Spatial Distribution and Enrichment Mechanisms of Major Trace Elements in Budonquan Salt Lake from Hoh Xil Basin, Northern Tibetan Plateau
Abstract
1. Introduction
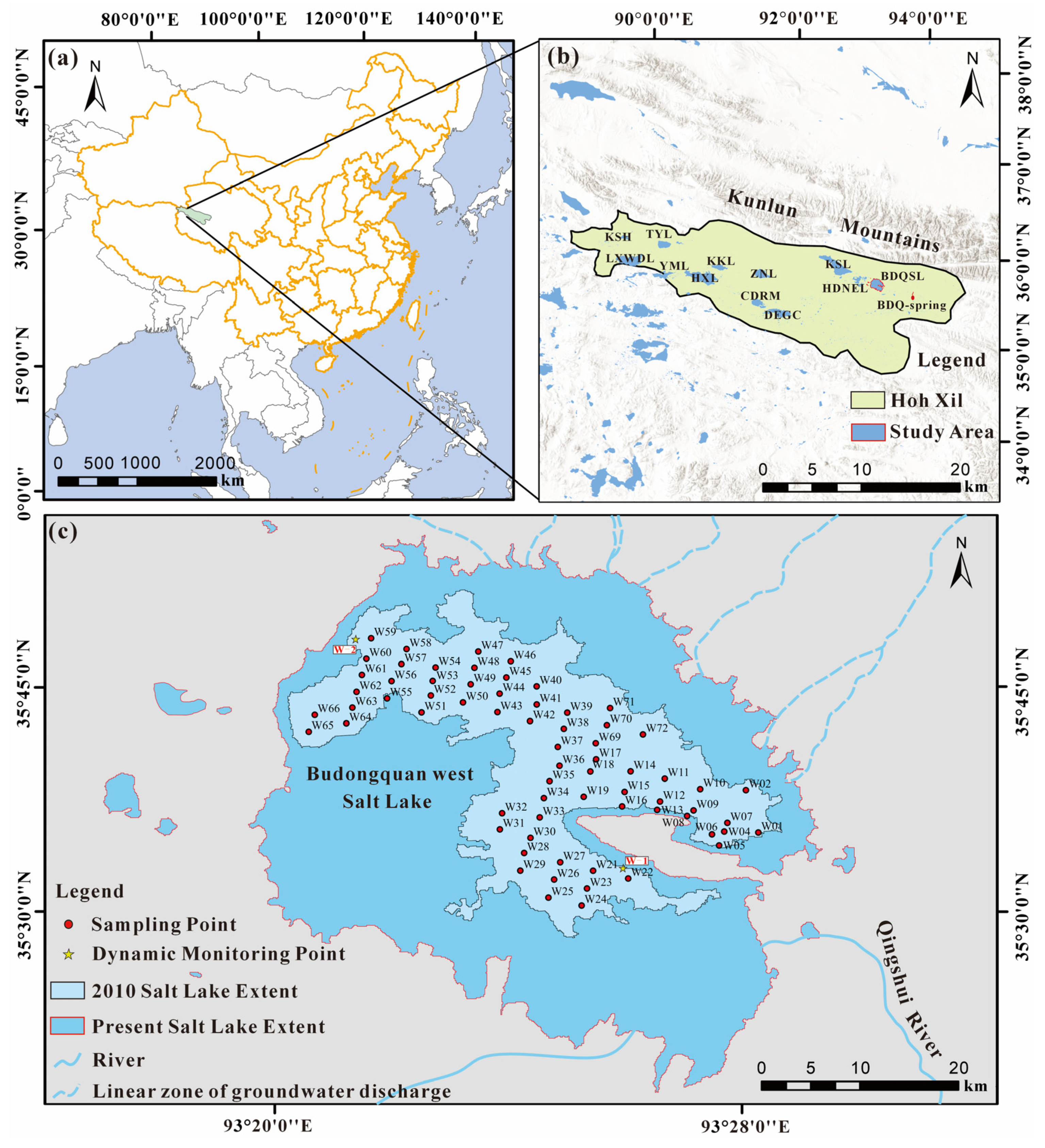
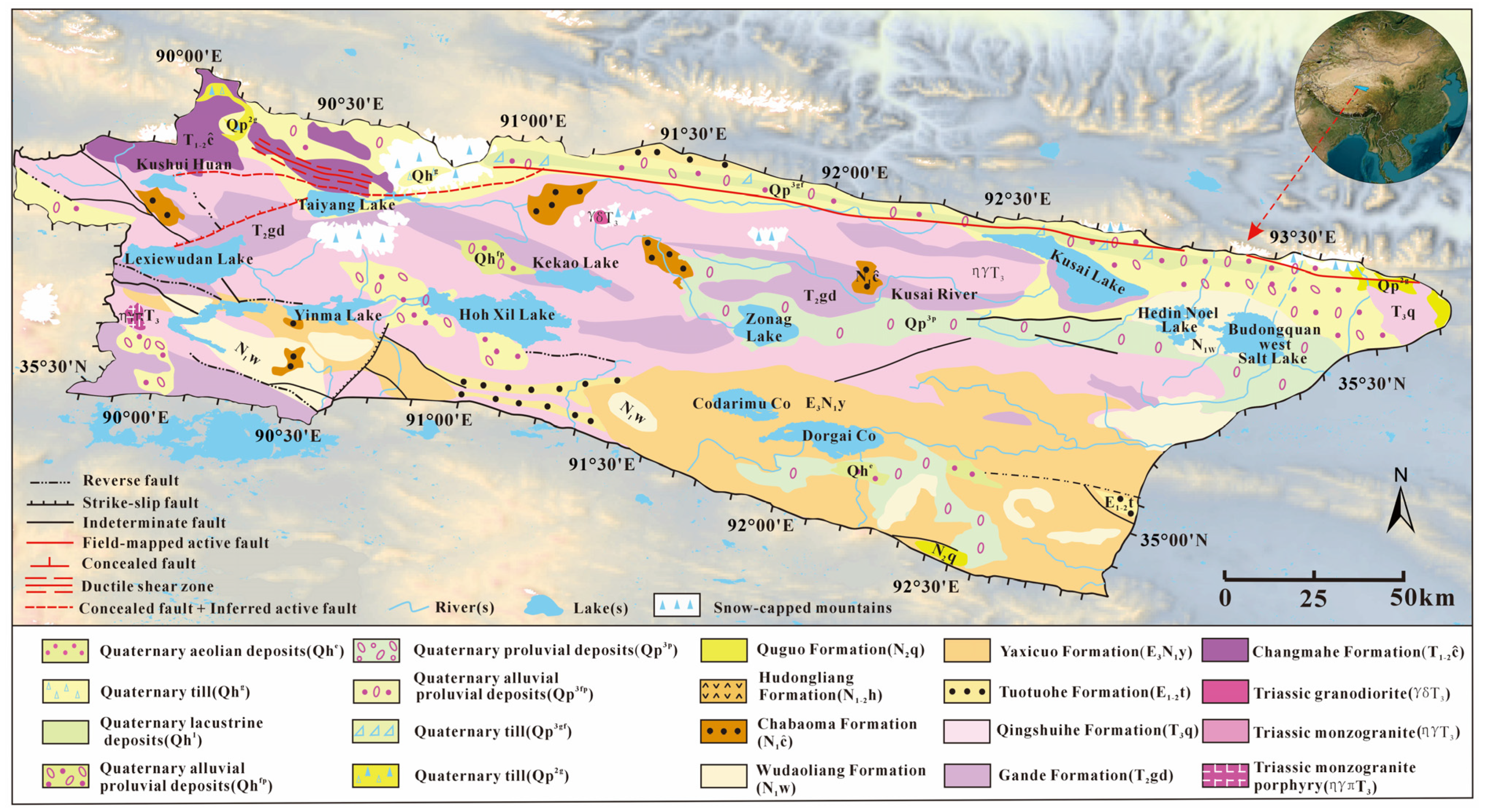
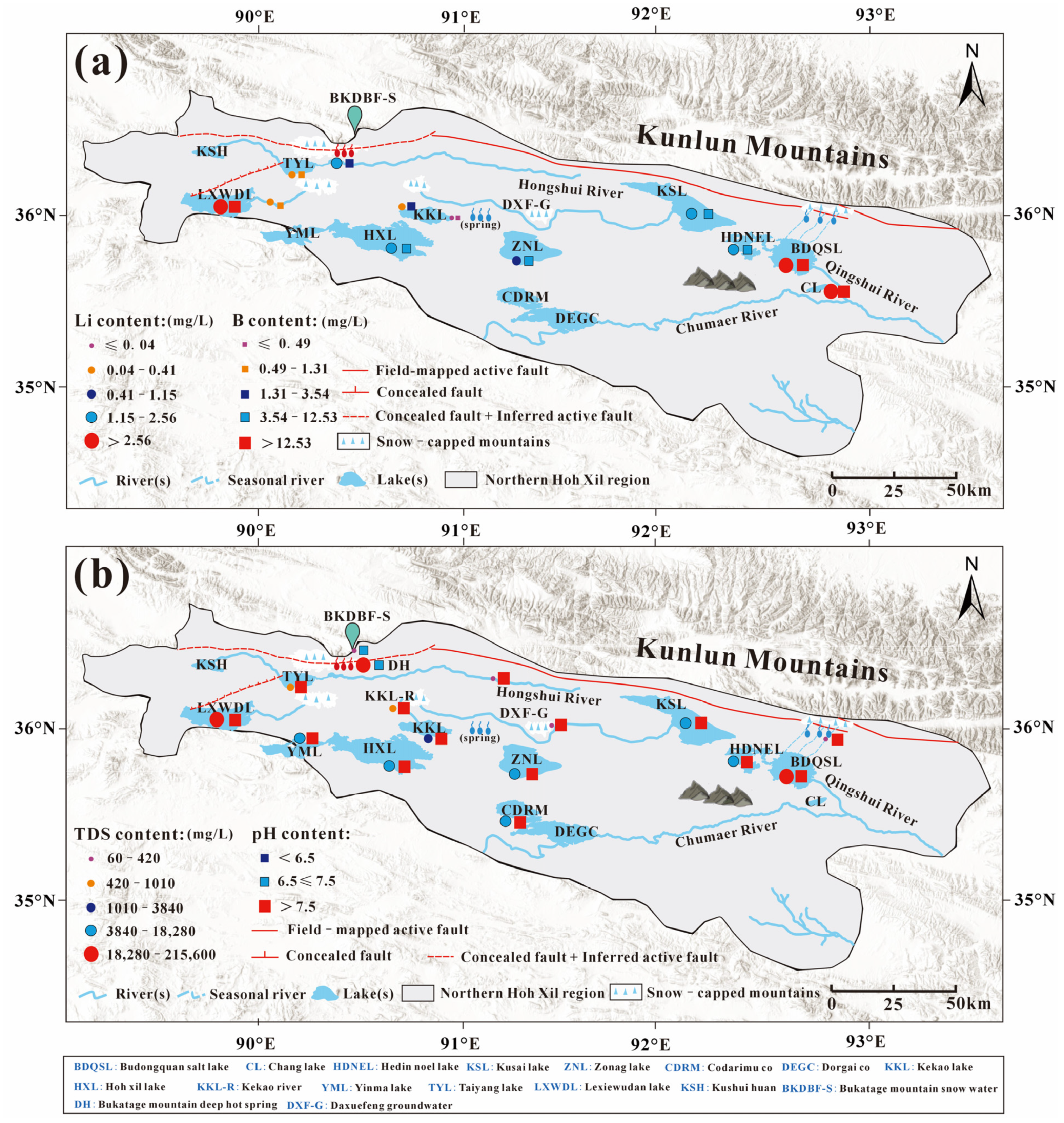
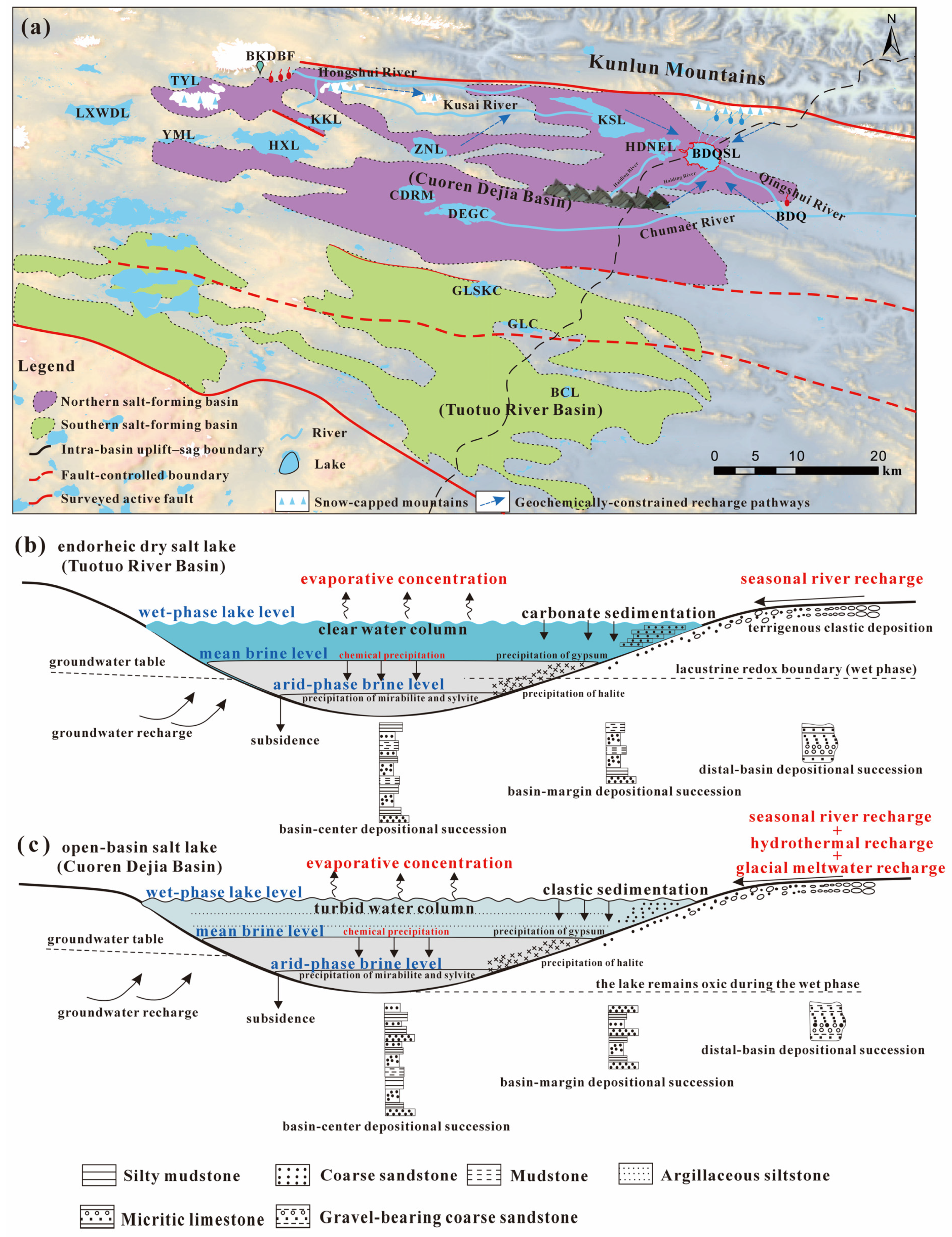
2. Geological Background and Hydrological Setting
3. Sampling Strategy and Analytical Methods
4. Results
4.1. Water Chemistry of Lake Water and Spatial–Vertical Variations
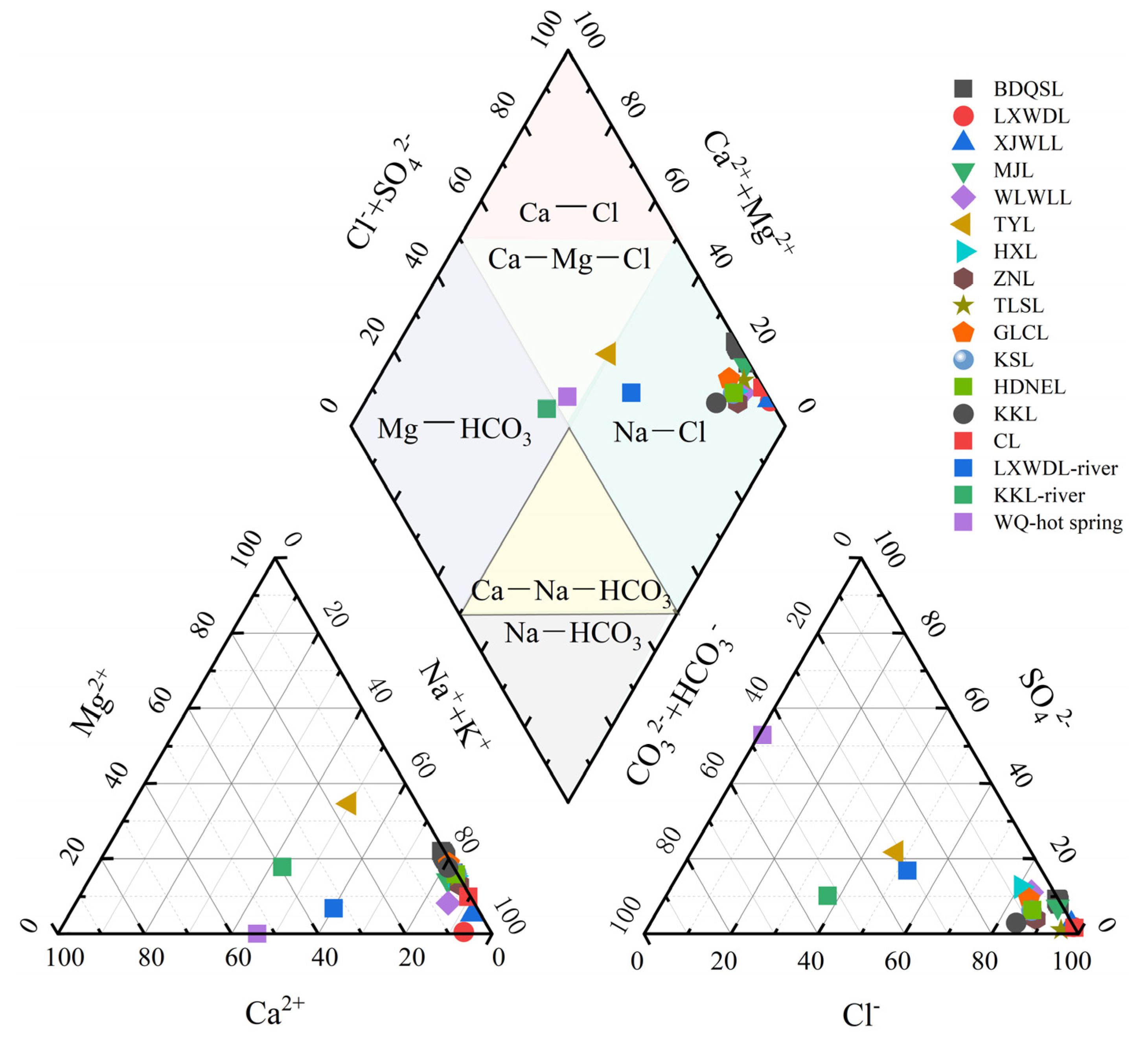
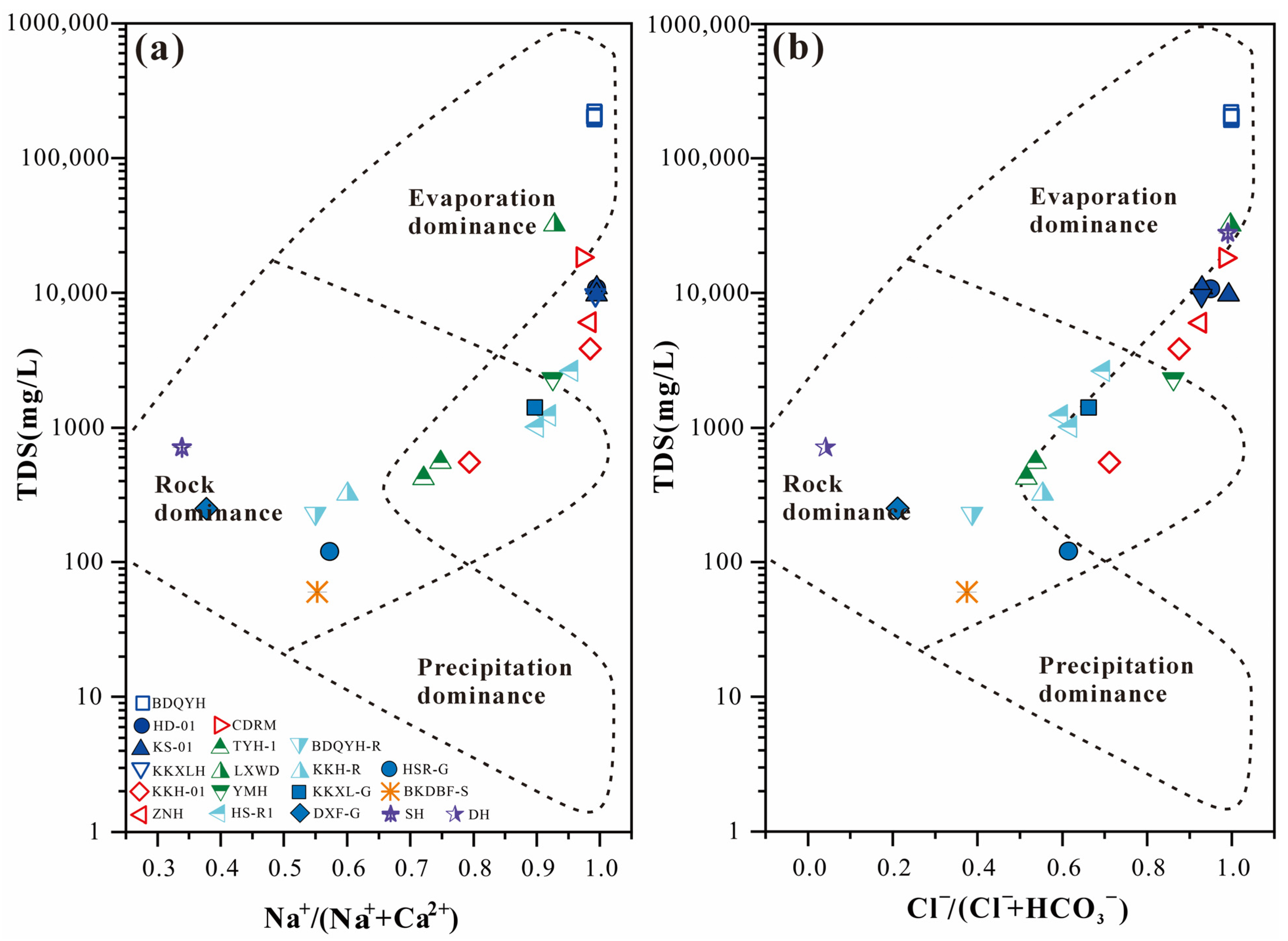
4.2. Ionic Relationships and Mineral Saturation
