Species-Specific Variations in the Nutritional Quality of Southern Ocean Phytoplankton in Response to Elevated pCO2
Abstract
:1. Introduction
2. Materials and Methods
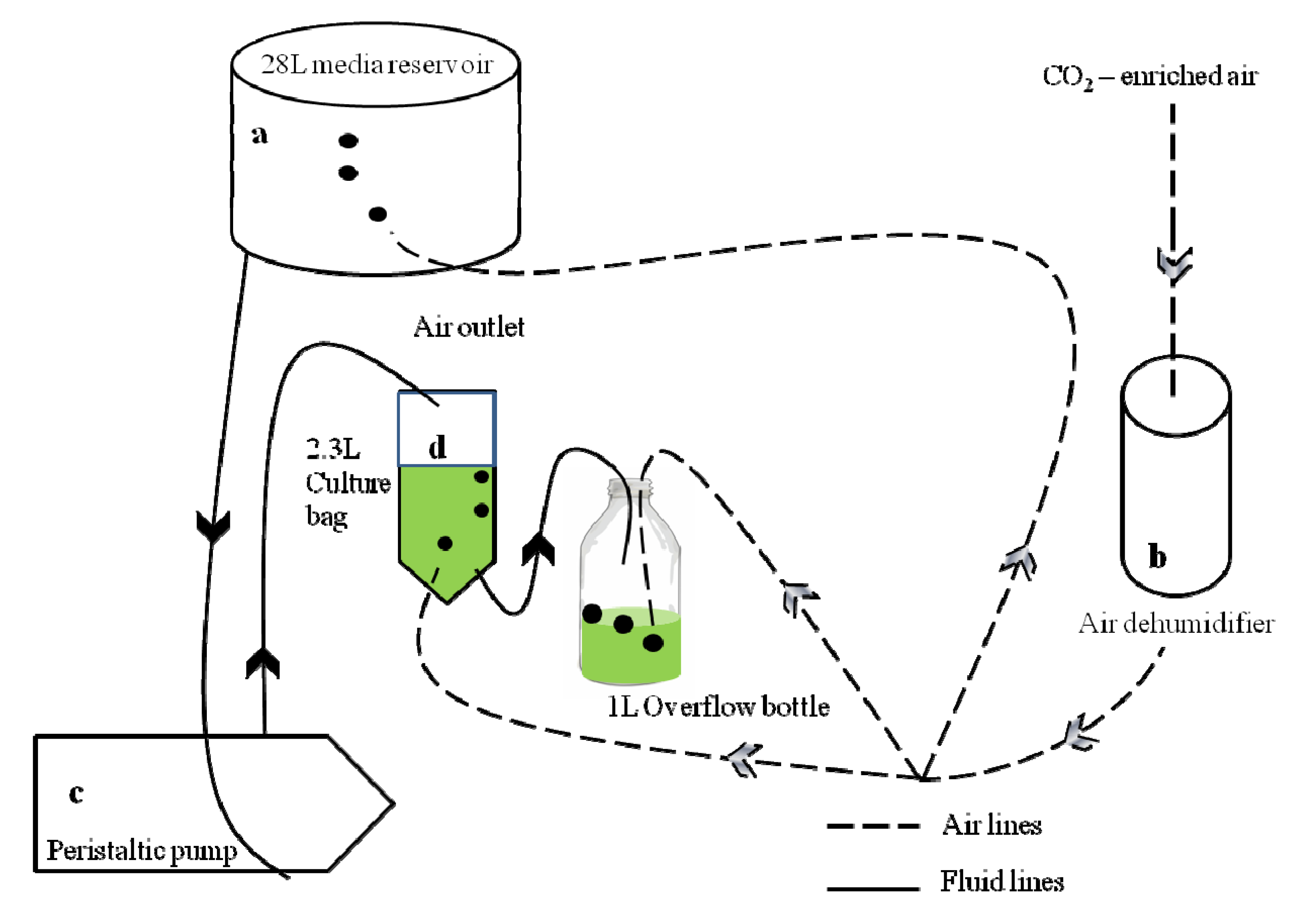
2.1. Experimental Conditions
| Species | Acclimation in generations | Nutrient concentrations (μM) | CO2 concentration (µatm) | |||||
|---|---|---|---|---|---|---|---|---|
| NOx | Si | P | Ambient | 1.5× | 2× | 2.5× | ||
| Pyramimonas gelidicola | ~5 | 17.5 (±4.5) | 24.4 (±5.2) | 5.7 (±1.3) | 400 (±41) | 612 (±54) | 806 (±80) | 977 (±106) |
| pH ± SD | 8.04 (±0.04) | 7.87 (±0.03) | 7.75 (±0.04) | 7.67 (±0.04) | ||||
| Phaeocystis antarctica | ~6 | 17.5 (±4.5) | 24.4 (±5.2) | 5.7 (±1.3) | 413 (±31) | 644 (±62) | - | 993 (±83) |
| pH ± SD | 8.02 (±0.03) | 7.86 (±0.04) | - | 7.67 (±0.03) | ||||
| Gymnodinium sp. | ~6 | 20.9 (±1.8) | 17.6 (±0.5) | 1.5 (±0.2) | 458 (±48) | 580 (±49) | 797 (±79) | 973 (±82) |
| pH ± SD | 8.02 (±0.04) | 7.89 (±0.03) | 7.75 (±0.04) | 7.70 (±0.03) | ||||
2.2. Carbonate Chemistry
2.3. Physiological and Biochemical Analyses
2.3.1. Cell Abundance
2.3.2. Cell Dimensions
2.3.3. Pigments
2.3.4. Cellular Carbohydrates and C:N Ratio
2.3.5. Fatty Acid Analysis
2.3.6. Statistical Analysis
3. Results and Discussion
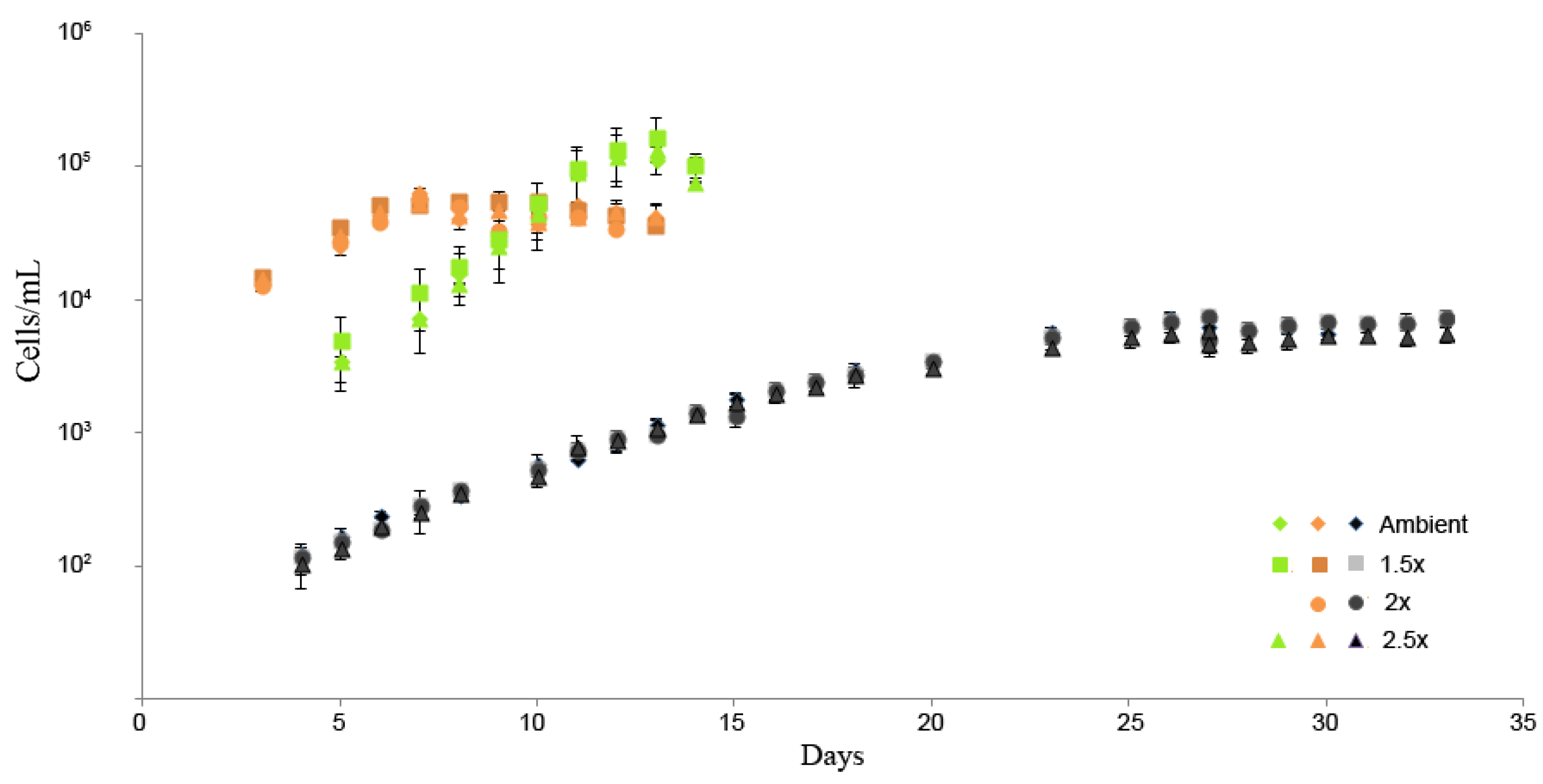
3.1. Cell Dimensions and Growth Rates
| CO2 treatment | Ambient | 1.5× | 2× | 2.5× |
|---|---|---|---|---|
| Pyramimonas gelidicola | ||||
