Removal of Sulphate Ions from Borehole Water Using Nanofiltration and Reverse Osmosis
Abstract
:1. Introduction
2. Materials and Methods
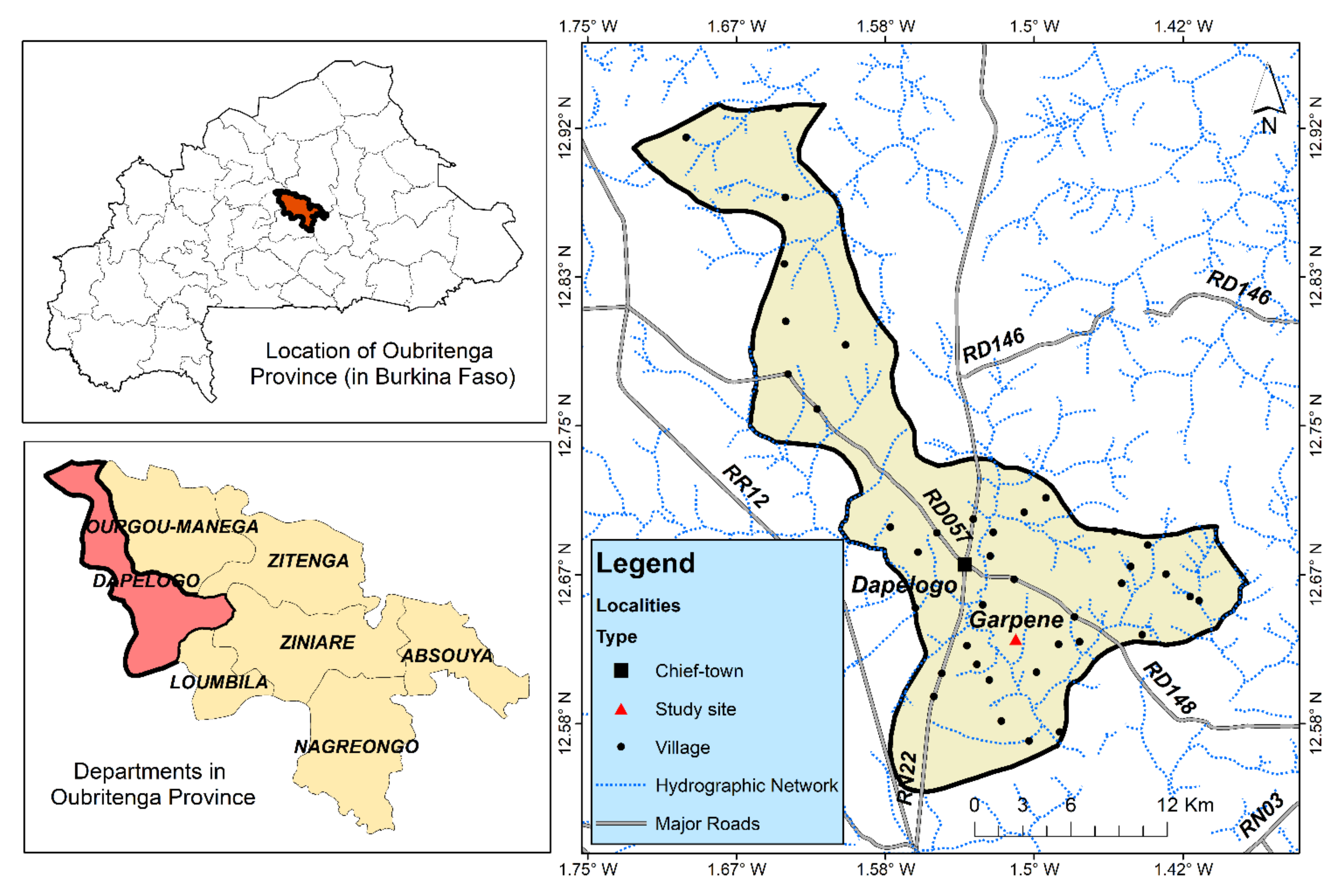
2.1. Presentation of Garpéné Village and Samples Collection from a Borehole
2.2. Design Configuration of the Nanofiltration and Reverse Osmosis Pilot
2.3. Characteristics of the Membranes Used
2.4. Filtration Processes
2.5. Evaluation of the Membrane System’s Performance
2.6. Analytical Methods for Solution Characterisation
2.7. Applied Pressures for Filtration
2.8. Feed Solutions of the Pilot
3. Results and Discussion
3.1. Characteristics of the Water from the Borehole
3.2. Performance of Reverse Osmosis and Nanofiltration for the Borehole Water Treatment
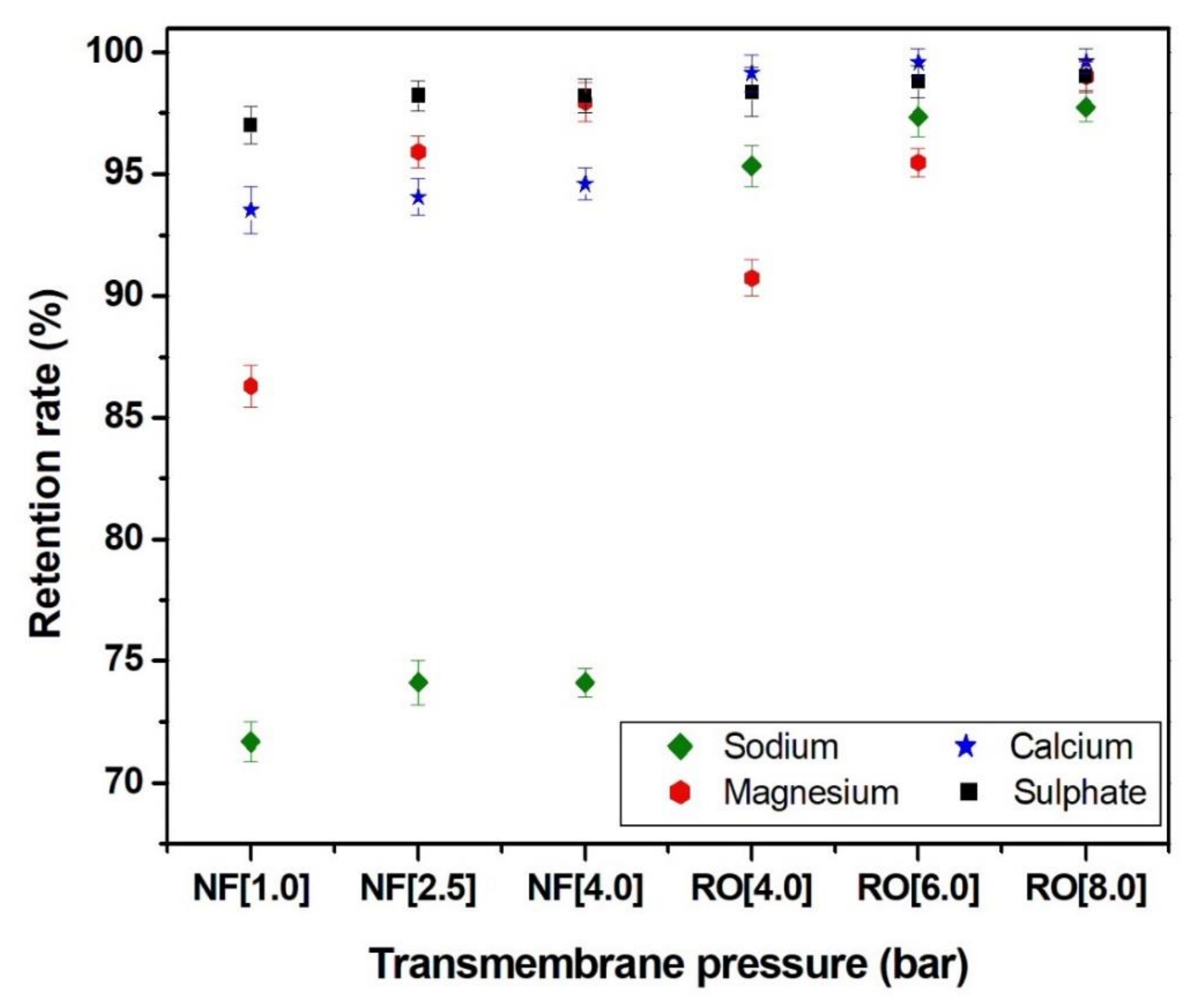
3.3. Effect of the Pressure on the Rejection of Main Ions
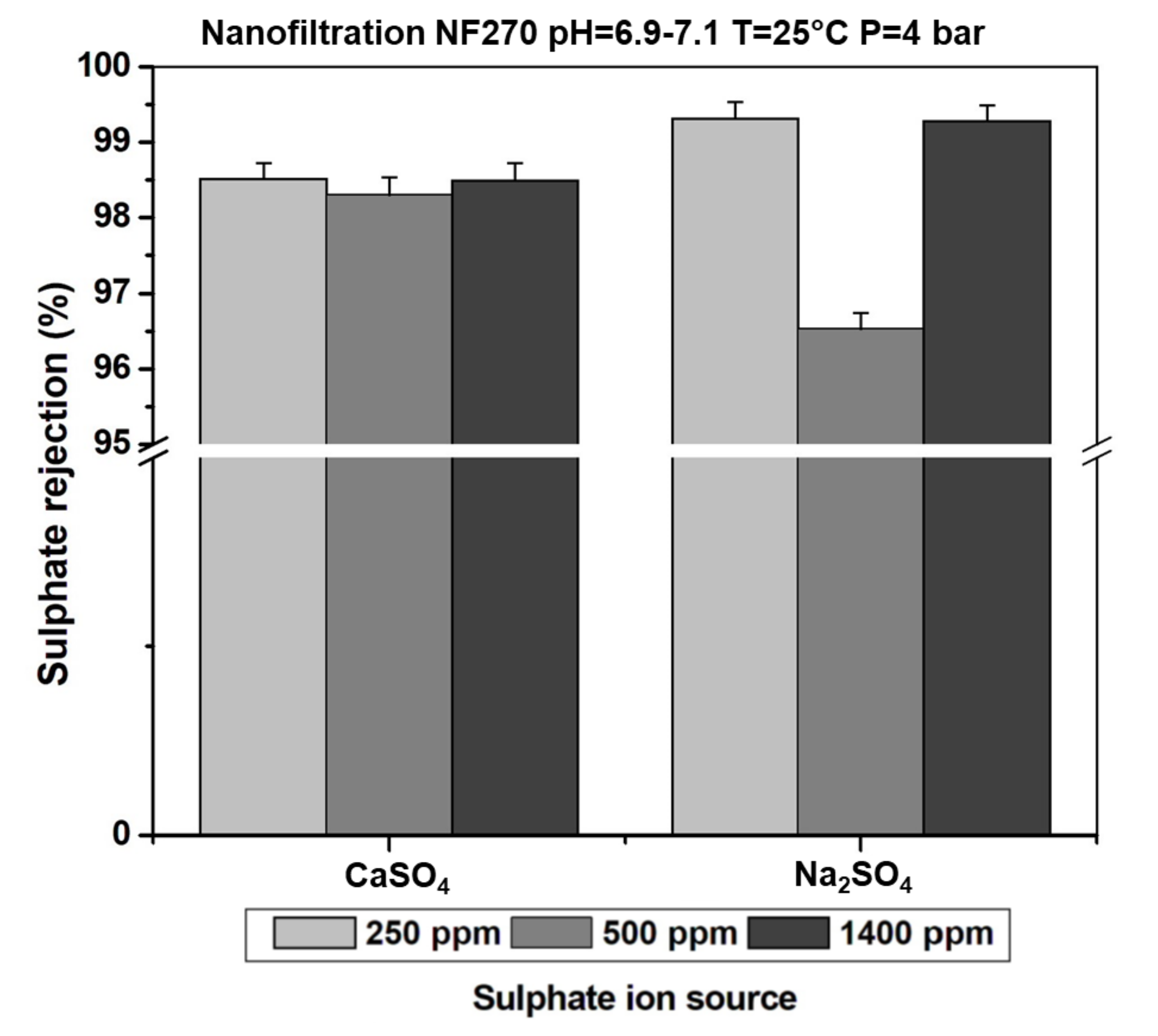
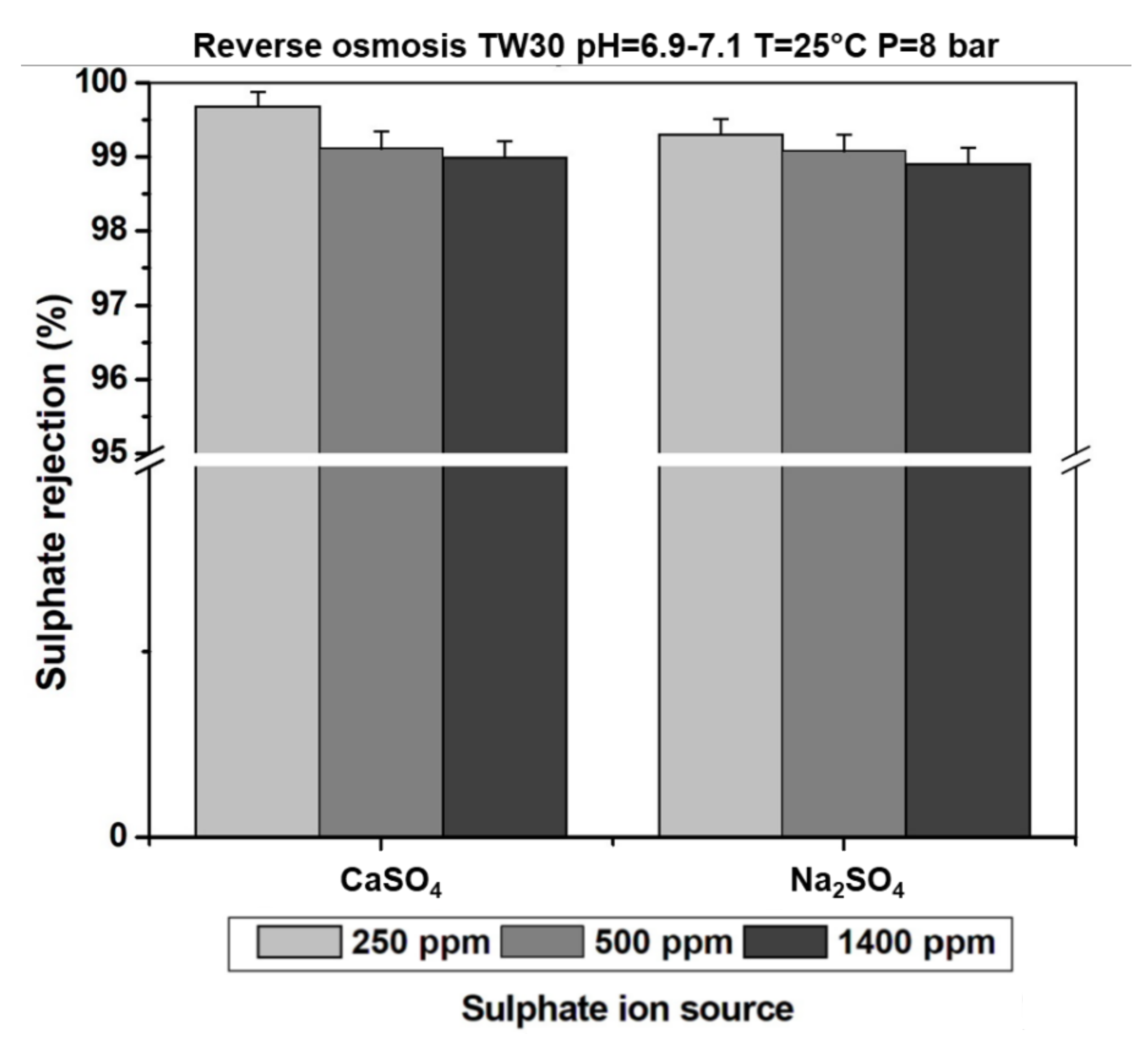
3.4. Effect of Sulphate Ion Concentration in the Feeding Solution on the Rejection Rate
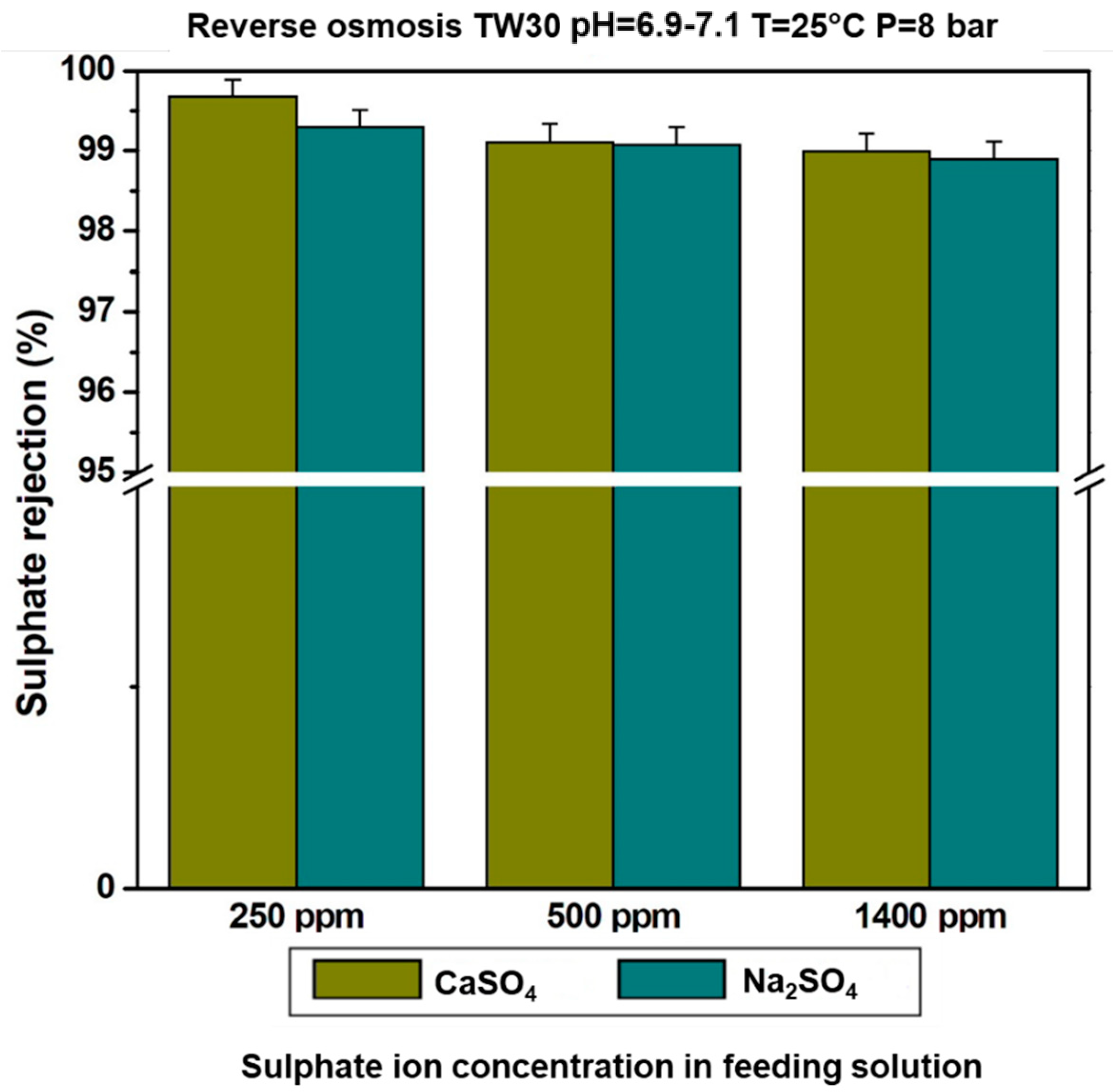
3.5. Effect of the Counter Ion on Sulphate Ion Retention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.; Cross, K.; Paden, M.; Laban, P. (Eds.) Spring: Managing Groundwater Sustainably; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2016; ISBN 978-2-8317-1789-0. [Google Scholar]
- Carvalho Resende, T.; Longuevergne, L.; Gurdak, J.; Leblanc, M.; Favreau, G.; Ansems, N.; van der Gun, J.; Gaye, C.B.; Aureli, A. Assessment of the impacts of climate variability on total water storage across Africa: Implications for groundwater resources management. Hydrogeol. J. 2019, 27, 493–512. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, A.M.; Lark, R.M.; Taylor, R.G.; Abiye, T.; Fallas, H.C.; Favreau, G.; Goni, I.B.; Kebede, S.; Scanlon, B.; Sorensen, J.P.R.; et al. Mapping groundwater recharge in Africa from ground observations and implications for water security. Environ. Res. Lett. 2021, 16, 034012. [Google Scholar] [CrossRef]