5. Discussion
5.1. Enrichment and Spatial Heterogeneity of Major Trace Elements in the Brine of BDQSL
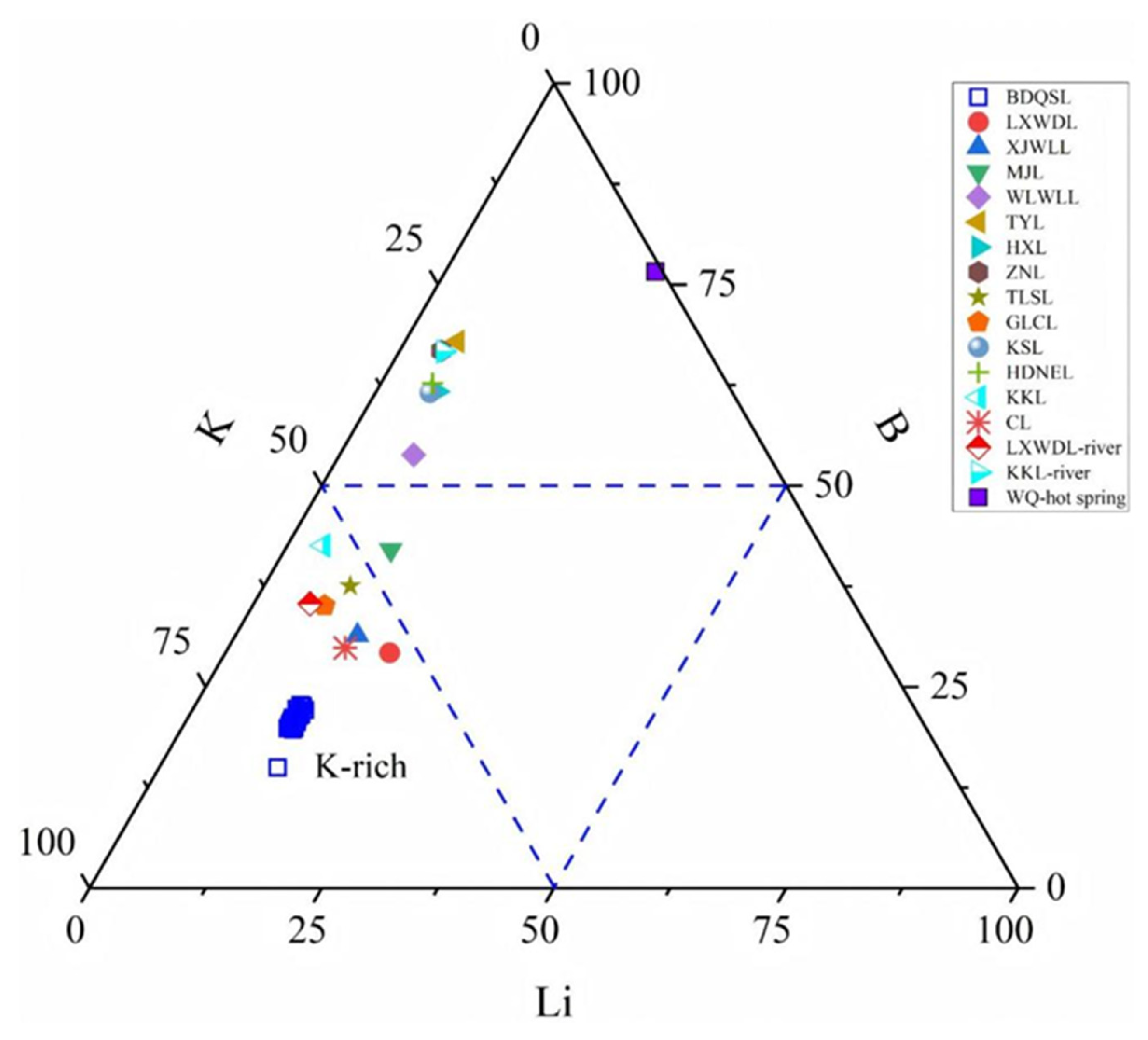
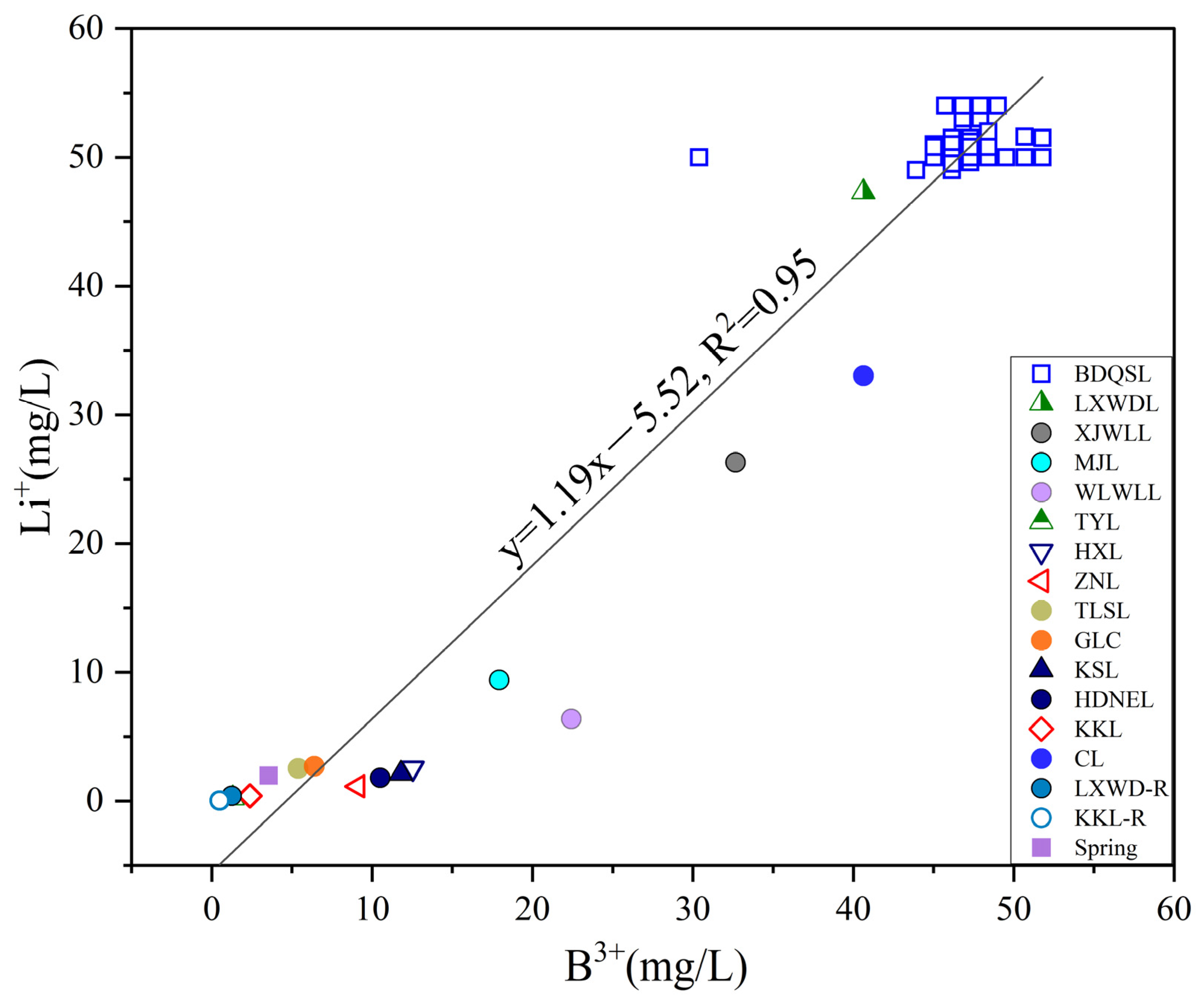
5.2. Sources and Enrichment Processes of Dissolved B-Li in Brine from the BDQSL
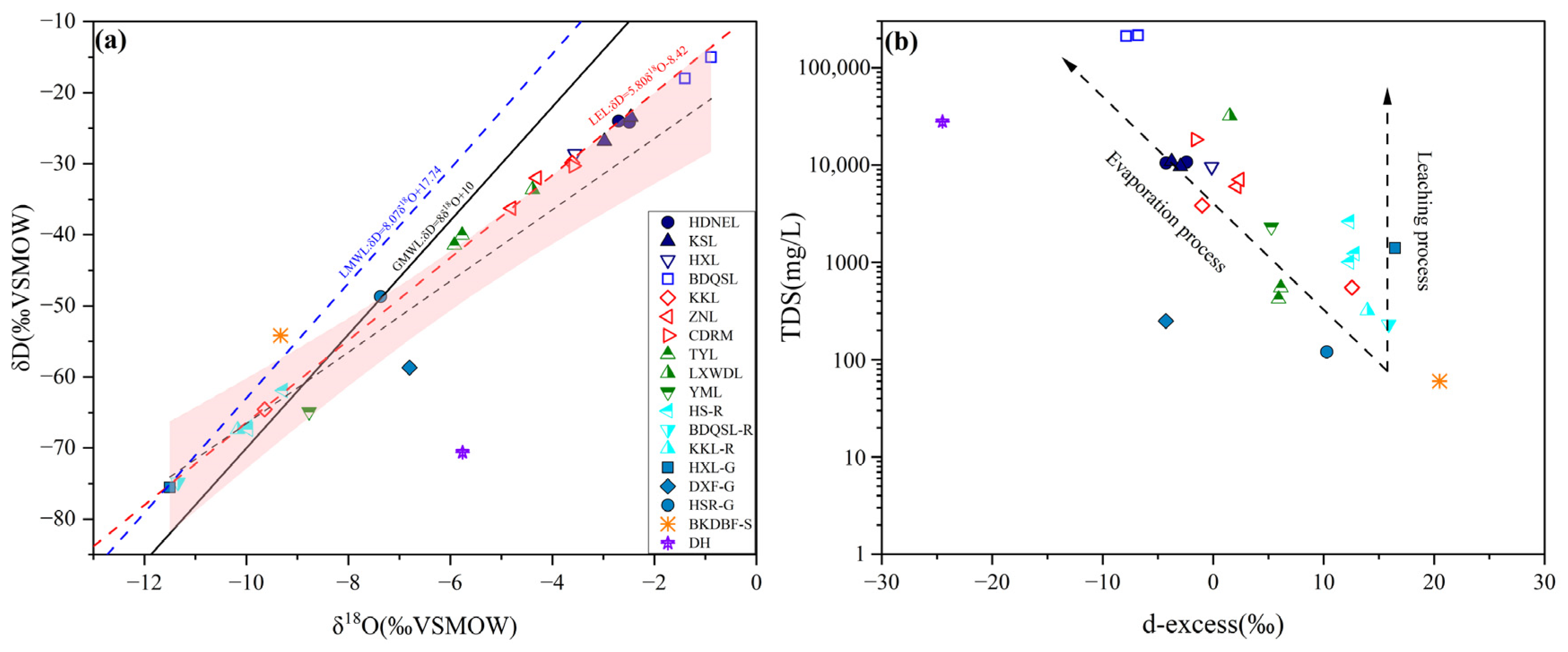
5.3. Evaporation and Hydrological Reorganization Freshening Effects: Evidence from H–O Isotopic Signatures at BDQSL
5.4. Salt Formation–Mineralization Model of Salt Lakes in the HXB
6. Conclusions
- (1)
- BDQSL brines demonstrate extreme hypersalinity (TDS 192.7–220.7 g/L), characterized by a Na-Cl hydrochemical field with substantial B and Li enrichment (45–51 mg/L and 50–54 mg/L, respectively). Spatial distribution patterns exhibit concurrent overall homogeneity with localized gradients, demonstrating progressive salinity increases from northwestern recharge zones toward southeastern evaporative sectors, yet lacking discrete geochemical zonation; vertical physicochemical parameters maintain remarkable consistency, indicating comprehensive water column mixing. This spatial architecture reflects water mass homogenization processes following rapid expansion triggered by the 2011 ZNL outburst flood event.