| Length (µm) | 6.72 (±1.01) | 7.04 (±1.23) | 6.82 (±1.03) | 7.01 (±1.37) |
| Width (µm) | 5.58 (±0.59) | 5.80 (±0.64) | 5.73 (±0.55) | 5.70 (±0.72) |
| n = 60 | n = 60 | n = 60 | n = 60 | |
| Gymnodinium sp. | ||||
| Length (µm) | 16.62 (±2.60) | 17.21 (±2.65) | 17.24 (±2.44) | 16.54 (±2.42) |
| Width (µm) | 8.16 (±1.08) | 8.59 (±1.49) | 8.97 (±1.63) | 8.07 (±1.37) |
| n = 45 | n = 45 | n = 58 | n = 57 | |
3.2. Pigments
| CO2 treatment | Ambient | 1.5× | 2× | 2.5× |
|---|---|---|---|---|
| Pyramimonas gelidicola | ||||
| Chl a | 0.62 (±0.18) | 0.47 (±0.11) | 0.53 (±0.14) | 0.70 (±0.06) |
| Chl a + b | 0.75 (±0.22) | 0.58 (±0.14) | 0.66 (±0.18) | 0.86 (±0.07) |
| (ant + violax + zea)/Chl a | 0.32 (±0.02) | 0.32 (±0.03) | 0.36 (±0.01) | 0.32 (±0.01) |
| γ-carotene/Chl a | 0.04 (±0.004) | 0.04 (±0.001) | 0.04 (±0.002) | 0.04 (±0.001) |
| Lutein/Chl a | 0.12 (±0.01) | 0.14 (±0.03) | 0.16 (±0.01) | 0.12 (±0.001) |
| n = 3 | n = 3 | n = 3 | n = 3 | |
| Phaeocystis antarctica | ||||
| Chl a | 0.15 (±0.06) | 0.10 (±0.02) | - | 0.08 (±0.02) |
| Chl a + c2 + c3 | 0.17 (±0.08) | 0.12 (±0.03) | - | 0.09 (±0.03) |
| (Ddx + dtx)/Chl a | 0.49 (±0.18) | 0.68 (±0.18) | - | 0.86 (±0.01) |
| n = 3 | n = 2 | - | n = 2 | |
| Gymnodinium sp. | ||||
| Chl a | 1.71 (±0.84) | 2.32 (±0.06) | 2.07 (±0.22) | 1.95 (±0.71) |
| Chl a + c2 | 1.80 (±0.89) | 2.43 (±0.06) | 2.19 (±0.23) | 2.04 (±0.74) |
| (Ddx + dtx)/Chl a | 0.82 (±0.12) | 0.77 (±0.07) | 0.77 (±0.11) | 0.84 (±0.09) |