- Xu, Y.; Seward, P.; Gaye, C.; Lin, L.; Olago, D.O. Preface: Groundwater in Sub-Saharan Africa. Hydrogeol. J. 2019, 27, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Gaye, C.B.; Tindimugaya, C. Review: Challenges and opportunities for sustainable groundwater management in Africa. Hydrogeol. J. 2018, 27, 1–12. [Google Scholar] [CrossRef]
- Grönwall, J.; Danert, K. Regarding Groundwater and DrinkingWater Access through A Human Rights Lens: Self-Supply as a Norm. Water 2020, 12, 419. [Google Scholar] [CrossRef] [Green Version]
- Faye, M.D.; Kafando, M.B.; Sawadogo, B.; Panga, R.; Ouédraogo, S.; Yacouba, H. Groundwater Characteristics and Quality in the Cascades Region of Burkina Faso. Resources 2022, 11, 61. [Google Scholar] [CrossRef]
- Kafando, M.B.; Koïta, M.; Le Coz, M.; Yonaba, O.R.; Fowe, T.; Zouré, C.O.; Faye, M.D.; Leye, B. Use of Multidisciplinary Approaches for Groundwater Recharge Mechanism Characterization in Basement Aquifers: Case of Sanon Experimental Catchment in Burkina Faso. Water 2021, 13, 3216. [Google Scholar] [CrossRef]
- Margat, J. Les Eaux Souterraines Dans le Monde; BRGM: Orléans, France, 1990; p. 44. [Google Scholar]
- Ayouba Mahamane, A.; Guel, B. Physico-Chemical Characterizations of Groundwater in the Locality of Yamtenga (Burkina Faso). Int. J. Biol. Chem. Sci. 2015, 9, 517–533. [Google Scholar] [CrossRef] [Green Version]
- Sako, A.; Bamba, O.; Gordio, A. Hydrogeochemical processes controlling groundwater quality around Bomboré gold mineralized zone, Central Burkina Faso. J. Geochem. Explor. 2016, 170, 58–71. [Google Scholar] [CrossRef]