- (2)
- Dissolved B-Li within BDQSL brines derives from synergistic hydrothermal inputs, water–rock weathering, and evaporative concentration processes (R2 = 0.95), demonstrating coupled migration pathways characterized by unified sources, concurrent transport, and co-located accumulation. Predominant hydrothermal contributions differentiate this lake’s B-Li enrichment mechanisms from purely evaporative saline systems.
- (3)
- Climate change-driven hydrological budgets transition toward positive balance conditions; H-O isotopic compositions are plotted systematically below the Local Evaporation Line (LEL), with d-excess values substantially depleted relative to input endmembers and inversely correlated with TDS, confirming evaporative fractionation as the dominant process. Concurrently, upstream cascade connectivity and recent warming–humidification trends drive lake expansion and brine dilution, signaling potential long-term evolution toward brackish conditions with profound implications for regional B-Li resource accumulation patterns.
- (4)
- Within the HXB’s open playa lake context, BDQSL mineralization integrates coupled “hydrothermal-evaporative composite” and “brine concentration-enrichment” mechanisms: fault-channeled thermal springs provide sustained B-Li fluxes, while intensive lacustrine evaporation amplifies concentration within the broad, shallow basin geometry, synergistically generating B-Li-enriched brines. This composite mineralization mechanism distinctly contrasts with mono-genetic evaporative systems exemplified by Bucha and Duoxiu lakes, representing archetypal mineralization processes characteristic of hydrothermally active plateau interior settings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample ID | pH | TDS (mg/L) | K+ (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | SO42− (mg/L) | Cl− (mg/L) | B2O3 (mg/L) | Li+ (mg/L) | CO32− (mg/L) | HCO3− (mg/L) | NO3− (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDQSL-01 | 8.05 | 215.6 | 1740 | 68,250 | 482.1 | 9331 | 15,620 | 119,600 | 154.3 | 54 | 300.7 | 238.1 | - |
| BDQSL-02 | 8.15 | 211.5 | 1720 | 68,250 | 462 | 9088 | 15,230 | 116,100 | 147.3 | 54 | 306.5 | 262.5 | - |
| BDQSL-03 | 8.26 | 211.8 | 1740 | 66,250 | 502.2 | 9185 | 15,530 | 117,900 | 157.8 | 54 | 283.8 | 230.2 | - |
| BDQSL-04 | 8.15 | 210.9 | 1720 | 67,500 | 482.1 | 9209 | 15,280 | 116,100 | 150.8 | 53 | 295.9 | 248.6 | - |
| BDQSL-05 | 8.25 | 212.9 | 1720 | 67,500 | 482.1 | 9209 | 15,410 | 117,900 | 154.3 | 53 | 305.1 | 261 | - |
| BDQSL-06 | 8.08 | 215 | 1740 | 69,500 | 482.1 | 9209 | 15,540 | 117,900 | 154.3 | 54 | 291.6 | 228.8 | - |
| BDQSL-07 | 8.14 | 215.4 | 1760 | 69,000 | 462 | 9307 | 15,480 | 118,800 | 150.8 | 52 | 299 | 245.7 | 17.4 |
| BDQSL-08 | 8.18 | 214.6 | 1720 | 69,000 | 482.1 | 9331 | 15,530 | 117,900 | 150.8 | 54 | 283.8 | 224 | - |
| BDQSL-09 | 8.12 | 215.4 | 1700 | 70,000 | 482.1 | 9185 | 15,570 | 117,900 | 154.3 | 53 | 283.3 | 227.1 | - |
| BDQSL-10 | 8.07 | 214.8 | 1740 | 68,250 | 502.2 | 9209 | 15,720 | 118,800 | 154.3 | 53 | 288.4 | 231.8 | - |
| BDQSL-11 | 8.05 | 217.4 | 1700 | 70,000 | 482.1 | 9331 | 15,670 | 119,600 | 147.3 | 54 | 311.1 | 267.2 | - |
| BDQSL-12 | 8.21 | 214.2 | 1720 | 67,750 | 502.2 | 9209 | 15,570 | 118,800 | 150.8 | 53 | 285.3 | 231.7 | - |
| BDQSL-13 | 8.15 | 215.7 | 1740 | 69,000 | 462 | 9331 | 15,740 | 118,800 | 150.8 | 54 | 292.8 | 251.6 | - |
| BDQSL-14 | 8.12 | 217 | 1780 | 68,250 | 482.1 | 9453 | 15,920 | 120,500 | 154.3 | 54 | 295.9 | 251.7 | - |
| BDQSL-15 | 8.24 | 220.7 | 1780 | 70,000 | 502.2 | 9550 | 16,020 | 122,200 | 154.3 | 54 | 302 | 263.9 | - |
| BDQSL-16 | 8.5 | 199.4 | 1690 | 63,750 | 483 | 8768 | 14,080 | 110,100 | 155.9 | 51 | 181.6 | 137 | 64 |
| BDQSL-17 | 8.53 | 205.9 | 1700 | 66,000 | 479 | 6848 | 15,720 | 112,800 | 155.9 | 52 | 236 | 179.6 | 64.5 |
| BDQSL-18 | 8.42 | 205.1 | 1690 | 66,000 | 492 | 8763 | 15,560 | 111,900 | 148.6 | 51 | 261.7 | 231.2 | 67 |
| BDQSL-19 | 7.8 | 200.5 | 1640 | 62,850 | 453 | 8545 | 14,280 | 112,200 | 152.3 | 51.5 | 228.2 | 200.3 | 51.8 |
| BDQSL-20 | 7.84 | 195.7 | 1630 | 59,450 | 457 | 8971 | 14,280 | 110,400 | 166.8 | 51.5 | 198.3 | 163.5 | 51.8 |
| BDQSL-21 | 7.79 | 199 | 1600 | 62,500 | 442 | 8426 | 14,320 | 111,300 | 148.6 | 49.6 | 113.7 | 98.21 | 51 |
| BDQSL-22 | 7.84 | 196 | 1630 | 60,000 | 439 | 8799 | 14,240 | 110,400 | 166.8 | 50 | 214.4 | 173.5 | 53.9 |
| BDQSL-23 | 7.85 | 198.8 | 1630 | 62,850 | 436 | 8494 | 14,450 | 110,400 | 152.3 | 50 | 210.8 | 176.2 | 51.6 |
| BDQSL-24 | 7.87 | 199.8 | 1640 | 60,700 | 428 | 8928 | 14,450 | 113,100 | 152.3 | 50 | 222 | 187.6 | 51.8 |
| BDQSL-25 | 7.86 | 201.8 | 1630 | 63,550 | 442 | 8917 | 14,490 | 112,200 | 155.9 | 51 | 220 | 169.6 | 51.7 |
| BDQSL-26 | 7.87 | 199.7 | 1630 | 62,150 | 446 | 9040 | 14,570 | 111,300 | 152.3 | 51 | 226.9 | 186.2 | 51.3 |
| BDQSL-27 | 7.83 | 193.9 | 1590 | 60,000 | 436 | 8556 | 14,240 | 108,700 | 97.9 | 50 | 0 | 332.4 | 92.1 |
| BDQSL-28 | 7.81 | 200.6 | 1610 | 62,850 | 453 | 8790 | 14,160 | 112,200 | 155.9 | 51 | 203.2 | 152.6 | 51.9 |
| BDQSL-29 | 7.78 | 199 | 1560 | 62,500 | 432 | 8739 | 14,740 | 110,500 | 152.3 | 49.6 | 113.4 | 96.28 | 50 |
| BDQSL-30 | 7.86 | 203 | 1630 | 63,550 | 450 | 9098 | 14,490 | 113,100 | 148.6 | 50 | 250.6 | 219.8 | 101.2 |
| BDQSL-31 | - | 204.3 | 1630 | 63,550 | 450 | 9221 | 14,860 | 114,000 | 152.3 | 51 | 226.9 | 186.2 | 52.9 |
| BDQSL-32 | - | 200.2 | 1630 | 62,150 | 450 | 8731 | 14,530 | 112,200 | 148.6 | 50 | 216.4 | 178.7 | 51 |
| BDQSL-33 | 7.93 | 202.8 | 1640 | 65,000 | 439 | 8799 | 14,980 | 111,300 | 145 | 51 | 249.9 | 209.6 | 49.9 |
| BDQSL-34 | 7.84 | 204.9 | 1640 | 64,300 | 436 | 9046 | 14,940 | 114,000 | 148.6 | 51 | 232.5 | 188.7 | 50.7 |
| BDQSL-35 | 7.86 | 204.2 | 1660 | 64,300 | 439 | 8799 | 14,360 | 114,000 | 148.6 | 49.5 | 244.3 | 207.1 | 50.6 |
| BDQSL-36 | 7.88 | 203.3 | 1640 | 64,300 | 446 | 8856 | 14,360 | 113,100 | 159.5 | 50 | 225.6 | 178.5 | 51.8 |
| BDQSL-37 | 7.84 | 197.3 | 1630 | 61,400 | 428 | 8622 | 14,320 | 110,400 | 152.3 | 50 | 205.9 | 177.6 | 51.9 |
| BDQSL-38 | 7.83 | 198.2 | 1630 | 62,150 | 436 | 8617 | 14,360 | 110,400 | 145 | 50 | 230.3 | 215 | 50.4 |
| BDQSL-39 | 7.87 | 199.9 | 1660 | 62,150 | 421 | 9055 | 13,790 | 112,200 | 152.3 | 50 | 232.5 | 191.9 | 52.5 |
| BDQSL-40 | 7.79 | 201 | 1660 | 62,150 | 432 | 9109 | 14,740 | 112,200 | 148.6 | 51.5 | 247 | 235.2 | 51.9 |
| BDQSL-41 | 7.86 | 201.6 | 1680 | 62,150 | 418 | 8934 | 14,740 | 113,100 | 152.3 | 50 | 244.3 | 210.3 | 50.6 |
| BDQSL-42 | 7.82 | 199.9 | 1680 | 62,150 | 453 | 8729 | 15,020 | 111,300 | 148.6 | 51 | 233.8 | 202.8 | 50.2 |
| BDQSL-43 | 7.84 | 200.2 | 1660 | 61,400 | 442 | 8797 | 15,100 | 112,200 | 148.6 | 50 | 245 | 214.1 | 51.4 |
| BDQSL-44 | 7.82 | 192.7 | 1610 | 58,900 | 425 | 8439 | 13,090 | 109,600 | 148.6 | 49 | 246.3 | 228.2 | 51.4 |
| BDQSL-45 | 7.76 | 198.1 | 1640 | 62,150 | 425 | 8684 | 14,200 | 110,400 | 141.4 | 49 | 263 | 238.9 | 49 |
| BDQSL-46 | 7.84 | 198.6 | 1660 | 61,400 | 436 | 8617 | 14,570 | 111,300 | 152.3 | 50 | 245 | 217.3 | 50.6 |
| BDQSL-47 | 7.81 | 203.6 | 1680 | 63,550 | 446 | 8795 | 14,530 | 114,000 | 155.9 | 50 | 233.1 | 202.1 | 51.9 |
| BDQSL-48 | 7.83 | 202.6 | 1660 | 63,550 | 439 | 8676 | 14,570 | 113,100 | 145 | 50 | 257.5 | 236.4 | 52.3 |