| β-carotene/Chl a | 0.07 (±0.01) | 0.06 (±0.02) | 0.04 (±0.02) | 0.04 (±0.04) |
| n = 3 | n = 3 | n = 3 | n = 3 | |
3.3. Elemental Composition
| CO2 treatment | Ambient | 1.5× | 2× | 2.5× |
|---|---|---|---|---|
| Pyramimonas gelidicola | ||||
| %C | 10.7 (±2.2) | 6.0 (±2.6) | 8.1 (±9.8) | 7.7 (±3.3) |
| %N | 1.1 (±0.1) | 0.7 (±0.0) | 0.9 (±0.6) | 0.9 (±0.1) |
| C:N | 9.9 (±2.4) | 8.6 (±4.1) | 6.6 (±5.6) | 8.5 (±3.9) |
| n = 3 | n = 3 | n = 3 | n = 3 | |
| Phaeocystis antarctica | ||||
| %C | 11.8 (±5.4) | 13.0 (±5.4) | - | 9.4 (±5.3) |
| %N | 1.2 (±0.03) | 1.1 (±0.1) | - | 1.4 (±0.6) |
| C:N | 9.4 (±4.1) | 11.6 (±4.1) | - | 6.7 (±1.0) |
| n = 3 | n = 2 | - | n = 2 | |
| Gymnodinium sp. | ||||
| %C | 14.1 (±2.6) | 14.2 (±2.8) | 15.6 (±4.5) | 10.5 (±2.5) |
| %N | 2.0 (±0.2) | 2.2 (±0.6) | 2.0 (±0.3) | 1.8 (±0.4) |
| C:N | 6.9 (±0.9) | 6.8 (±1.5) | 7.6 (±1.1) | 5.7 (±0.3) |
| n = 3 | n = 3 | n = 3 | n = 3 | |
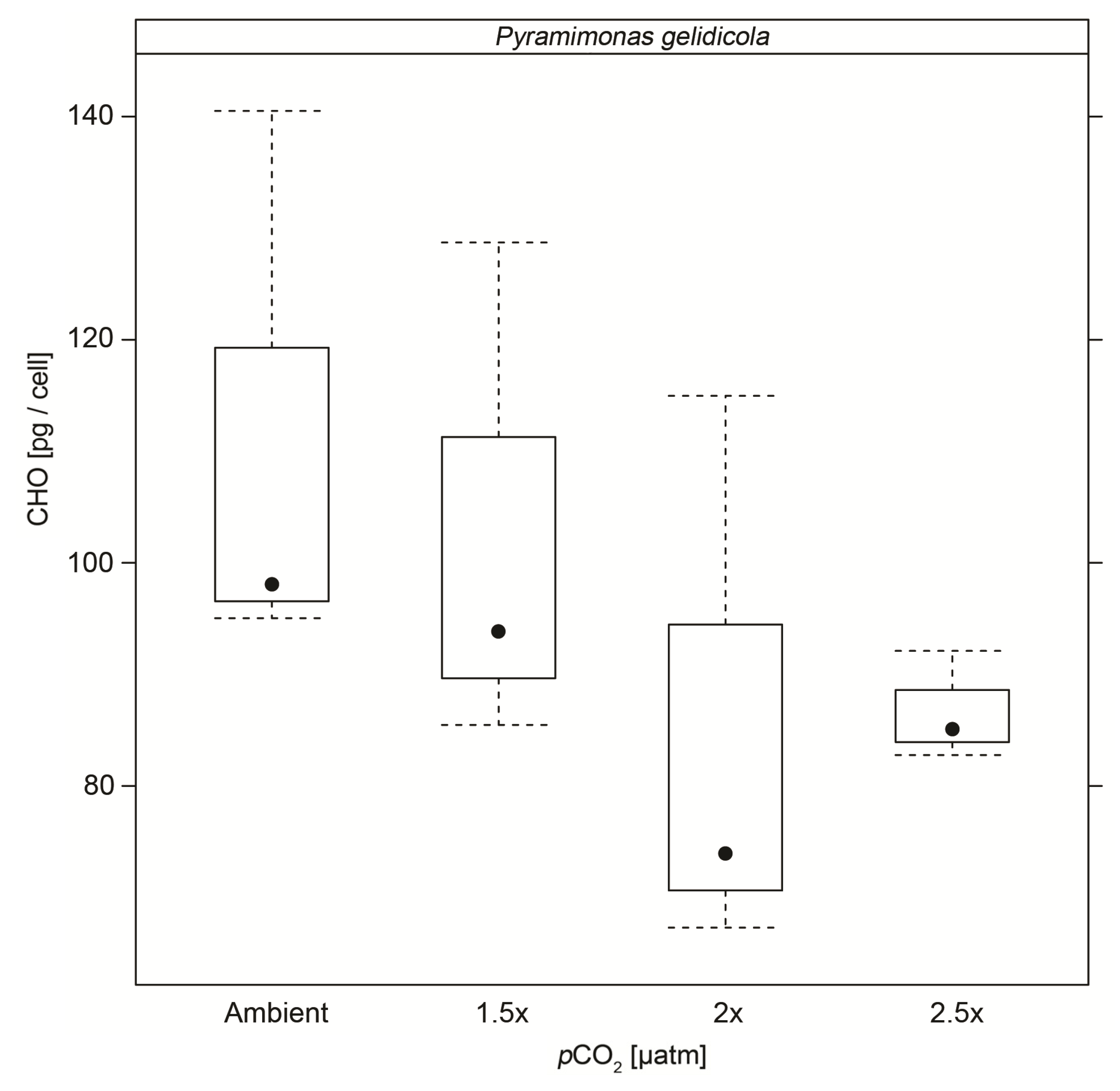
3.4. Particulate Carbohydrates
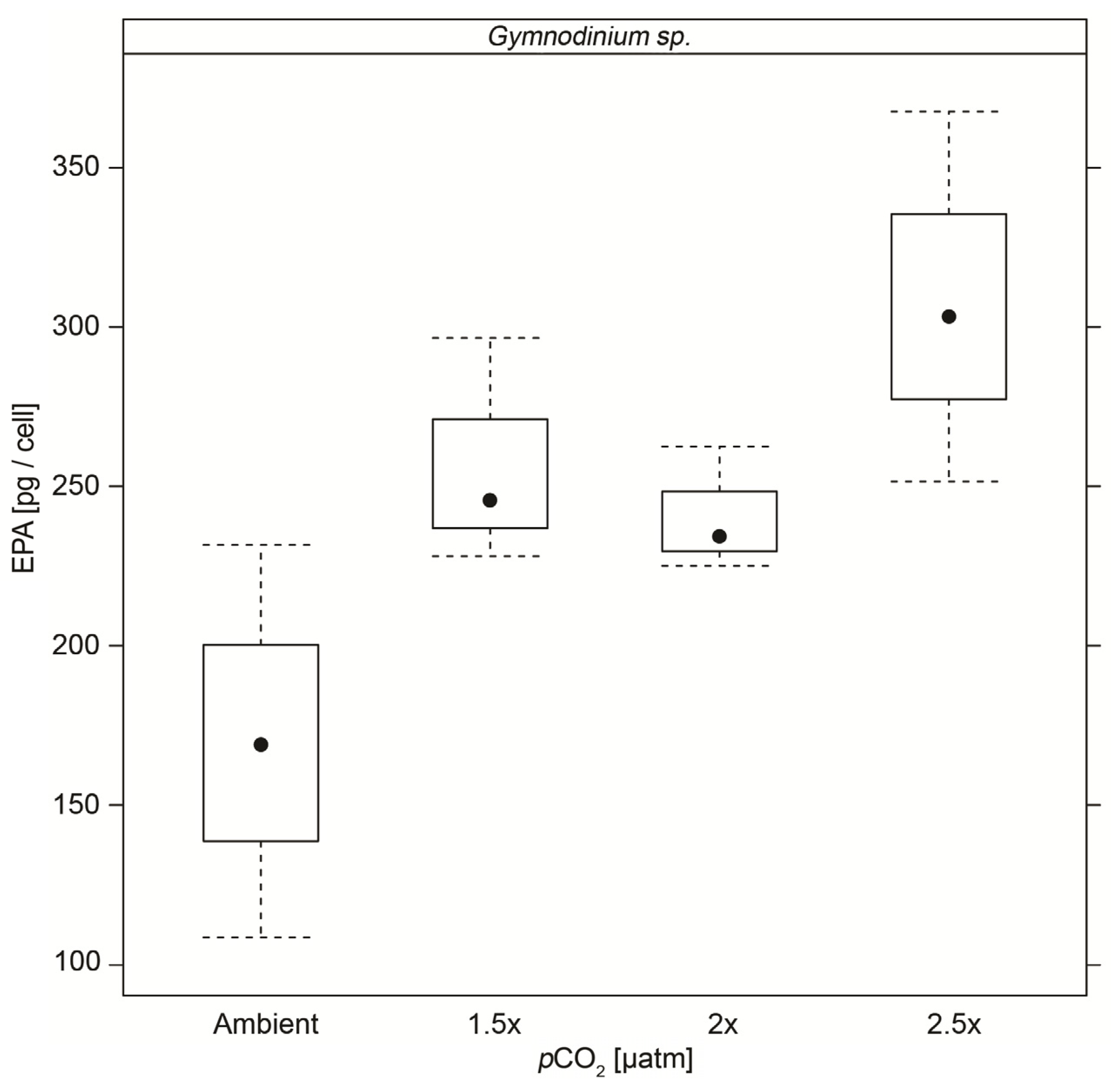
3.5. Fatty Acids
| CO2 treatment | Ambient | 1.5× | 2× | 2.5× |
|---|---|---|---|---|
| Pyramimonas gelidicola | ||||
| CHO | 111.2 (±25.5) | 102.7 (±22.9) | 85.4 (±25.8) * | 86.7 (±4.9) * |
| n = 3 | n = 3 | n = 3 | n = 3 | |
| Phaeocystis antarctica | ||||
| CHO | 7.6 (±0.5) | 8.3 (±0.7) | - | 9.9 (±0.5) * |
| n = 3 | n = 2 | - | n = 2 | |
| Gymnodinium sp. | ||||
