, 244–249. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Guo, H.; Hu, Q.; Peng, W.; Wang, J. Extraction of valuable metals from lepidolite. Hydrometallurgy 2012, 117, 116–118. [Google Scholar] [CrossRef]
- Radoslovich, E. The structure of muscovite, KAl2(Si3Al)O10(OH)2. Acta Crystallogr. 1960, 13, 919–932. [Google Scholar] [CrossRef]
- Jackson, W.; West, J. The Crystal Structure of Muscovite—ΚAl2(AlSi3)O10(OH)2. Z. Krist.–Cryst. Mater. 1931, 76, 211–227. [Google Scholar] [CrossRef]
- Güven, Ν.; Burnham, C.W. The crystal structure of 3Τ muscovite. Z. Krist.–Cryst. Mater. 1967, 125, 163–183. [Google Scholar] [CrossRef]
- Tressaud, A.; Labrugère, C.; Durand, E.; Serier, H.; Demyanova, L.P. Surface modification of phyllosilicate minerals by fluorination methods. J. Vac. Sci. Technol. A 2010, 28, 373–381. [Google Scholar] [CrossRef]
- Evans, S.; Raftery, E. X-ray photoelectron diffraction studies of lepidolite. Clay Miner. 1982, 17, 443–452. [Google Scholar] [CrossRef]
- Stevens, R.E. New analyses of lepidolites and their interpretation. Am. Mineral. J. Earth Planet. Mater. 1938, 23, 607–628. [Google Scholar]
- Winchell, A. Further studies of the lepidolite system. Am. Mineral. J. Earth Planet. Mater. 1942, 27, 114–130. [Google Scholar]
- Korbel, C.; Demeusy, B.; Kahou, Z.S.; Filippova, I.V.; Dehaine, Q.; Filippov, L.O. Flowsheet development for the selective flotation of lepidolite from the Beauvoir granite from mineralogical insights. Miner. Eng. 2025, 225, 109207. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Merigot, K.; Quadir, Z.; Rickard, W.D.A.; Evans, N.J.; McDonald, B.J.; Catovic, E.; Spitalny, P. Applications of advanced analytical and mass spectrometry techniques to the characterisation of micaceous lithium-bearing ores. Miner. Eng. 2018, 116, 182–195. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.; Zhang, J.; Cao, Y.; He, J.; Li, G. Selective Inhibition Mechanisms of Fe (III) in the Flotation of Lepidolite. Minerals 2024, 14, 851. [Google Scholar] [CrossRef]
- He, J.; Xu, H.; Yang, B.; Gong, W.; Zhang, Z.; Shan, Y.; Song, T. Adsorption properties of an efficient combined collector and its enhanced separation of lepidolite from feldspar. J. Ind. Eng. Chem. 2025, 148, 797–808. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Hu, Y.; Yu, X.; He, G.; Fu, W. Froth flotation separation of lepidolite ore using a new Gemini surfactant as the flotation collector. Sep. Purif. Technol. 2022, 282, 119122. [Google Scholar] [CrossRef]
- Belyankina, Y.D.; Petrov, V. Geochemical role of micas in mineral associations: Classification, chemistry, and genesis of micas. Int. Geol. Rev. 1983, 25, 993–1003. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Kile, D.E.; Poppi, M. Crystal structure and crystal chemistry of lithium-bearing muscovite-2M1. Can. Mineral. 2001, 39, 1171–1180. [Google Scholar] [CrossRef]