- Das, S. Groundwater. J. Geol. Soc. India 2018, 91, 505. [Google Scholar] [CrossRef]
- Sinha Ray, S.P.; Elango, L. Deterioration of Groundwater Quality: Implications and Management. Water Governance: Challenges and ProspectsSingh, A., Saha, D., Tyagi, A.C., Eds.; Springer WaterSpringer: Singapore, 2019; pp. 87–101. ISBN 9789811327001. [Google Scholar]
- Wang, H.; Zhang, Q. Research Advances in Identifying Sulfate Contamination Sources of Water Environment by Using Stable Isotopes. Int. J. Environ. Res. Public. Health 2019, 16, 1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luczaj, J. Groundwater Quantity and Quality. Resources 2016, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Naseem, F.; Zobia Zia, H.; Ishtiaq Tariq, M.; Amjad Bashir, M.; Amber Hameed, S.; Samiullah, K.; Qayyoom, A.; Farooq, H.; Mehroz Afzal, R.; Hashem, M.; et al. Role of chemical composition of drinking water in human health of the community. J. King Saud Univ. -Sci. 2022, 34, 102232. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Fernando, W.A.M.; Ilankoon, I.M.S.K.; Syed, T.H.; Yellishetty, M. Challenges and opportunities in the removal of sulphate ions in contaminated mine water: A review. Miner. Eng. 2018, 117, 74–90. [Google Scholar] [CrossRef]
- Sharma, M.K.; Kumar, M. Sulphate contamination in groundwater and its remediation: An overview. Environ. Monit. Assess. 2020, 192, 74. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, M.; Hawari, A.H.; Alfahel, R.; Hassan, M.K.; Altaee, A. Comparison of Nanofiltration with Reverse Osmosis in Reclaiming Tertiary Treated Municipal Wastewater for Irrigation Purposes. Membranes 2021, 11, 32. [Google Scholar] [CrossRef]