| BDQSL-49 | 7.82 | 202.6 | 1600 | 62,500 | 450 | 8360 | 15,230 | 114,000 | 145 | 50.8 | 146.2 | 142.2 | 53 |
| BDQSL-50 | 7.84 | 202.1 | 1680 | 62,850 | 428 | 9050 | 14,410 | 113,100 | 148.6 | 50 | 250.6 | 219.8 | 51.7 |
| BDQSL-51 | 7.85 | 198.8 | 1660 | 63,550 | 436 | 8556 | 14,410 | 109,600 | 148.6 | 49.5 | 228.9 | 204.1 | 50.9 |
| BDQSL-52 | 7.78 | 199.8 | 1640 | 63,550 | 432 | 8496 | 13,790 | 111,300 | 148.6 | 49.5 | 228.2 | 197.1 | 50.2 |
| BDQSL-53 | 7.86 | 206.6 | 1680 | 65,700 | 446 | 9162 | 14,940 | 114,000 | 152.3 | 50 | 267.3 | 240 | 52.9 |
| BDQSL-54 | 7.83 | 200.8 | 1600 | 62,500 | 439 | 8857 | 14,740 | 112,200 | 152.3 | 50.8 | 121.5 | 98.1 | 52.3 |
| BDQSL-55 | 7.83 | 206 | 1690 | 65,000 | 450 | 8851 | 14,530 | 114,900 | 155.9 | 51.5 | 232.5 | 195.1 | 51.8 |
| BDQSL-56 | 7.84 | 200.9 | 1600 | 62,000 | 436 | 8859 | 15,390 | 112,200 | 145 | 50.8 | 115.7 | 114.5 | 50 |
| BDQSL-57 | 7.81 | 199.5 | 1600 | 60,500 | 450 | 8728 | 14,740 | 113,100 | 155.9 | 50.8 | 97.37 | 83.11 | 52 |
| BDQSL-58 | 7.83 | 201.2 | 1620 | 62,000 | 442 | 8794 | 14,860 | 113,100 | 155.9 | 50.8 | 97.37 | 83.11 | 52 |
| BDQSL-59 | 7.85 | 205.6 | 1640 | 62,000 | 432 | 8432 | 15,110 | 117,600 | 152.3 | 50.8 | 115 | 110.6 | 51 |
| BDQSL-60 | 7.86 | 200.3 | 1640 | 62,000 | 432 | 8432 | 15,150 | 112,200 | 152.3 | 50.8 | 106.4 | 98.66 | 52 |
| BDQSL-61 | 7.81 | 201.7 | 1560 | 62,500 | 446 | 8363 | 15,310 | 113,100 | 155.9 | 51.2 | 106.7 | 103.8 | 53 |
| BDQSL-62 | 7.84 | 206.3 | 1640 | 62,500 | 425 | 8926 | 15,680 | 116,700 | 152.3 | 51.2 | 100.8 | 92.99 | 52 |
| BDQSL-63 | 7.82 | 202.2 | 1640 | 62,000 | 442 | 8304 | 15,390 | 114,000 | 152.3 | 50.8 | 97.37 | 83.11 | 53 |
| BDQSL-64 | 7.62 | 203.4 | 1600 | 62,000 | 439 | 8735 | 15,270 | 114,900 | 155.9 | 50.8 | 111.6 | 102.4 | 53 |
| BDQSL-65 | 8.48 | 204.1 | 1680 | 62,500 | 472 | 8776 | 14,320 | 115,800 | 166.8 | 50 | 267.5 | 202.1 | - |
| BDQSL-66 | 8.46 | 197.6 | 1600 | 62,000 | 456 | 8418 | 14,980 | 109,600 | 163.2 | 50 | 245.8 | 183.2 | - |
| BDQSL-67 | 8.45 | 201.9 | 1680 | 62,000 | 484 | 8769 | 15,310 | 113,100 | 163.2 | 51.6 | 257 | 194.6 | - |
| BDQSL-68 | 8.45 | 204.1 | 1620 | 65,000 | 486 | 9137 | 13,010 | 114,000 | 155.9 | 52 | 293.2 | 250.5 | 56.8 |
| Sample ID | Depth (m) | pH | TDS (mg/L) | K+ (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | SO42− (mg/L) | Cl− (mg/L) | B2O3 (mg/L) | Li+ (mg/L) | CO32− (mg/L) | HCO3− (mg/L) | NO3− (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDQSL1 | 0.5 | 8.25 | 212,994.5 | 1720 | 67,500 | 482.1 | 9209 | 15,410 | 117,900 | 154.3 | 53 | 305.1 | 261 | - |
| BDQSL1-1 | >1 | 8.25 | 215,560.7 | 1740 | 69,000 | 482.1 | 9088 | 15,430 | 118,800 | 154.3 | 300 | 308.2 | 258.1 | - |
| BDQSL2 | 0.5 | 8.08 | 215,099.8 | 1740 | 69,500 | 482.1 | 9209 | 15,540 | 117,900 | 154.3 | 54 | 291.6 | 228.8 | - |
| BDQSL2-1 | >1 | 8.14 | 216,600.8 | 1720 | 70,000 | 482.1 | 9331 | 15,510 | 118,800 | 150.8 | 53 | 303.6 | 250.3 | - |
| BDQSL3 | 0.5 | 8.18 | 214,675.7 | 1720 | 69,000 | 482.1 | 9331 | 15,530 | 117,900 | 150.8 | 54 | 283.8 | 224 | - |
| BDQSL3-1 | >1 | 8.25 | 217,240.3 | 1720 | 70,000 | 482.1 | 9209 | 15,510 | 119,600 | 154.3 | 54 | 283.8 | 227.1 | - |
| BDQSL4 | 0.5 | 8.05 | 217,562.7 | 1700 | 70,000 | 482.1 | 9331 | 15,670 | 119,600 | 147.3 | 54 | 311.1 | 267.2 | - |
| BDQSL4-1 | >1 | 8.2 | 214,044.5 | 1720 | 67,750 | 462 | 9185 | 15,400 | 118,800 | 154.3 | 53 | 288.4 | 231.8 | - |
| BDQSL5 | 0.5 | 8.12 | 217,141 | 1780 | 68,250 | 482.1 | 9453 | 15,920 | 120,500 | 154.3 | 54 | 295.9 | 251.7 | - |
| BDQSL5-1 | >1 | 8.14 | 216,700.9 | 1760 | 67,750 | 462 | 9453 | 16,020 | 120,500 | 154.3 | 54 | 295.9 | 251.7 | - |
| BDQSL6 | 0.5 | 8.53 | 204,235 | 1700 | 66,000 | 479 | 6848 | 15,720 | 112,800 | 155.9 | 52 | 236 | 179.6 | 64.5 |
| BDQSL6-1 | >1 | 8.46 | 204,435.2 | 1690 | 65,750 | 506 | 8999 | 13,910 | 112,800 | 152.3 | 53 | 263 | 248.4 | 63.5 |
| BDQSL7 | 0.5 | 8.42 | 205,164.5 | 1690 | 66,000 | 492 | 8763 | 15,560 | 111,900 | 148.6 | 51 | 261.7 | 231.2 | 67 |
| BDQSL7-1 | >1 | 8.42 | 205,271.8 | 1690 | 66,250 | 510 | 8752 | 16,260 | 111,000 | 148.6 | 51 | 275.6 | 270.6 | 64 |
| BDQSL8 | 0.5 | 7.8 | 197,398.39 | 1600 | 60,000 | 439 | 8612 | 14,980 | 111,300 | 155.9 | 50.4 | 113.4 | 94.69 | 53 |
| BDQSL8-1 | >1 | 7.82 | 198,398.57 | 1560 | 60,000 | 450 | 8989 | 14,740 | 112,200 | 155.9 | 50.4 | 113.1 | 91.17 | 49 |
| BDQSL9 | 0.5 | 7.81 | 200,677.6 | 1610 | 62,850 | 453 | 8790 | 14,160 | 112,200 | 155.9 | 51 | 203.2 | 152.6 | 51.9 |
| BDQSL9-1 | >1 | 7.87 | 201,842.5 | 1630 | 63,550 | 446 | 8978 | 14,360 | 112,200 | 155.9 | 51 | 231.8 | 188 | 51.8 |
| BDQSL10 | 0.5 | 7.86 | 203,088.2 | 1630 | 63,550 | 450 | 9098 | 14,490 | 113,100 | 148.6 | 50 | 250.6 | 219.8 | 101.2 |
| BDQSL10-1 | >1 | 7.86 | 204,380.3 | 1630 | 63,550 | 450 | 9221 | 14,860 | 114,000 | 152.3 | 51 | 226.9 | 186.2 | 52.9 |
| BDQSL11 | 0.5 | 7.86 | 200,335.7 | 1630 | 62,150 | 450 | 8731 | 14,530 | 112,200 | 148.6 | 50 | 216.4 | 178.7 | 51 |
| BDQSL11-1 | >1 | 7.88 | 203,772.2 | 1630 | 64,300 | 453 | 8812 | 14,780 | 113,100 | 155.9 | 49.5 | 238 | 200.7 | 53.1 |
| BDQSL12 | 0.5 | 7.93 | 202,863.4 | 1640 | 65,000 | 439 | 8799 | 14,980 | 111,300 | 145 | 51 | 249.9 | 209.6 | 49.9 |
| BDQSL12-1 | >1 | 7.84 | 202,530.7 | 1640 | 62,850 | 442 | 8980 | 14,860 | 113,100 | 152.3 | 51 | 226.2 | 179.2 | 50 |
| BDQSL13 | 0.5 | 7.86 | 204,258.1 | 1660 | 64,300 | 439 | 8799 | 14,360 | 114,000 | 148.6 | 49.5 | 244.3 | 207.1 | 50.6 |
| BDQSL13-1 | >1 | 7.87 | 202,667.7 | 1660 | 63,550 | 439 | 8676 | 14,610 | 113,100 | 148.6 | 50 | 210.8 | 173 | 50.3 |
| BDQSL14 | 0.5 | 7.79 | 201,025.2 | 1660 | 62,150 | 432 | 9109 | 14,740 | 112,200 | 148.6 | 51.5 | 247 | 235.2 | 51.9 |
| BDQSL14-1 | >1 | 7.84 | 198,907 | 1660 | 60,700 | 436 | 8678 | 14,610 | 112,200 | 152.3 | 51 | 204.6 | 163.5 | 51.6 |
| Sample ID | pH | TDS (mg/L) | K+ (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | SO42− (mg/L) | Cl− (mg/L) | B2O3 (mg/L) | Li+ (mg/L) | CO32− (mg/L) | HCO3− (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W 1-1 | 8.4 | 216.7 | 1720 | 68,500 | 386 | 9073 | 16,960 | 119,400 | 166.8 | 52.4 | 298.1 | 258.6 |
| W 1-2 | 7.6 | 216.2 | 2080 | 66,500 | 448.5 | 9598 | 16,710 | 119,400 | 234.4 | 53 | 851.7 | 734.2 |
| W 1-3 | 7.57 | 208.7 | 2100 | 64,500 | 482.5 | 9848 | 7343 | 123,800 | 178.4 | 55.5 | 255.6 | 170.9 |
| W 1-4 | 7.51 | 209.1 | 2140 | 64,500 | 489.5 | 10,390 | 5433 | 125,600 | 178.4 | 55.5 | 240.2 | 173.7 |
| W 1-5 | 8.63 | 216.2 | 2070 | 65,800 | 550 | 10,130 | 5392 | 131,700 | 188.6 | 75 | 212.5 | 143.4 |
| W 1-6 | 8.41 | 220.2 | 2040 | 68,000 | 452.1 | 10,750 | 5565 | 132,700 | 196.9 | 53 | 221.3 | 194.3 |
| W 1-7 | 8.4 | 239.9 | 2220 | 71,000 | 552 | 10,940 | 9796 | 144,800 | 241.2 | 55.5 | 207.2 | 127.8 |
| W 1-8 | 8.36 | 193.3 | 1890 | 61,000 | 541 | 8942 | 11,530 | 109,100 | 145.3 | 51 | 0 | 307.8 |
| W 1-9 | 8.31 | 167.7 | 1400 | 52,000 | 110.5 | 7146 | 13,110 | 93,480 | 109.4 | 53 | 0 | 461.8 |
| W 1-10 | 8.57 | 220.1 | 1680 | 67,000 | 439.9 | 10,110 | 18,520 | 121,700 | 171.6 | 57 | 269.1 | 223.5 |
| W 1-11 | 8.04 | 209.8 | 1620 | 65,500 | 410.6 | 6995 | 15,710 | 119,000 | 147.5 | 53.2 | 224.6 | 177.3 |
| W 1-12 | 8.05 | 185.6 | 1520 | 56,750 | 325.5 | 8222 | 13,210 | 105,100 | 143.4 | 48.2 | 215.7 | 161.1 |