- Chardon, E.S.; Livens, F.R.; Vaughan, D.J. Reactions of feldspar surfaces with aqueous solutions. Earth-Sci. Rev. 2006, 78, 1–26. [Google Scholar] [CrossRef]
- Smith, J.V. Feldspar Minerals: 1 Crystal Structure and Physical Properties; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Blum, A.E.; Stillings, L.L. Feldspar dissolution kinetics. Rev. Mineral. 1995, 31, 291–352. [Google Scholar]
- Heyes, G.; Allan, G.; Bruckard, W.; Sparrow, G. Review of flotation of feldspar. Miner. Process. Extr. Metall. 2012, 121, 72–78. [Google Scholar] [CrossRef]
- Ribbe, P. Chemistry, structure and nomenclature of feldspars. Feldspar Mineral. 1983, 2, 1–20. [Google Scholar]
- Smith, J.V. Feldspar Minerals: 2 Chemical and Textural Properties; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Harris, M.J.; Salje, E.K.; Guttler, B.K.; Carpenter, M.A. Structural states of natural potassium feldspar: An infrared spectroscopic study. Phys. Chem. Miner. 1989, 16, 649–658. [Google Scholar] [CrossRef]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and mineral chemistry of quartz: A review. Mineral. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Pan, X.; Li, S.; Li, Y.; Guo, P.; Zhao, X.; Cai, Y. Resource, characteristic, purification and application of quartz: A review. Miner. Eng. 2022, 183, 107600. [Google Scholar] [CrossRef]
- Rahimi, M.; Dehghani, F.; Rezai, B.; Aslani, M.R. Influence of the roughness and shape of quartz particles on their flotation kinetics. Int. J. Miner. Metall. Mater. 2012, 19, 284–289. [Google Scholar] [CrossRef]
- Larsen, E.; Kleiv, R. Flotation of quartz from quartz-feldspar mixtures by the HF method. Miner. Eng. 2016, 98, 49–51. [Google Scholar] [CrossRef]
- Swanepoel, A.J.; Rees, D.; Renton, K.; Swanepoel, C.; Kromhout, H.; Gardiner, K. Quartz exposure in agriculture: Literature review and South African survey. Ann. Occup. Hyg. 2010, 54, 281–292. [Google Scholar] [CrossRef]
- Raman, C.; Nedungadi, T. The α-β transformation of quartz. Nature 1940, 145, 147. [Google Scholar] [CrossRef]
- Bhappu, R.; Fuerstenau, D. Recovery of valuable minerals from pegmatitic ores. In Circular; New Mexico Bureau of Geology & Mineral Resources: Socorro, NM, USA, 1964; Volume 70, p. 29. [Google Scholar]
- Bai, Y.; Cui, W.; Gao, Y.; Wen, W.; Sun, Y.; Yan, P. Synergistic mechanism of mixed cationic/anionic collectors on lepidolite flotation from the perspective of improving the performance of flotation foam. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130354. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, F.; Qin, W.; Wei, Q. Collision–attachment law of lepidolite, feldspar and quartz with bubbles in the combined cationic and anionic collector system. Physicochem. Probl. Miner. Process. 2022, 58, 155324. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Q.; Jiao, F.; Qin, W. Role of nanobubbles on the fine lepidolite flotation with mixed cationic/anionic collector. Powder Technol. 2023, 427, 118785. [Google Scholar] [CrossRef]