- Ainscough, T.J.; Oatley-Radcliffe, D.L.; Barron, A.R. Groundwater Remediation of Volatile Organic Compounds Using Nanofiltration and Reverse Osmosis Membranes—A Field Study. Membranes 2021, 11, 61. [Google Scholar] [CrossRef]
- Fernández-Medrano, V.; Cuartas-Uribe, B.; Bes-Piá, M.-A.; Mendoza-Roca, J.-A. Application of Nanofiltration and Reverse Osmosis Membranes for Tannery Wastewater Reuse. Water 2022, 14, 2035. [Google Scholar] [CrossRef]
- Dykes, G.M.; Conlon, W.J. Use of Membrane Technology in Florida. J. AWWA 1989, 81, 43–46. [Google Scholar] [CrossRef]
- Dach, H. Comparison of nanofiltration and reverse osmosis processes for a selective desalination of brackish water feeds. Ph.D. Thesis, Université d’Angers, Angers, France, 2008. [Google Scholar]
- INSD. 5e Recensement Général de la Population et de l’habitat Burkina Faso: Synthèse des Résultats Définitifs; INSD: Ouagadougou, Burkina Faso, 2020. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, Incorporating the First Addendum, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- BUMIGEB. Notice Explicative de la Carte Géologique et Minière du Burkina Faso; BUMIGEB: Ouagadougou, Burkina Faso, 2003. [Google Scholar]
- Boualem, B.; Kherici, N.; Hadj-Said, S. Qualité des eaux des aquifères du Sahara septentrionale cas des eaux des aquifères d’El-Oued (Algérie). Int. J. Environ. Glob. Clim. Change 2015, 2, 21–31. [Google Scholar]
- Mansouri, Z.; Leghrieb, Y.; Kouadri, S.; Al-Ansari, N.; Najm, H.M.; Mashaan, N.S.; Eldirderi, M.M.A.; Khedher, K.M. Hydro-Geochemistry and Groundwater Quality Assessment of Ouargla Basin, South of Algeria. Water 2022, 14, 2441. [Google Scholar] [CrossRef]