| W 2-1 | 8.45 | 206.8 | 1720 | 65,500 | 513 | 9850 | 16,460 | 112,200 | 163.2 | 52.8 | 257.7 | 201.6 |
| W 2-2 | 7.65 | 204.4 | 1960 | 65,000 | 452 | 8773 | 15,510 | 112,200 | 171.4 | 51.5 | 222.5 | 137.3 |
| W 2-3 | 7.56 | 207.7 | 2080 | 64,000 | 475.5 | 9852 | 9849 | 121,100 | 178.4 | 54 | 293.9 | 277.4 |
| W 2-4 | 7.53 | 206.6 | 2000 | 65,000 | 496 | 10,390 | 6108 | 122,000 | 181.9 | 55 | 243.9 | 156.1 |
| W 2-5 | 8.67 | 216.7 | 2020 | 64,400 | 540 | 10,980 | 5347 | 132,700 | 188.6 | 75 | 247.8 | 192.5 |
| W 2-6 | 8.35 | 219.7 | 2040 | 67,750 | 452.1 | 10,750 | 5515 | 132,700 | 203.9 | 53.22 | 185.6 | 133.5 |
| W 2-7 | 8.39 | 234.4 | 2120 | 68,500 | 542 | 10,620 | 10,210 | 141,800 | 222.9 | 53.8 | 220.5 | 184.1 |
| W 2-8 | 8.38 | 238.3 | 2080 | 71,950 | 387.3 | 10,640 | 13,800 | 139,100 | 209.8 | 54.6 | 0 | 257 |
| W 2-9 | 8.37 | 246 | 2000 | 75,800 | 147.3 | 10,660 | 17,550 | 139,300 | 195.6 | 56 | 0 | 622.5 |
| W 2-10 | 8.22 | 231.9 | 1760 | 69,000 | 488.8 | 10,670 | 16,920 | 132,400 | 187.6 | 64.2 | 279.4 | 270.2 |
| W 2-11 | 6.06 | 192.8 | 1560 | 58,500 | 345.8 | 8458 | 14,080 | 109,500 | 349.8 | 49.6 | 0 | 0 |
| Sample Type | Sample ID | pH | TDS (mg/L) | K+ (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | SO42− (mg/L) | Cl− (mg/L) | B3+ (mg/L) | Li+ (mg/L) | CO32− (mg/L) | HCO3− (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eastern lakes | HDNEL-01 | 8.92 | 10.51 | 66.30 | 3383 | 14.64 | 353.2 | 512.5 | 5315 | 10.50 | 1.81 | 154.80 | 491.70 |
| HDNEL-02 | 8.78 | 10.71 | 57.50 | 3400 | 14.44 | 337.30 | 543.30 | 5469 | - | - | - | - | |
| KSL-01 | 8.96 | 10.93 | 78.97 | 3489 | 14.79 | 375.0 | 589.5 | 5463 | 11.79 | 2.13 | 212.80 | 531.10 | |
| KSL-02 | 8.97 | 9.68 | 67.53 | 3092 | 13.68 | 329.9 | 489.9 | 48,488 | - | - | - | - | |
| HXL | 8.57 | 9.59 | 80.48 | 3025 | 18.77 | 318.7 | 951.1 | 4443 | 12.53 | 2.56 | - | 137.70 | |
| Central lakes | KKL-01 | 9.32 | 3.84 | 35.65 | 1143 | 15.3 | 149.4 | 94.93 | 1860 | 2.39 | 0.41 | 96.74 | 334.40 |
| KKL-02 | 7.95 | 0.55 | 6.16 | 115.7 | 26.29 | 23.63 | 25.28 | 198.4 | - | - | - | - | |
| ZNL | 8.66 | 6.01 | 40.31 | 1980 | 28.52 | 154.8 | 243.0 | 3049 | 9.11 | 1.15 | 77.39 | 413.10 | |
| CDRM | 8.44 | 18.28 | 77.16 | 6040 | 151.2 | 496.1 | 1083 | 10,073 | - | - | - | - | |
| TLSL | 8.82 | 14,300 | 82.50 | 4650.0 | 25.28 | 440.20 | 126.80 | 8508.0 | 5.38 | 2.54 | - | - | |
| GLC | 8.86 | 11,070 | 112.50 | 3400.0 | 21.67 | 418.30 | 839.70 | 5621.0 | 6.39 | 2.71 | - | - | |
| Western lakes | TYL-1 | 9.03 | 0.55 | 6.36 | 86.94 | 25.5 | 36.45 | 82.93 | 122.3 | 1.31 | 0.23 | - | - |
| TYL-2 | 8.74 | 0.42 | 4.37 | 64.24 | 21.68 | 26.28 | 67.16 | 87.05 | - | - | - | - | |
| LXWDL | 7.69 | 31.99 | 598.1 | 10,555 | 712.8 | 432.7 | 516.9 | 18,972 | 40.63 | 47.30 | - | 98.35 | |
| YML | 9.01 | 2.30 | 18.36 | 607.6 | 42.44 | 112.7 | 266.8 | 946.9 | - | - | - | - | |
| XJWLL | 7.74 | 60,600 | 625.00 | 21,250 | 406.20 | 629.60 | 1358.00 | 36,083.0 | 32.66 | 26.30 | - | 157.40 | |
| MJL | 8.33 | 21,790 | 215.00 | 6875.0 | 225.70 | 629.60 | 1317.00 | 12,239.0 | 17.92 | 9.40 | 19.35 | 216.40 | |
| WLWLL | 8.87 | 16,000 | 172.50 | 5100.0 | 316.00 | 258.40 | 1441.00 | 8002.0 | 22.41 | 6.39 | 212.80 | 432.70 | |
| River water | HSR-1 | 8.17 | 1.23 | 13.99 | 275.7 | 20.84 | 52.92 | 95.78 | 339.1 | - | - | - | - |
| HSR-2 | 7.83 | 2.63 | 52.89 | 726.7 | 29.69 | 70.81 | 95.53 | 917.6 | - | - | - | - | |
| HSR-3 | 8.02 | 1.01 | 11.82 | 219.6 | 21.18 | 45.06 | 79.44 | 286.6 | - | - | - | - | |
| BDQSL-R | 9.02 | 0.23 | 2.31 | 25.69 | 18.34 | 15.43 | 30.42 | 36.41 | - | - | - | - | |
| LXWDL-R | 8.05 | 0.64 | 22.80 | 116.00 | 61.75 | 7.66 | 75.73 | 173.90 | 1.25 | 0.40 | - | 177.00 | |
| KKL-R | 8.29 | 0.32 | 4.54 | 46.76 | 27.11 | 14.91 | 20.72 | 83.01 | - | - | - | - | |
| Ground water | HXL-G | 8.24 | 1.40 | 11.07 | 350.6 | 35.08 | 26.86 | 215 | 395.3 | - | - | - | - |
| DXF-G | 8.11 | 0.25 | 2.52 | 21.39 | 30.79 | 8.65 | 13.78 | 20.34 | - | - | - | - | |
| HSR-G | 6.93 | 0.12 | 1.29 | 17.92 | 11.66 | 4.14 | 6.60 | 36.04 | - | - | - | - | |
| Snowmelt water | BKDBF-S | 7.31 | 0.06 | 0.17 | 5.15 | 3.63 | 1.48 | 2.65 | 10.51 | - | - | - | - |
| Hot-spring | KKL-spring | 7.72 | 0.22 | 2.30 | 28.00 | 23.47 | 6.35 | 14.82 | 40.51 | 0.49 | 0.04 | - | 98.35 |
| DH | 6.73 | 27.88 | 1133.9 | 9341 | 590.82 | 9.93 | 60.68 | 16,234.6 | - | - | - | - | |
| SH | 6.76 | 0.71 | 3.14 | 6.05 | 10.31 | 1.94 | 449.48 | 5.17 | - | - | - | - |
References
- Zheng, M.P.; Liu, X.F. Hydrochemistry of salt lakes of the Qinghai-Tibet Plateau, China. Aquat. Geochem. 2009, 15, 293–320. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gao, C.L.; Cheng, A.Y.; Liu, Y.; Zhang, L.S.; He, X.H. Hydrochemistry, distribution and formation of lithium-rich brines in salt lakes on the Qinghai-Tibetan Plateau. Minerals 2019, 9, 528. [Google Scholar]
- Zhang, G.Q.; Yao, T.D.; Xie, H.J.; Yang, K.; Zhu, L.P.; Shum, C.K.; Bolch, T.; Yi, S.; Allen, S.; Jiang, L.G.; et al. Response of Tibetan Plateau lakes to climate change: Trends, patterns, and mechanisms. Earth-Sci. Rev. 2020, 208, 103269. [Google Scholar] [CrossRef]
- He, M.Y.; Luo, C.G.; Yang, H.J.; Kong, F.C.; Li, Y.L.; Deng, L.; Zhang, X.Y.; Yang, K.Y. Sources and a proposal for comprehensive exploitation of lithium brine deposits in the Qaidam Basin on the northern Tibetan Plateau, China: Evidence from Li isotopes. Ore Geol. Rev. 2020, 117, 103277. [Google Scholar] [CrossRef]
- Kong, F.C.; Li, Q.K.; Liu, D.; Xie, L.N.; Wang, X.Y.; Song, J.M.; Shan, F.S.; Fan, Q.S. Deep hydrothermal and shallow groundwater borne lithium and boron loadings to a mega brine lake in Qinghai Tibet Plateau based on multi-tracer models. J. Hydrol. 2021, 598, 126310. [Google Scholar] [CrossRef]
- Li, J.S.; Chen, F.K.; Ling, Z.Y.; Li, T.W. Lithium sources in oilfield waters from the Qaidam Basin, Tibetan Plateau: Geochemical and Li isotopic evidence. Ore Geol. Rev. 2021, 139, 104481. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Kong, F.C.; Lei, Z.C.; Duan, J.H.; Han, G.; Shi, H.Y.; Wang, J.P. Distribution characteristics and formation mechanisms of rubidium and cesium in the water bodies of the northern Hoh Xil region. J. Salt Lake Res. 2024, 32, 19–28, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Munk, L.A.; Hynek, S.A.; Bradley, D.C.; Boutt, D.; Labay, K.; Jochens, H. Lithium brines: A global perspective. Rev. Econ. Geol. 2016, 18, 339–365. [Google Scholar]
- Song, H.L.; Li, Q.K.; Fan, Q.S.; Han, G.; Wang, J.P.; Li, J.S.; Ling, Z.Y.; Wang, T.L.; Han, J.J.; Jiao, Y.; et al. Climate change-driven evolution of Li-B resources in representative Qinghai-Tibet Plateau salt lake. Sci. Total Environ. 2024, 935, 173456. [Google Scholar]
- Hu, D.S. Hydrochemical characteristics of salt lakes in Hoh Xil area. J. Salt Lake Res. 1997, 5, 1–15, (In Chinese with English abstract). [Google Scholar]
- Tan, H.B.; Chen, J.; Rao, W.B.; Zhang, W.J.; Zhou, H.F. Geothermal constraints on enrichment of boron and lithium in salt lakes: An example from a river-salt lake system on the northern slope of the eastern Kunlun Mountains, China. J. Asian Earth Sci. 2012, 51, 21–29. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Liu, X.F. Hydrogeochemistry and mineral assemblages of salt lakes in the Qinghai-Tibet Plateau. Acta Geol. Sin. 2010, 84, 1585–1600. [Google Scholar]