- Chen, H.; Sun, A.; Liao, M.; Zhao, H.; Zhong, H. A Method for Flotation of Lepidolite Without Desliming. CN Patent CN115608520A, 17 January 2023. [Google Scholar]
- Ai, W.; Xiong, H.; Yang, J.; Yan, Z.; Yi, Y.; Xiong, S. A Method for Recovering Lepidolite Concentrate from Lepidolite Ore Tailings. CN Patent CN114713364B, 24 November 2023. [Google Scholar]
- Peng, S.; Wang, Z.; Zhao, H.; Liu, F.; Wang, Q.; Lu, H.; Wei, S.; Dou, H.; Wang, B.; Bai, Z.; et al. Experimental research on compre-hensive mineral processing of lepidolite and feldspar in Hunan Province. Ceramics 2024, 1, 38–41. [Google Scholar] [CrossRef]
- Sousa, R.; Ramos, V.; Guedes, A.; Noronha, F.; Botelho de Sousa, A.; Machado Leite, M.; Seltmann, R.; Dolgopolova, A. The Alvarrões-Gonçalo Li project: An example of sustainable lithium mining. Adv. Geosci. 2018, 45, 1–5. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, S.; Cheng, C.; Wang, H.; Liu, R.; Hu, Y.; He, G.; Yu, X.; Fu, W. Recycling lepidolite from tantalum–niobium mine tailings by a combined magnetic–flotation process using a novel gemini surfactant: From tailings dams to the “bling” raw material of lithium. ACS Sustain. Chem. Eng. 2020, 8, 18206–18214. [Google Scholar] [CrossRef]
- Lombe, W. The Surface Chemistry Spodumene, Lepidolite and in the Presence of and Flotation of Associate d Silicates Dodecylamine. Ph.D. Thesis, University of London, London, UK, 1983. [Google Scholar]
- Yang, Z.; Xu, H.; Tang, X.; Zhou, H.; Xie, T.; Shen, L.; Guo, L.; Luo, X. Application of a novel mixed anionic/cationic collector in the selective flotation separation of lepidolite and quartz. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134919. [Google Scholar] [CrossRef]
- Deng, Y.; Ou, L. Mechanism for the selective separation of lepidolite from albite by sodium lauroyl glutamate as a green environment collector. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134922. [Google Scholar] [CrossRef]
- Xu, R.; Liu, Y.; Sun, N.; Kang, J.; Sun, W.; Tang, H.; Wang, L. The activation role of Mg2+ in the lepidolite flotation using NaOL. Sep. Purif. Technol. 2024, 351, 128035. [Google Scholar] [CrossRef]
- Silva, E.M.S.; Peres, A.E.C.; Silva, A.C.; Florêncio, D.L.; Caixeta, V.H. Sorghum starch as depressant in mineral flotation: Part 2—Flotation tests. J. Mater. Res. Technol. 2019, 8, 403–410. [Google Scholar] [CrossRef]