| CHO | 93.4 (±30.3) | 92.1 (±12.3) | 110.4 (±26.5) | 91.9 (±16.5) |
| n = 3 | n = 3 | n = 3 | n = 3 | |
| CO2 treatment | Ambient | 1.5× | 2× | 2.5× |
|---|---|---|---|---|
| Pyramimonas gelidicola | ||||
| SFA | 0.3 (±0.08) | 0.4 (±0.12) | 0.4 (±0.12) | 0.3 (±0.21) |
| MUFA | 3.0 (±0.36) | 4.6 (±0.92) * | 3.8 (±0.72) | 2.2 (±0.85) |
| PUFA | 9.9 (±1.10) | 9.4 (±0.90) | 9.5 (±1.38) | 6.5 (±2.72) * |
| ω3 | 4.9 (±0.67) | 4.3 (±0.36) | 4.7 (±0.63) | 3.4 (±1.29) * |
| ω6 | 2.5 (±0.31) | 2.7 (±0.34) | 2.6 (±0.47) | 1.5 (±1.00) * |
| DHA | 1.2 (±0.19) | 1.1 (±0.10) | 1.2 (±0.15) | 0.8 (±0.36) * |
| EPA | 0.1 (±0.01) | 0.1 (±0.02) | 0.1 (±0.01) | 0.1 (±0.03) |
| Total FA | 14.9 (±1.5) | 16.3 (±2.3) | 15.4 (±2.2) | 10.2 (±3.9) * |
| n = 3 | n = 3 | n = 3 | n = 3 | |
| Phaeocystis antarctica | ||||
| SFA | 0.2 (±0.15) | 0.2 (±0.03) | - | 0.3 (±0.06) |
| MUFA | 0.7 (±0.58) | 0.5 (±0.17) | - | 0.7 (±0.11) |
| PUFA | 1.9 (±1.42) | 1.4 (±0.04) | - | 1.6 (±0.25) |
| ω3 | 1.1 (±0.81) | 0.8 (±0.00) | - | 0.9 (±0.13) |
| ω6 | 0.8 (±0.61) | 0.6 (±0.04) | - | 0.7 (±0.11) |
| DHA | 0.5 (±0.37) | 0.4 (±0.02) | - | 0.5 (±0.08) |
| EPA | 0.1 (±0.09) | 0.1 (±0.00) | - | 0.1 (±0.01) |
| Total FA | 3.1 (±2.37) | 2.3 (±0.28) | - | 2.9 (±0.48) |
| n = 3 | n = 2 | - | n = 2 | |
| Gymnodinium sp. | ||||
| SFA | 28.0 (±16.47) | 26.4 (±3.93) | 35.4 (±30.07) | 20.0 (±9.40) |
| MUFA | 27.0 (±8.65) | 27.0 (±2.86) | 37.0 (±7.98) * | 31.2 (±5.31) |
| PUFA | 80.6 (±38.52) | 96.8 (±7.58) | 117.1 (±52.75) | 94.1 (±27.36) |