- Du, Y.S.; Fan, Q.S.; Gao, D.L.; Wei, H.C.; Shan, F.S.; Li, B.K.; Zhang, X.R.; Yuan, Q.; Qin, Z.J.; Ren, Q.H. Evaluation of boron isotopes in halite as an indicator of the salinity of Qarhan paleolake water in the eastern Qaidam Basin, western China. Geosci. Front. 2019, 10, 253–262. [Google Scholar] [CrossRef]
- Deng, W.M.; Jin, K.L.; Zhao, J.X.; Guo, Z.F.; Zheng, X.F.; Guo, F.S. Cenozoic volcanism and geochemical characteristics in Hoh Xil area, Qinghai Province. Acta Petrol. Sin. 1996, 12, 530–548, (In Chinese with English abstract). [Google Scholar]
- Hu, D.S. Hydrochemical types and zoning of salt lakes in Hoh Xil region. Oceanol. Limnol. Sin. 1992, 23, 245–253, (In Chinese with English abstract). [Google Scholar]
- Hu, D.S. Formation and evolution of salt lakes in Hoh Xil Basin. J. Salt Lake Res. 1992, 1, 7–15, (In Chinese with English abstract). [Google Scholar]
- Hu, D.S. Salt lake resources and development prospects in Hoh Xil area. Resour. Sci. 1997, 19, 22–28, (In Chinese with English abstract). [Google Scholar]
- Zhang, G.M.; Wu, Z.H.; Li, D.W.; Zhu, H.P.; Zhang, Q.L. Structural deformation and basin evolution in Hoh Xil region. Geol. Rev. 2003, 49, 285–293, (In Chinese with English abstract). [Google Scholar]
- Liu, J.Y.; Fang, X.M.; Song, C.H.; Gao, J.P.; Zhang, W.L.; Meng, Q.Q. Late Cenozoic tectonic-sedimentary evolution of the Hoh Xil Basin, northern Tibet. Basin Res. 2016, 28, 273–291. [Google Scholar]
- Li, X.Y.; Cai, Y.J. Application of hydrogen and oxygen isotopes in hydrological cycle of Qinghai Lake basin. J. Lake Sci. 2017, 29, 922–931, (In Chinese with English abstract). [Google Scholar]
- Boschetti, T.; Toscani, L.; Shouakar-Stash, O.; Iacumin, P.; Venturelli, G.; Mucchino, C.; Frape, S.K. Salt waters of the Northern Apennine Foredeep Basin (Italy): Origin and evolution. Aquat. Geochem. 2011, 17, 71–108. [Google Scholar] [CrossRef]
- Yu, J.Q.; Zhang, H.A.; Yu, F.J.; Liu, D.P. Oxygen and hydrogen isotopic compositions of meteoric waters in the eastern part of Xizang. Geochemistry 1984, 3, 93–101. [Google Scholar] [CrossRef]
- Yu, W.S.; Yao, T.D.; Tian, L.D.; Wang, Y.; Yin, C.L. Isotopic composition of atmospheric water vapor before and after the monsoon’s end in the Nagqu River Basin. Chin. Sci. Bull. 2005, 50, 2755–2760. [Google Scholar] [CrossRef]
- Xiao, Y.; Shao, J.L.; Frape, S.K.; Cui, Y.L.; Dang, X.Y.; Wang, S.B.; Ji, Y.H. Groundwater origin, flow regime and geochemical evolution in arid endorheic watersheds: A case study from the Qaidam Basin, northwestern China. Hydrol. Earth Syst. Sci. 2018, 22, 4381–4400. [Google Scholar] [CrossRef]
- Gong, D.X.; Yi, H.S.; Zhou, J.Y.; Wu, C.H.; Xia, G.Q. Discussion on Sedimentary Characteristics of the Paleogene Salt-bearing Formation and Saltforming Model of the Paleo-Saline in Hoh Xil Area. J. Salt Lake Res. 2014, 22, 21–25, (In Chinese with English abstract). [Google Scholar]
- Song, X.F.; Liu, X.C.; Xia, J.; Yu, J.J.; Tang, C.Y. A study of interaction between surface water and groundwater using environmental isotope in Huaisha River basin. Sci. China Ser. D Earth Sci. 2006, 49, 1299–1310. [Google Scholar] [CrossRef]
- Song, C.H.; Fang, X.M.; Gao, J.P.; Nie, J.S.; Yan, M.D.; Xu, X.H.; Sun, D. Magnetostratigraphy of Late Cenozoic fossil mammals in the northeastern margin of the Tibetan Plateau. Chin. Sci. Bull. 2003, 48, 188–193. [Google Scholar] [CrossRef]
- Song, Y.G.; Fang, X.M.; Li, J.J.; An, Z.S.; Miao, X.D. The Late Cenozoic uplift of Liupan Shan, China. Sci. China Ser. D Earth Sci. 2001, 44, 176–184. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, C.Y.; Wang, H.Y.; Dong, H.L.; Li, T.W. Hydrochemical characteristics and controlling factors of natural water in the Qaidam Basin. Arid Zone Res. 2023, 40, 737–746, (In Chinese with English abstract). [Google Scholar]
- Han, G.; Liu, C.Q. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem. Geol. 2004, 204, 1–21. [Google Scholar] [CrossRef]
- Xue, J.B.; Zhong, W.; Cao, J.X. Changes in physicochemical parameters, dissolved organic matter, and microbial communities in lake water from prolonged circulation. J. Clean. Prod. 2024, 451, 142123. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Gibson, J.J.; Birks, S.J.; Yi, Y. Stable isotope mass balance of lakes: A contemporary perspective. Quat. Sci. Rev. 2016, 131, 316–328. [Google Scholar] [CrossRef]
- Horita, J.; Wesolowski, D.J. Liquid-vapor fractionation of oxygen and hydrogen isotopes of water from the freezing to the critical temperature. Geochim. Cosmochim. Acta 1994, 58, 3425–3437. [Google Scholar] [CrossRef]
- Pang, X.P. A Study of the Hot Springs Water Chemistry and Sinter Deposition in the Bukedaban, Hoh Xil Region. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2009; pp. 24–26, (In Chinese with English abstract). [Google Scholar]
- Li, Y.F.; Pan, T.; Li, H.; Cheng, H.D.; Zhang, P.X.; Han, W.; Li, B.K.; Yuan, Q.; Ma, X.F.; Ma, H.Z. Source and genesis of Ca-Cl type brines in Qaidam Basin, Qinghai-Tibetan Plateau: Evidence from hydrochemistry as well as B and Li isotopes. Front. Environ. Sci. 2024, 11, 1248294. [Google Scholar] [CrossRef]
- Flexer, V.; Baspineiro, C.F.; Galli, C.I. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef]
- Yin, A.; Harrison, T.M. Geologic evolution of the Himalayan-Tibetan orogen. Annu. Rev. Earth Planet. Sci. 2000, 28, 211–280. [Google Scholar] [CrossRef]
- Tapponnier, P.; Xu, Z.Q.; Roger, F.; Meyer, B.; Arnaud, N.; Wittlinger, G.; Yang, J.S. Oblique stepwise rise and growth of the Tibet Plateau. Science 2001, 294, 1671–1677. [Google Scholar] [CrossRef]
- Wang, G.X.; Li, Y.S.; Wu, Q.B.; Wang, Y.B. Impacts of permafrost changes on alpine ecosystem in Qinghai-Tibet Plateau. Sci. China Ser. D Earth Sci. 2006, 49, 1156–1169. [Google Scholar] [CrossRef]
- Fang, X.M.; Zhang, W.L.; Meng, Q.Q.; Gao, J.P.; Wang, X.M.; King, J.; Song, C.H.; Dai, S.; Miao, Y.F. High-resolution magnetostratigraphy of the Neogene Huaitoutala section in the eastern Qaidam Basin on the NE Tibetan Plateau, Qinghai Province, China and its implication on tectonic uplift of the NE Tibetan Plateau. Earth Planet. Sci. Lett. 2007, 258, 293–306. [Google Scholar] [CrossRef]
- Li, X.D.; Xu, J.X.; Han, W.H.; Han, J.B. Hydrochemical Characteristics and Influencing Factors of Lakes in Hoh Xil. Earth Environ. 2024, 52, 056, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Lei, Y.B.; Yang, K.; Wang, B.; Sheng, Y.W.; Bird, B.W.; Zhang, G.Q.; Tian, L.D. Response of inland lake dynamics over the Tibetan Plateau to climate change. Clim. Chang. 2014, 125, 281–290. [Google Scholar] [CrossRef]
- Fan, Q.S.; Ma, Y.; Cheng, H.; Wei, H.C.; Yuan, Q.; Qin, Z.; Shan, F.S. Boron occurrence in halite and boron isotope geochemistry of halite in the Qarhan Salt Lake, western China. Sediment. Geol. 2015, 322, 34–42. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zheng, H.B.; Zhang, B.; Chen, D.; Lei, L.P. Long-term changes of lake level and water balance in the Nam Co Lake Basin, central Tibetan Plateau. J. Geogr. Sci. 2014, 24, 497–507. [Google Scholar]
- Roger, F.; Tapponnier, P.; Arnaud, N.; Schärer, U.; Brunel, M.; Xu, Z.Q.; Yang, J.S. An Eocene magmatic belt across central Tibet: Mantle subduction triggered by the Indian collision? Terra Nova 2000, 12, 102–108. [Google Scholar] [CrossRef]