- Porowski, A.; Porowska, D.; Halas, S. Identification of Sulfate Sources and Biogeochemical Processes in an Aquifer Affected by Peatland: Insights from Monitoring the Isotopic Composition of Groundwater Sulfate in Kampinos National Park, Poland. Water 2019, 11, 1388. [Google Scholar] [CrossRef] [Green Version]
- Harrak, N.E.; Elazhar, F.; Belhamidi, S.; Elazhar, M.; Touir, J.; Elmidaoui, A. Comparaison des performances des deux procédés membranaires: La Nanofiltration et de l’Osmose inverse dans le Dessalement des eaux saumâtres (Performances comparison of two membranes processes: Nanofiltration and Reverse Osmosis in brackish water Desalination). Mater. Environ. Sci. J. 2015, 6, 383–390. [Google Scholar]
- Ahoulé, D. Performances Comparatives des Techniques de Nanofiltration et d’osmose Inverse Pour le Traitement d’eau de Consommation Contaminée à l’arsenic au Burkina Faso. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2016. [Google Scholar]
- Sawadogo, B.; Konaté, Y.; Lesage, G.; Zaviska, F.; Monnot, M.; Heran, M.; Karambiri, H. Brewery wastewater treatment using MBR coupled with nanofiltration or electrodialysis: Biomass acclimation and treatment efficiency. Water Sci. Technol. 2018, 77, 2624–2634. [Google Scholar] [CrossRef]
- Castaño Osorio, S.; Biesheuvel, P.M.; Dykstra, J.E.; Virga, E. Nanofiltration of complex mixtures: The effect of the adsorption of divalent ions on membrane retention. Desalination 2022, 527, 115552. [Google Scholar] [CrossRef]
- Ouali, S.; Loulergue, P.; Biard, P.-F.; Nasrallah, N.; Szymczyk, A. Ozone compatibility with polymer nanofiltration membranes. J. Membr. Sci. 2021, 618, 118656. [Google Scholar] [CrossRef]
- Vaseghi, G.; Ghassemi, A.; Loya, J. Characterization of reverse osmosis and nanofiltration membranes: Effects of operating conditions and specific ion rejection. Desalination Water Treat. 2016, 57, 23461–23472. [Google Scholar] [CrossRef]