| ω3 | 33.7 (±12.41) | 39.7 (±2.55) | 44.7 (±14.12) | 38.2 (±10.79) |
| ω6 | 47.0 (±26.42) | 57.2 (±5.14) | 72.6 (±38.80) | 55.7 (±16.60) |
| DHA | 18.0 (±7.75) | 22.0 (±1.92) | 25.3 (±9.77) | 22.6 (±5.13) |
| EPA | 0.17 (±0.061) | 0.26 (±0.036) ** | 0.24 (±0.020) ** | 0.31 (±0.058) ** |
| Total FA | 153.6 (±72.45) | 170.5 (±11.91) | 216.2 (±104.09) | 164.9 (±44.92) |
| n = 3 | n = 3 | n = 3 | n = 3 | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeebe, R.E.; Zachos, J.C.; Caldeira, K.; Tyrrell, T. Carbon Emissions and Acidification. Science 2008, 321, 51–52. [Google Scholar] [CrossRef]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The oceanic sink for anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Summary for Policymakers. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chem, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; p. 18. [Google Scholar]
- The Royal Society. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide. Policy Document 12/05; The Royal Society: Cardiff, UK, 2005; p. 57. [Google Scholar]
- Caldeira, K.; Wickett, M.E. Oceanography: Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef]
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008, 65, 414–432. [Google Scholar] [CrossRef]
- IPCC. Working Group I Report “The physical basis”, 2007. Available online: http://ipcc.ch/publications_and_data/ar4/wg1/en/contents.html (accessed on 10 June 2014).