- Ayedzi, L.D.; Zanin, M.; Skinner, W.; Wood, G.B.A. Minimizing entrainment recovery of ultrafine silicate minerals in pentlandite flotation using carboxymethyl cellulose. Miner. Eng. 2024, 217, 108942. [Google Scholar] [CrossRef]
- Ma, S.; Deng, J.; Xing, D.; Huang, G.; Cheng, T.; Zhang, D.; Deng, J.; Zhong, W.; Leng, X.; Quan, B. Study on the influence of graphite and sphalerite flotation separation by carboxymethyl cellulose. Sep. Purif. Technol. 2025, 355, 129577. [Google Scholar] [CrossRef]
- Cruz, D.G.d.; Alves, M.O.; Lima, R.M.F. Effect of Sodium Hexametaphosphate on Flotation of Quartz and Hematite with Starch/Etheramine in the Presence of Ca and Mg Ions. Miner. Process. Extr. Metall. Rev. 2024, 45, 950–956. [Google Scholar] [CrossRef]
- Kumar, S.J.; Han-Seung, L. Enhanced corrosion resistance properties of 15Al-85Zn coating by post-treatment with sodium hexa-meta phosphate in saline solution. Corros. Sci. 2024, 226, 111684. [Google Scholar]
- Silva, J.P.; Baltar, C.; Gonzaga, R.; Peres, A.; Leite, J. Identification of sodium silicate species used as flotation depressants. Min. Metall. Explor. 2012, 29, 207–210. [Google Scholar] [CrossRef]
- Krishnan, R.; Gopan, G. A comprehensive review of lithium extraction: From historical perspectives to emerging technologies, storage, and environmental considerations. Clean. Eng. Technol. 2024, 20, 100749. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Xu, J.; Wang, H.; Chao, W.; Lu, W.; Zhou, B.; Yang, S.; Hu, N. Lithium occurrences and enrichment of granite regolith-hosted Li deposits in Jiangxi Province, South China: An example of the Xikeng Li deposit. Ore Geol. Rev. 2024, 169, 106104. [Google Scholar] [CrossRef]
- Ramirez, A.; Rojas, A.; Gutierrez, L.; Laskowski, J.S. Sodium hexametaphosphate and sodium silicate as dispersants to reduce the negative effect of kaolinite on the flotation of chalcopyrite in seawater. Miner. Eng. 2018, 125, 10–14. [Google Scholar] [CrossRef]
- Qin, W.; Hu, J.; Zhu, H.; Jiao, F.; Jia, W.; Han, J.; Chen, C. Effect of depressants on flotation separation of magnesite from dolomite and calcite. Int. J. Min. Sci. Technol. 2023, 33, 83–91. [Google Scholar] [CrossRef]
- Ding, H.; Lin, H.; Deng, Y. Depressing effect of sodium hexametaphosphate on apatite in flotation of rutile. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2007, 14, 200–203. [Google Scholar] [CrossRef]