- Żyłła, R.; Foszpańczyk, M.; Olak-Kucharczyk, M.; Marszałek, J.; Ledakowicz, S. Removal of Organic Compounds with an Amino Group during the Nanofiltration Process. Membranes 2022, 12, 58. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Natural organic matter fouling and chemical cleaning of nanofiltration membranes. Water Supply 2004, 4, 245–251. [Google Scholar] [CrossRef]
- Lu, D.; Yao, Z.; Jiao, L.; Waheed, M.; Sun, Z.; Zhang, L. Separation mechanism, selectivity enhancement strategies and advanced materials for mono-/multivalent ion-selective nanofiltration membrane. Adv. Membr. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Lee, J.; Woo, Y.C.; Kim, H.S. Effect of driving pressure and recovery rate on the performance of nanofiltration and reverse osmosis membranes for the treatment of the effluent from MBR. Desalination Water Treat. 2015, 54, 3589–3595. [Google Scholar] [CrossRef]
- Alsalhy, Q.; Albayati, T.; Zablouk, M. A Study of the Effect of Operating Conditions on Reverse Osmosis Membrane Performance with and without Air Sparging Technique. Chem. Eng. Commun. 2013, 200, 1–19. [Google Scholar] [CrossRef]
- Ramdani, A.; Gassara, S.; Deratani, A.; Taleb, S. Nanofiltration Performance for Synthetic and Natural Water Defluorination: Application to South-Algeria Groundwater; Springer: Cham, Switzerland, 2018; pp. 481–491. [Google Scholar]
- Abouzaid, A.; Mouzdahir, A.; Rumeau, M. Étude de la rétention des sels monovalents et bivalents par nanofiltration. Comptes Rendus Chim. 2003, 6, 431–436. [Google Scholar] [CrossRef]


| Characteristics | NF | RO |
|---|---|---|
| Material | Polyamide Thin-film composite | |