- Tortell, P.D.; Payne, C.D.; Li, Y.; Trimborn, S.; Rost, B.; Smith, W.O.; Riesselman, C.; Dunbar, R.B.; Sedwick, P.; DiTullio, G.R. CO2 sensitivity of Southern Ocean phytoplankton. Geophys. Res. Lett. 2008, 35, 1–5. [Google Scholar]
- McNeil, B.I.; Matear, R.J. Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2. Proc. Natl. Acad. Sci. USA 2008, 104, 18860–18864. [Google Scholar] [CrossRef]
- Fabry, V.J.; McClintock, J.B.; Mathis, J.T.; Grebmeier, J.M. Ocean acidification at high latitudes: The bellwether. Oceanography 2009, 22, 160–171. [Google Scholar]
- Riebesell, U. Effects of CO2 enrichment on marine phytoplankton. J. Oceanogr. 2004, 60, 719–729. [Google Scholar] [CrossRef]
- Montes-Hugo, M.; Doney, S.C.; Ducklow, H.W.; Fraser, W.; Martinson, D.; Stammerjohn, S.E.; Schofield, O. Recent changes in phytoplankton communities associated with rapid regional climate change along the Western Antarctic Peninsula. Science 2009, 323, 1470–1473. [Google Scholar] [CrossRef]
- Riebesell, U.; Schulz, K.G.; Bellerby, R.G.J.; Botros, M.; Fritsche, P.; Meyerhoefer, M.; Neill, C.; Nondal, G.; Oschlies, A.; Wohlers, J.; et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature 2007, 450, 545–549. [Google Scholar] [CrossRef] [Green Version]
- Burkhardt, S.; Riebesell, U. CO2 availability affects elemental composition (C:N:P) of the marine diatom Skeletonema costatum. Mar. Ecol. Prog. Ser. 1997, 155, 67–76. [Google Scholar] [CrossRef]
- Bellerby, R.G.J.; Schulz, K.G.; Riebesell, U.; Neill, C.; Nondal, G.; Heegaard, E.; Johannessen, T.; Brown, K.R. Marine ecosystem community carbon and nutrient uptake stoichiometry under varying ocean acidification during the PeECE III experiment. Biogeosciences 2008, 5, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Paulino, A.I.; Egge, J.K.; Larsen, A. Effects of increased atmospheric CO2 on small and intermediate sized osmotrophs during a nutrient induced phytoplankton bloom. Biogeosciences 2008, 5, 739–748. [Google Scholar] [CrossRef]
- Schoo, K.L.; Malzahn, A.M.; Krause, E.; Boersma, M. Increased carbon dioxide availability alters phytoplankton stoichiometry and affects carbon cycling and growth of a marine planktonic herbivore. Mar. Biol. 2013, 160, 2145–2155. [Google Scholar] [CrossRef]
- Iglesias-Rodriguez, M.D.; Halloran, P.R.; Rickaby, R.E.M.; Hall, I.R.; Colmenero-Hidalgo, E.; Gittins, J.R.; Green, D.R.H.; Tyrrell, T.; Gibbs, S.J.; von Dassow, P.; et al. Phytoplankton calcification in a high-CO2 world. Science 2008, 320, 336–340. [Google Scholar] [CrossRef]
- Hoogstraten, A.; Timmermans, K.R. Morphological and physiological effects in Proboscia alata (Bacillariophyceae) grown under different light and CO2 conditions of the modern Southern Ocean. J. Phycol. 2012, 48, 559–568. [Google Scholar] [CrossRef]
- Rossoll, D.; Bermudez, R.; Hauss, H.; Schulz, K.G.; Riebesell, U.; Sommer, U.; Winder, M. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS One 2012, 7, e34737. [Google Scholar]
- Hinga, K.R. Effects of pH on coastal marine phytoplankton. Mar. Ecol. Prog. Ser. 2002, 238, 281–300. [Google Scholar] [CrossRef]
- Tortell, P.D.; DiTullio, G.R.; Sigman, D.M.; Morel, F.M.M. CO2 effects on taxonomic compositionand nutrient utilization in an Equatorial Pacific phytoplankton assemblage. Mar. Ecol. Prog. Ser. 2002, 236, 37–43. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lee, K.; Shin, K.; Kang, J.-H.; Lee, H.-W.; Kim, M.; Jang, P.-G.; Jang, M.-C. The effect of seawater CO2 concentration on growth of a natural phytoplankton assemblage in a controlled mesocosm experiment. Limnol. Oceanogr. 2006, 51, 1629–1636. [Google Scholar] [CrossRef]
- Gregg, W.W.; Conkright, M.E.; Ginoux, P.; O’Reilly, J.E.; Casey, N.W. Ocean primary production and climate: Global decadal changes. Geophys. Res. Lett. 2003, 30, 1809–18012. [Google Scholar] [CrossRef]
- Boyce, D.G.; Lewis, M.R.; Worm, B. Global phytoplankton decline over the past century. Nature 2010, 466, 591–596. [Google Scholar] [CrossRef]
- Hauri, C.; Gruber, N.; Plattner, G.-K.; Alin, S.; Feely, R.A.; Hales, B.; Wheeler, P.A. Ocean acidification in the California Current System. Oceanography 2009, 22, 60–71. [Google Scholar] [CrossRef]
- Urabe, J.; Togari, J.; Elser, J.J. Stoichiometric impacts of increased carbon dioxide on a planktonic herbivore. Glob. Chang. Biol. 2003, 9, 818–825. [Google Scholar] [CrossRef]
- Carotenuto, Y.; Putzeys, S.; Simonelli, P.; Paulino, A.; Meyerhoefer, M.; Suffrian, K.; Antia, A.; Nejstgaards, J.C. Copepod feeding and reproduction in relation to phytoplankton development during the PeECE III mesocosm experiment. Biogeosci. Discuss. 2007, 4, 3913–3936. [Google Scholar] [CrossRef]
- Urabe, J.; Waki, N. Mitigation of adverse effects of rising CO2 on a planktonic herbivore by mixed algal diets. Glob. Chang. Biol. 2009, 15, 523–531. [Google Scholar] [CrossRef]
- Yoshida, T.; Virtue, P.; Kawaguchi, S.; Nichols, P.D. Factors determining the hatching success of Antarctic krill Euphausia superba embryo: Lipid and fatty acid composition. Mar. Biol. 2011, 158, 2313–2325. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Chen, B. Effects of dietary essential fatty acids on reproduction rates of a subtropical calanoid copepod, Acartia erythraea. Mar. Ecol. Prog. Ser. 2012, 455, 95–110. [Google Scholar] [CrossRef]
- Harrison, P.J.; Thompson, P.A.; Calderwood, G.S. Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J. Appl. Phycol. 1990, 2, 45–56. [Google Scholar] [CrossRef]
- Bell, M.V.; Dick, J.R.; Anderson, T.R.; Pond, D. Application of liposome and stable isotope tracer techniques to study polyunsaturated fatty acid biosynthesis in marine zooplankton. J. Plankton Res. 2007, 29, 417–422. [Google Scholar] [CrossRef]
- Carvalho, A.; Malcata, F. Optimization of W-3 fatty acid production by microalgae: Crossover effects of CO2 and light intensity under batch and continuous cultivation modes. J. Mar. Biotechnol. 2005, 7, 381–388. [Google Scholar] [CrossRef]
- Koven, W.M.; Kissil, G.W.; Tandler, A. Lipid and n-3 requirement of Sparus aurata larvae during starvation and feeding. Aquaculture 1989, 79, 185–191. [Google Scholar] [CrossRef]
- Thornton, D.C.O. Effect of low pH on carbohydrate production by a marine planktonic diatom (Chaetoceros muelleri). Res. Lett. Ecol. 2009, 2009. [Google Scholar] [CrossRef]
- Barry, J.P.; Tyrrell, T.; Hansson, L.; Plattner, G.; Gattuso, J.P. Atmospheric CO2 targets for ocean acidification perturbation experiments. In Guide to Best Practices in Ocean Acidification Research and Data Reporting; Riebesell, U., Fabry, V.J., Hansson, L., Gattuso, J., Eds.; Publications Office of the European Union: Luxembourg, 2010; pp. 53–64. [Google Scholar]
- Riebesell, U.; Fabry, V.J.; Hansson, L.; Gattuso, J.-P. (Eds.) Guide to Best Practices for Ocean Acidification Research and Data Reporting; Publications Office of the European Union: Luxembourg, 2010; p. 260.