- Matveeva, T.; Gromova, N.; Lantsova, L. Effect of tannin on compound collector adsorption and stibnite and arsenopyrite flotation from complex ore. J. Min. Sci. 2017, 53, 1108–1115. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; Guo, J.; Yang, B.; Sun, H.; Yin, W.; Wang, Y.; Fu, Y. Role of tannin pretreatment in flotation separation of magnesite and dolomite. Int. J. Miner. Metall. Mater. 2024, 31, 452–461. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Y.; Zhu, Y.; Sun, N.; Wang, W. Investigating the performance of oxalic acid for separating bastnaesite from calcium-bearing gangue minerals based on experiment and theoretical calculation. Miner. Eng. 2021, 170, 107047. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, S.; Han, Y.; Li, Y. The effects of various activators on flotation performance of lime-depressed pyrrhotite. Can. Metall. Q. 2019, 58, 132–139. [Google Scholar] [CrossRef]
- Li, H.; Chai, W.; Cao, Y.; Yang, S. Flotation enhancement of low-grade bauxite using oxalic acid as surface pretreatment agent. Appl. Surf. Sci. 2022, 577, 151964. [Google Scholar] [CrossRef]
- Yang, X.; Bao, J.; Liu, Z.; Wu, D.; Cao, Y.; Xu, C.C.; Hao, H.; Zhang, Y. Synthesis of sulfomethylated depolymerized alkali lignin and its application in scheelite flotation. J. Mol. Liq. 2025, 424, 127102. [Google Scholar] [CrossRef]
- Qian, H.; Bao, J.; Shen, C.; Wu, D.; Wang, J.; Hao, H.; Zhang, Y. Improved flotation separation of scheelite from calcite by sulfomethylated kraft lignin. Materials 2023, 16, 4690. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Lei, M.; Liu, Y.; Du, X.; Geng, L. Flotation separation test of zinnwaldite in Yichun of Jiangxi. Non Met. Ores 2019, 42, 64–67. [Google Scholar]
- Ahmad, S.S.; Yongjun, S.; Yu, Z.; Hongtao, Z.; Lianjie, Z. Texture and Trace Element Geochemistry of Quartz: A Review. Minerals 2022, 12, 1042. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, L.; Huang, Y.; Fu, L.; Ma, L.; Chen, K.; Gu, Z. Review on K-Feldspar Mineral Processing for Extracting Metallic Potassium as a Fertilizer Resource. Minerals 2024, 14, 168. [Google Scholar] [CrossRef]
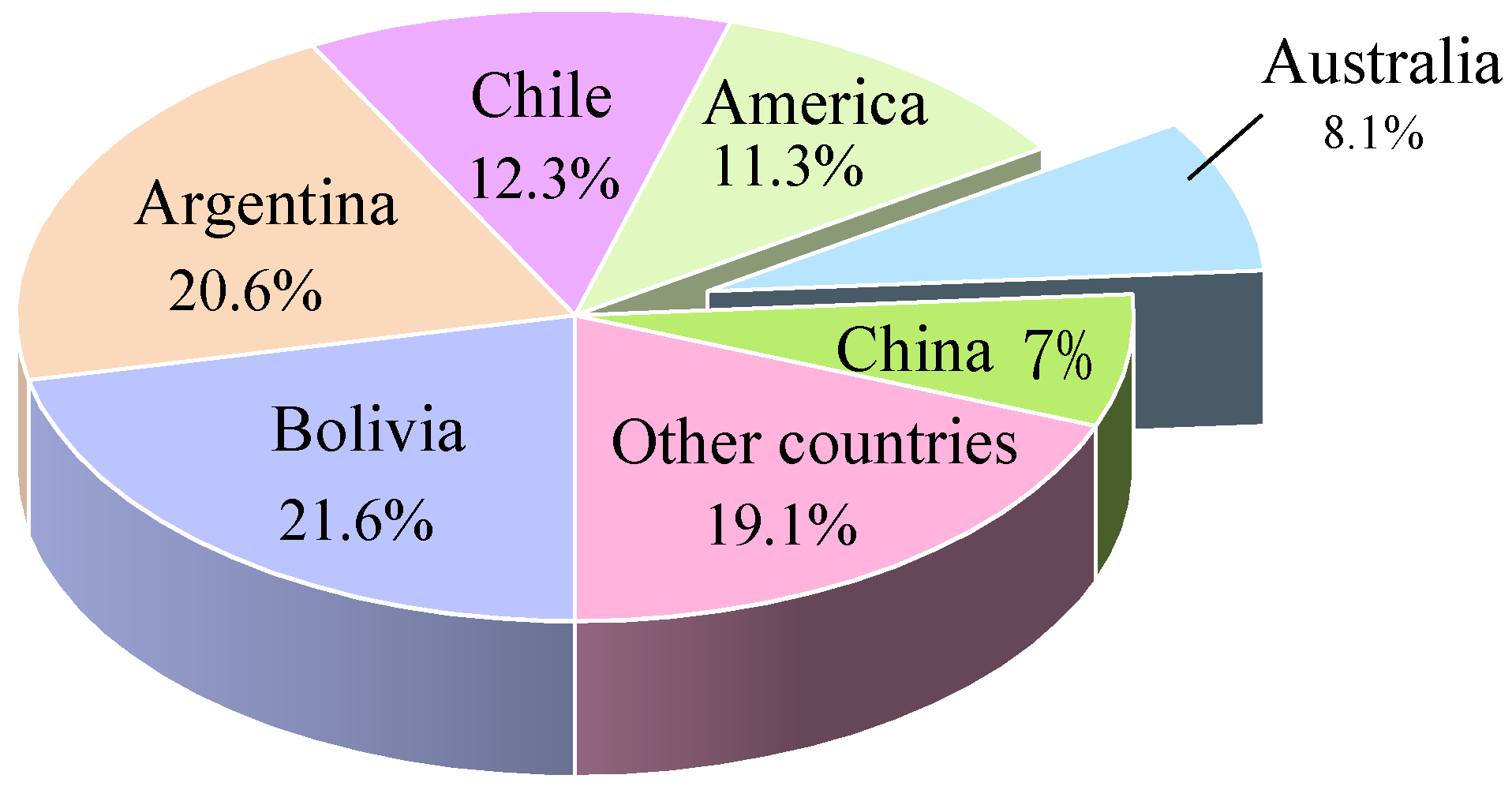




| Mine Production | Reserve | |||
|---|---|---|---|---|
| 2022 | 2023 | 2024 (Prediction) | ||
| Australia | 16,000 | 22,400 | 27,000 | 1,500,000 |
| Argentina | 6400 | 7200 | 9400 | 2,000,000 |
| Chile | 15,300 | 18,000 | 22,500 | 7,600,000 |
| China | 3300 | 6300 | 9000 | 3,300,000 |
| Zimbabwe | 2000 | 3500 | 5200 | 22,000 |
| Brazil | 550 | 600 | 750 | 47,000 |
| World total | 43,550 | 62,200 | 73,850 | 14,000,000 |
| Polytype | Crystal System | Frequency | Space Group |
|---|---|---|---|
| 2M1 | Monoclinic | Common | C2/c |
| 2M2 | Monoclinic | Uncommon | C2/c |
| 3T | Trigonal | Rare | P3112 |
| Minerals | Colors | Density (g/cm3) | Chemical Formula | Crystal Structure |
|---|---|---|---|---|
| Lepidolite [50] | Light white, lavender or purple | 2.6–3.0 | K(Li,Al)3(Al,Si)4O10(F,OH)2 | Monoclinic Crystal |
| Muscovite [48] | Colorless, white or gray | 2.6–3.0 | K2Al4(Si6Al2)O20(OH)4 | Monoclinic crystals |