| Type | NF270-2540 | TW30-2540 |
| Total filtration area (m2) | 2.6 | 2.6 |
| Membrane length (mm) | 1016 | 1016 |
| Diameter (mm) | 61 | 61 |
| Pure water permeability (LMH/bar) | 12.5 | 5.7 |
| Pressure (bar) | 5 | 16 |
| Manufacturer | Dow Filmtec | |
| Parameters | Unit | Concentration | WHO Drinking Water Standards [26] |
|---|---|---|---|
| pH | 6.9 ± 0.1 | 6.5–8.5 | |
| Turbidité (NTU) | NTU | 0.15 ± 0.01 | ≤5 |
| Temperature | °C | 27 ± 1 | - |
| Electric conductivity (EC) | µS/cm | 1520 ± 4 | ≤1200 |
| Total alkalinity | mg/L CaCO3 | 16 ± 2 | - |
| Total hardness | mg/L CaCO3 | 1220 ± 8 | - |
| Sulfate (SO42−) | mg/L | 1400 ± 28 | ≤250 |
| Calcium (Ca2+) | mg/L | 371.2 ± 9.3 | ≤100 |
| Sodium (Na+) | mg/L | 138.8 ± 3.7 | ≤200 |
| Potassium (K+) | mg/L | 9.3 ± 0.3 | ≤200 |
| Ammonium (NH4+) | mg/L | 0.38 ± 0.02 | ≤1.5 |
| Iron (Fe2+) | mg/L | 0.22 ± 0.03 | ≤0.3 |
| Magnesium (Mg2+) | mg/L | 70.1 ± 2.8 | ≤50 |
| Nitrite (NO2−) | mg/L | 0.063 ± 0.001 | ≤3 |
| Nitrate (NO3−) | mg/L | 9.6 ± 0.3 | ≤50 |
| Orthophosphate (PO43−) | mg/L | 0.11 ± 0.01 | - |
| Chloride (Cl−) | mg/L | 0.028 ± 0.002 | 250 |
| Sulfide (S2−) | mg/L | 0.009 ± 0.000 | - |
| Floride (F−) | mg/L | 0.40 ± 0.00 | ≤1.5 |
| Bicarbonate (HCO3−) | mg/L | 19.5 ± 0.9 | - |
| Parameters | Unit | Borehole Water | Permeate | Rejection Rate (%) | ||
|---|---|---|---|---|---|---|
| NF | RO | NF | RO | |||
| pH | 6.9 ± 0.1 | 7.0 | 7.0 | - | - | |
| Turbidity | NTU | 0.15 ± 0.01 | 0.06 | 0.01 | 60.0 | 93.3 |
| Temperature | °C | 27 ± 1 | 35 | 35 | - | - |
| Electric conductivity | µS/cm | 1520 ± 4 | 13 | 10 | 99.1 | 99.4 |
| Total alkalinity | mg CaCO3/L | 16 ± 2 | 7 | 2 | 56.3 | 87.5 |
| Total hardness | mg CaCO3/L | 1220 ± 8 | 56 | 19 | 95.4 | 98.4 |