- Chen, S.; Gao, K. Solar ultraviolet radiation and CO2-induced ocean acidification interacts to influence the photosynthetic performance of the red tide alga Phaeocystis globosa (Prymnesiophycea). Hydrobiologia 2011, 675, 105–117. [Google Scholar] [CrossRef]
- Gao, K.; Xu, J.; Gao, G.; Li, Y.; Hutchins, D.A.; Huang, B.; Wang, L.; Zheng, Y.; Jin, P.; Cai, X.; et al. Rising CO2 and increased light exposure synergistically reduce marine primary production. Nat. Clim. Chang. 2012, 2, 519–523. [Google Scholar]
- Li, G.; Campbell, D.A. Rising CO2 interacts with growth light and growth rate to alter photosystem II photoinactivation of the coastal diatom Thalassiosira pseudonana. PLoS One 2013, 8, e55562. [Google Scholar]
- Burkhardt, S.; Zondervan, I.; Riebesell, U. Effect of CO2 concentration on C:N:P ratio in marine phytoplankton: A species comparison. Limnol. Oceanogr. 1999, 44, 683–690. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacae Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phyoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Shi, D.; Xu, Y.; Morel, F.M.M. Effects of the pH/pCO2 control method on medium chemistry and phytoplankton growth. Biogeosciences 2009, 6, 1199–1207. [Google Scholar] [CrossRef]
- Sugie, K.; Yoshimura, T. Effects of pCO2 and iron on the elemental composition and cell geometry of the marine diatom Pseudo-nitzschia pseudodelicatissima (Bacillariophyceae). J. Phycol. 2013, 49, 475–488. [Google Scholar] [CrossRef]
- Gibson, J.A. Carbon Flow through Marine Environments of the Vestfold Hills, East Antarctica; ANARE Reports 139; Australian Antarctic Division, Hobart: Kingston, Australia, 1998; pp. 174–178. [Google Scholar]
- Roden, N.P.; Shadwick, E.H.; Tilbrook, B.; Trull, T.W. Annual cycle of carbonate chemistry and decadal change in coastal Prydz Bay, East Antarctica. Mar. Chem. 2013, 155, 135–147. [Google Scholar] [CrossRef]
- Egge, J.K.; Aksnes, D.L. Silicate as regulating nutrient in phytoplankton competition. Mar. Ecol. Prog. Ser. 1992, 83, 281–289. [Google Scholar] [CrossRef]
- Harrison, P.J.; Conway, H.L.; Holmes, R.W.; Davis, C.O. Marine diatoms grown in chemostats under silicate or ammonium limitation. III. Cellular composition and morphology of Chaetoceros debilis, Skeletonema costatum, and Thalassiosira gravida. Mar. Biol. 1977, 43, 19–31. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Lavigne, H. Technical Note: Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 2009, 6, 2121–2133. [Google Scholar] [CrossRef]
- Wynn-Edwards, C.; King, R.; Kawaguchi, S.; Davidson, A.T.; Wright, S.W.; Virtue, P. Development of a continuous phytoplankton culture system for ocean acidification experiments. Water 2014, 6. in press. [Google Scholar]
- Feng, Y.; Hare, C.E.; Leblanc, K.; Rose, J.M.; Zhang, Y.; DiTullio, G.R.; Lee, P.A.; Wilhelm, S.W.; Rowe, J.M.; Sun, J.; et al. Effects of increased pCO2 and temperature on the North Atlantic spring bloom. I. The phytoplankton community and biogeochemical response. Mar. Ecol. Prog. Ser. 2009, 388, 13–25. [Google Scholar] [CrossRef]
- Lomas, M.W.; Gilbert, P.M. Comparisons of nitrate uptake, storage, and reduction in marine diatoms and flagellates. J. Phycol. 2000, 36, 903–913. [Google Scholar] [CrossRef]
- Thomson, P.G.; Davidson, A.T.; Cadman, N. Seasonal changes in effects of ambient UVR on natural communities of Antarctic marine protists. Aquat. Microb. Ecol. 2008, 52, 131–147. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. (Eds.) Guide to Best Practices for Ocean CO2 Measurements. PICES Special Publication 3. Available online: http://cdiac.ornl.gov/oceans/Handbook_2007.html (acessed on 12 June 2014).