| Quartz [68] | Colorless or creamy white | 2.6 | SiO2 | Tetragonal Crystal System |
| Feldspar [66] | Colorless or off-white | 2.6–2.75 | (Na,K)(Al,Si)4O8 | Monoclinic Crystal |
| Flotation Processes | Characteristics |
|---|---|
| Acid flotation [74] | Lower cost, but serious equipment corrosion, high foam viscosity |
| Neutral flotation [75] | Environmentally friendly, but mud interference, need to rely on dispersants to inhibit sludge |
| Desliming flotation [78] | Simple process, good results, but irreversible loss of fine-grained lepidolite |
| Selective flocculation flotation [78] | Strengthen the sorting efficiency, reduce the loss of desliming, high sensitivity to chemicals, industrialization is difficult |
| Combined reciprocal flotation [80] | Suitable for complex lepidolite system, high comprehensive recovery rate, complex process, industrialization difficulties |
| Reagent Name | Molecular Structure | Characteristics |
|---|---|---|
| Primary amine [58] | 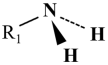 | High collectability |
| Secondary amine [34] |  | Low consumption |
| Tertiary amine [81] |  | Low sensitivity to slurry |
| Quaternary ammonium salt [82] | 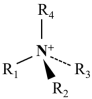 | Low sensitivity to slurry, good foaming properties |
| Type | Name | Depressing Mechanism |
|---|---|---|
| Inorganic depressant [92] | Sodium Silicate | Hydrolyzed to form colloidal silicate polymers, hindering the adsorption of traps. |
| Sodium hexametaphosphate | Generation of refractory complexes on the surface of chalcopyrite, hindering the adsorption of traps. | |
| Organic Depressants [98,99] | Tannin | Generation of strong hydrophilic film on mineral surface |
| Oxalic acid | Generation of insoluble metal complexes, enhancing hydrophilicity of minerals | |
| Lignin | Disperses sludge and acts selectively on gangue minerals | |
| Combined depressant [105] | Tartaric acid-starch-kerosene | Combines the advantages of various depressants to increase the difference in floatability between lepidolite and chondrites. |
| Sodium hexametaphosphate-sodium silicate | ||
| Sodium Carbonate-Sodium Silicate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Feng, B.; Jiang, L. Froth Flotation of Lepidolite—A Review. Minerals 2025, 15, 750. https://doi.org/10.3390/min15070750
Yang X, Feng B, Jiang L. Froth Flotation of Lepidolite—A Review. Minerals. 2025; 15(7):750. https://doi.org/10.3390/min15070750
Chicago/Turabian StyleYang, Xusheng, Bo Feng, and Longxia Jiang. 2025. "Froth Flotation of Lepidolite—A Review" Minerals 15, no. 7: 750. https://doi.org/10.3390/min15070750
APA StyleYang, X., Feng, B., & Jiang, L. (2025). Froth Flotation of Lepidolite—A Review. Minerals, 15(7), 750. https://doi.org/10.3390/min15070750