- Liu, Z.F.; Wang, C.S.; Yi, H.S. Evolution and mass accumulation of the Cenozoic Hoh Xil basin, northern Tibet. J. Sediment. Res. 2001, 71, 971–984. [Google Scholar] [CrossRef]
- Wang, C.S.; Zhao, X.X.; Liu, Z.F.; Lippert, P.C.; Graham, S.A.; Coe, R.S.; Yi, H.S.; Zhu, L.D.; Liu, S.; Li, Y.L. Constraints on the early uplift history of the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2008, 105, 4987–4992. [Google Scholar] [CrossRef]
- Wu, Z.H.; Ye, P.S.; Barosh, P.J.; Wu, Z.H. The October 6, 2008, Mw 6.3 magnitude Damxung earthquake, Yadong-Gulu rift, Tibet, and implications for present-day crustal deformation within Tibet. J. Asian Earth Sci. 2011, 40, 943–957. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Yang, Z.M.; Lu, Y.J.; Kemp, A.; Zheng, Y.C.; Li, Q.Y.; Tang, J.X.; Yang, Z.S.; Duan, L.F. A genetic linkage between subduction- and collision-related porphyry Cu deposits in continental collision zones. Geology 2015, 43, 247–250. [Google Scholar] [CrossRef]
- Ma, H.Z.; Fan, Q.S.; Wei, H.C.; Han, W.; Shan, F.S.; Chen, F.K. Geochemical characteristics and origin of Tertiary evaporites in the Qaidam Basin. Acta Sedimentol. Sin. 2010, 28, 1138–1145, (In Chinese with English abstract). [Google Scholar]
- Yao, T.D.; Thompson, L.; Yang, W.; Yu, W.S.; Gao, Y.; Guo, X.J.; Yang, X.X.; Duan, K.Q.; Zhao, H.B.; Xu, B.Q. Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings. Nat. Clim. Chang. 2012, 2, 663–667. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Li, T.; Wang, B. Decadal change of the spring snow depth over the Tibetan Plateau: The associated circulation and influence on the East Asian summer monsoon. J. Clim. 2004, 17, 2780–2793. [Google Scholar] [CrossRef]
- Wu, G.J.; Yao, T.D.; Xu, B.Q.; Tian, L.D.; Zhang, C.L.; Zhang, X.L. Volume-size distribution of microparticles in ice cores from the Tibetan Plateau. J. Glaciol. 2009, 55, 859–868. [Google Scholar] [CrossRef]
- Li, M.H.; Kang, S.C.; Zhu, L.P.; You, Q.L.; Zhang, Q.G.; Wang, J.B. Mineralogy and geochemistry of the Holocene lacustrine sediments in Nam Co, Tibet. Quat. Int. 2008, 187, 105–116. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, L.P.; Ju, J.T.; Wang, J.B.; Ma, Q.F. Temporal and spatial variations of lake water balance components in the Qinghai-Tibet Plateau. J. Great Lakes Res. 2019, 45, 1099–1112. [Google Scholar]
- Gao, Q.Z.; Guo, Y.Q.; Xu, H.M.; Ganjurjav, H.; Li, Y.; Wan, Y.F.; Qin, X.B.; Ma, X.; Liu, S. Climate change and its impacts on vegetation distribution and net primary productivity of the alpine ecosystem in the Qinghai-Tibetan Plateau. Sci. Total Environ. 2016, 554, 34–41. [Google Scholar] [CrossRef]
- Wu, Q.B.; Zhang, T.J. Recent permafrost warming on the Qinghai-Tibetan Plateau. J. Geophys. Res. Atmos. 2008, 113, D13108. [Google Scholar] [CrossRef]
- Cui, B.L.; Li, X.Y. Stable isotopes reveal sources of precipitation in the Qinghai Lake Basin of the northeastern Tibetan Plateau. Sci. Total Environ. 2015, 527, 26–37. [Google Scholar] [CrossRef]
- Jin, Z.D.; You, C.F.; Wang, Y.; Shi, Y.W. Hydrological and solute budgets of Lake Qinghai, the largest lake on the Tibetan Plateau. Quat. Int. 2010, 218, 151–156. [Google Scholar] [CrossRef]
- Fan, Q.S.; Ma, H.Z.; Wei, H.C.; An, F.Y. Comprehensive chemical and isotopic analyses of basaltic-trachyandesitic Holocene lavas: Petrogenesis and geodynamic implications for the northern Tibetan Plateau. Lithos 2018, 318, 386–399. [Google Scholar]
- Warren, J.K. Evaporites: Sediments, Resources and Hydrocarbons; Springer: Berlin/Heidelberg, Germany, 2006; 1035p. [Google Scholar]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Department of the Interior, US Geological Survey: Reston, VA, USA, 1985; Volume 2254. [Google Scholar]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 126. [Google Scholar]
- Williams, W.D. Salinisation: A major threat to water resources in the arid and semi-arid regions of the world. Lakes Reserv. Res. Manag. 1999, 4, 85–91. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water—Analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Zhao, Y.; Wu, Z.H.; Liu, F.T.; Zhang, H.; Xu, T. Lithospheric structure and dynamic processes of the Tianshan orogenic belt and the Junggar basin. Tectonophysics 2003, 376, 199–239. [Google Scholar] [CrossRef]
- Liu, W.G.; Xiao, Y.K.; Peng, Z.C.; An, Z.S.; He, X.X. Boron concentration and isotopic composition of halite from experiments and salt lakes in the Qaidam Basin. Geochim. Cosmochim. Acta 2000, 64, 2177–2183. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Risacher, F. Closed basin brine evolution and the influence of Ca–Cl inflow waters: Death Valley and Bristol Dry Lake California, Qaidam Basin, China, and Salar de Atacama, Chile. Aquat. Geochem. 2009, 15, 71–94. [Google Scholar] [CrossRef]
- Eugster, H.P.; Hardie, L.A. Saline lakes. In Lakes; Springer: New York, NY, USA, 1978; pp. 237–293. [Google Scholar]
- Spencer, R.J.; Baedecker, M.J.; Eugster, H.P.; Forester, R.M.; Goldhaber, M.B.; Jones, B.F.; Kelts, K.; McKenzie, J.; Madsen, D.B.; Rettig, S.L. Great Salt Lake, and precursors, Utah: The last 30,000 years. Contrib. Mineral. Petrol. 1984, 86, 321–334. [Google Scholar] [CrossRef]
- Hardie, L.A.; Eugster, H.P. The evolution of closed-basin brines. Mineral. Soc. Am. Spec. Pap. 1970, 3, 273–290. [Google Scholar]
- Drever, J.I. The Geochemistry of Natural Waters: Surface and Groundwater Environments; Prentice Hall: Upper Saddle River, NJ, USA, 1997; 436p. [Google Scholar]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Zhang, P.X. Salt Lake of Qaidam Basin; Scientific Publishing House: Beijing, China, 1987; pp. 47–73, (In Chinese with English abstract). [Google Scholar]
- Yu, J.Q.; Gao, C.L.; Cheng, A.Y.; Liu, Y.; Zhang, L.S.; He, X.H. Geomorphic, hydroclimatic and hydrothermal controls on the formation of lithium brine deposits in the Qaidam Basin, northern Tibetan Plateau, China. Ore Geol. Rev. 2013, 50, 171–183. [Google Scholar] [CrossRef]
- Risacher, F.; Fritz, B. Geochemistry of Bolivian salars, Lipez, southern Altiplano: Origin of solutes and brine evolution. Geochim. Cosmochim. Acta 1991, 55, 687–705. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Hu, Y.; Cui, Q.; Yang, Y.; Lu, C.; Zhang, J. Spatial Distribution and Enrichment Mechanisms of Major Trace Elements in Budonquan Salt Lake from Hoh Xil Basin, Northern Tibetan Plateau. Water 2025, 17, 3210. https://doi.org/10.3390/w17223210
Han G, Hu Y, Cui Q, Yang Y, Lu C, Zhang J. Spatial Distribution and Enrichment Mechanisms of Major Trace Elements in Budonquan Salt Lake from Hoh Xil Basin, Northern Tibetan Plateau. Water. 2025; 17(22):3210. https://doi.org/10.3390/w17223210
Chicago/Turabian StyleHan, Guang, Yan Hu, Qiangqiang Cui, Yuzhen Yang, Chao Lu, and Jianjian Zhang. 2025. "Spatial Distribution and Enrichment Mechanisms of Major Trace Elements in Budonquan Salt Lake from Hoh Xil Basin, Northern Tibetan Plateau" Water 17, no. 22: 3210. https://doi.org/10.3390/w17223210
APA StyleHan, G., Hu, Y., Cui, Q., Yang, Y., Lu, C., & Zhang, J. (2025). Spatial Distribution and Enrichment Mechanisms of Major Trace Elements in Budonquan Salt Lake from Hoh Xil Basin, Northern Tibetan Plateau. Water, 17(22), 3210. https://doi.org/10.3390/w17223210