- Lewis, E.; Wallace, D.W.R. Program Developed for CO2 System Calculations; Oak Ridge National Laboratory, U.S. Department of Energy: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Mehrbach, C.; Culberson, C.; Hawley, J.; Pytkowicz, R. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973, 18, 897–907. [Google Scholar] [CrossRef]
- Dickson, A.G.; Millero, F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. A 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- Mock, T.; Hoch, N. Long-term temperature acclimation of photosynthesis in steady-state cultures of the polar dioatm Fragilariopsis cylindrus. Photosynth. Res. 2005, 85, 307–317. [Google Scholar] [CrossRef]
- Zapata, M.; Rodriguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar]
- Wright, S.W.; Jeffrey, S.W. High resolution system for chlorophylls and carotenoids of marine phytoplankton. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffrey, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; UNESCO: Paris, France, 1997; pp. 327–341. [Google Scholar]
- Mantoura, R.F.C.; Repeta, D.J. Calibration methods for HPLC. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffrey, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; UNESCO: Paris, France, 1997; pp. 407–428. [Google Scholar]
- Wright, S.W.; van den Enden, R.L.; Pearce, I.; Davidson, A.T.; Scott, F.J.; Westwood, K.J. Phytoplankton community structure and stocks in the Southern Ocean (30–80° E) determined by CHEMTAX analysis of HPLC pigment signatures. Deep Sea Res. II 2010, 57, 758–778. [Google Scholar] [CrossRef]
- Brown, M.R.; McClausland, M.A.; Kowalski, K. The nutritional valule of four Australian microalgal strains fed to Pacific oyster Crassostrea gigas spat. Aquaculture 1998, 165, 281–293. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colometric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar]
- R Project for Statistical Computing, 2012. Available online: http://www.r-project.org/index.html (accessed on 6 February 2014).
- Fiorini, S.; Gattuso, J.-P.; van Rijswijk, P.; Middelburg, J. Coccolithophores lipid and carbon isotope composition and their variability related to changes in seawater carbonate chemistry. J. Exp. Mar. Biol. Ecol. 2010, 394, 74–85. [Google Scholar] [CrossRef]
- Engel, A. Direct relationship between CO2 uptake and transparent exopolymer particles production in natural phtyoplankton. J. Plankton Res. 2002, 24, 49–53. [Google Scholar] [CrossRef]
- Taraldsvik, M.; Myklestad, S.M. The effect of pH on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom Skeletonema costatum. Eur. J. Phycol. 2000, 35, 189–194. [Google Scholar] [CrossRef]
- Leu, E.; Daase, M.; Schulz, K.G.; Stuhr, A.; Riebesell, U. Effect of ocean acidification on the fatty acid composition of a natural plankton community. Biogeosciences 2013, 10, 1143–1153. [Google Scholar] [CrossRef] [Green Version]
- Wynn-Edwards, C.; Kawaguchi, S.; King, R.; Davidson, A.T.; Wright, S.W.; Nichols, D.P.; Candy, S.; Wotherspoon, S.; Virtue, P. Can increased pCO2 alter the biochemistry of Antarctic phytoplankton and thus affect the survival of Antarctic krill larvae? Polar Biol. 2014. submitted. [Google Scholar]
- Lane, A.E.; Burris, J.E. Effects of environmental pH on the internal pH of Chlorella pyrenoidosa, Scenedesmus quadricauda, and Euglena mutabilis. Plant Physiol. 1981, 68, 439–442. [Google Scholar] [CrossRef]
- Young, B.P.; Shin, J.J.H.; Orij, R.; Chao, J.T.; Li, S.C.; Guan, X.L.; Khong, A.; Jan, E.; Wenk, M.R.; Prinz, W.A.; et al. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science 2010, 329, 1085–1088. [Google Scholar] [CrossRef]
- Sato, N.; Tsuzuki, M.; Kawaguchi, A. Glycerolipid synthesis in Chlorellakessleri 11 h: Effect of CO2 concentration during growth. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2003, 1633, 35–42. [Google Scholar] [CrossRef]
- Torstensson, A.; Hedblom, M.; Andersson, J.; Andersson, M.X.; Wulff, A. Synergism between elevated pCO2 and temperature on the Antarctic sea ice diatom Nitzschia lecointei. Biogeosciences 2013, 10, 6391–6401. [Google Scholar] [CrossRef]
- Arnold, H.E.; Kerrison, P.; Steinke, M. Interacting effects of ocean acidification and warming on growth and DMS-production in the haptophyte coccolithophore Emiliania huxleyi. Glob. Chang. Biol. 2013, 19, 1007–1016. [Google Scholar] [CrossRef]
- Sobrino, C.; Ward, M.L.; Neale, P.J. Acclimation to elevated CO2 and ultraviolet radiation in the diatom Thalassiosira pseudonana: Effects on growth, photosynthesis and spectral sensitivity of photoinhibition. Limnol. Oceanogr. 2008, 53, 494–505. [Google Scholar] [CrossRef]
- Gervais, F.; Riebesell, U. Effect of phosphorus limitation on elemental composition and stable carbon isotope fractionation in a marine diatom growing under different CO2 concentrations. Limnol. Oceanogr. 2001, 46, 497–504. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wynn-Edwards, C.; King, R.; Davidson, A.; Wright, S.; Nichols, P.D.; Wotherspoon, S.; Kawaguchi, S.; Virtue, P. Species-Specific Variations in the Nutritional Quality of Southern Ocean Phytoplankton in Response to Elevated pCO2. Water 2014, 6, 1840-1859. https://doi.org/10.3390/w6061840
Wynn-Edwards C, King R, Davidson A, Wright S, Nichols PD, Wotherspoon S, Kawaguchi S, Virtue P. Species-Specific Variations in the Nutritional Quality of Southern Ocean Phytoplankton in Response to Elevated pCO2. Water. 2014; 6(6):1840-1859. https://doi.org/10.3390/w6061840
Chicago/Turabian StyleWynn-Edwards, Cathryn, Rob King, Andrew Davidson, Simon Wright, Peter D. Nichols, Simon Wotherspoon, So Kawaguchi, and Patti Virtue. 2014. "Species-Specific Variations in the Nutritional Quality of Southern Ocean Phytoplankton in Response to Elevated pCO2" Water 6, no. 6: 1840-1859. https://doi.org/10.3390/w6061840