| Sulfate (SO42−) | mg/L | 1400 ± 28 | 18 | 14 | 98.7 | 99.0 |
| Calcium (Ca2+) | mg/L | 371.2 ± 9.3 | 20 | 2 | 94.6 | 99.5 |
| Sodium (Na+) | mg/L | 138.8 ± 3.7 | 34.2 | 3.7 | 75.3 | 97.3 |
| Potassium (K+) | mg/L | 9.3 ± 0.3 | 2.0 | 0.0 | 78.5 | 100.0 |
| Ammonium (NH4+) | mg/L | 0.38 ± 0.02 | 0.18 | 0.03 | 52.6 | 92.1 |
| Iron (Fe2+) | mg/L | 0.22 ± 0.03 | 0.03 | 0.02 | 86.4 | 90.9 |
| Magnesium (Mg2+) | mg/L | 70.1 ± 2.8 | 1.4 | 3.4 | 98.0 | 95.1 |
| Nitrite (NO2−) | mg/L | 0.063 ± 0.001 | 0.016 | 0.014 | 74.6 | 77.8 |
| Nitrate (NO3−) | mg/L | 9.6 ± 0.3 | 5.0 | 2.6 | 47.9 | 72.9 |
| Orthophosphate (PO43−) | mg/L | 0.11 ± 0.01 | 0.04 | 0.01 | 63.6 | 90.9 |
| Chloride (Cl−) | mg/L | 0.028 ± 0.002 | 0.0004 | 0.0003 | 98.6 | 98.9 |
| Sulfide (S2−) | mg/L | 0.009 ± 0.000 | 0.003 | 0.001 | 66.7 | 88.9 |
| Floride (F−) | mg/L | 0.40 ± 0.00 | 0.27 | 0.01 | 32.5 | 97.5 |
| Bicarbonate (HCO3−) | mg/L | 19.5 ± 0.9 | 8.5 | 2.4 | 56.4 | 87.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawadogo, B.; Konaté, Y.; Sossou, S.K.; Ado Saidou, N.F.; Nouhou Moussa, A.W.; Karambiri, H. Removal of Sulphate Ions from Borehole Water Using Nanofiltration and Reverse Osmosis. Water 2022, 14, 3422. https://doi.org/10.3390/w14213422
Sawadogo B, Konaté Y, Sossou SK, Ado Saidou NF, Nouhou Moussa AW, Karambiri H. Removal of Sulphate Ions from Borehole Water Using Nanofiltration and Reverse Osmosis. Water. 2022; 14(21):3422. https://doi.org/10.3390/w14213422
Chicago/Turabian StyleSawadogo, Boukary, Yacouba Konaté, Seyram Kossi Sossou, Nana Fassouma Ado Saidou, Abdoul Wahab Nouhou Moussa, and Harouna Karambiri. 2022. "Removal of Sulphate Ions from Borehole Water Using Nanofiltration and Reverse Osmosis" Water 14, no. 21: 3422. https://doi.org/10.3390/w14213422




