The Suitability of Hybrid Fe0/Aggregate Filtration Systems for Water Treatment
Abstract
:1. Introduction
2. The Remediation Fe0/Aggregate Filtration System and Its Proper Investigation
2.1. Historical and Fundamental Aspects
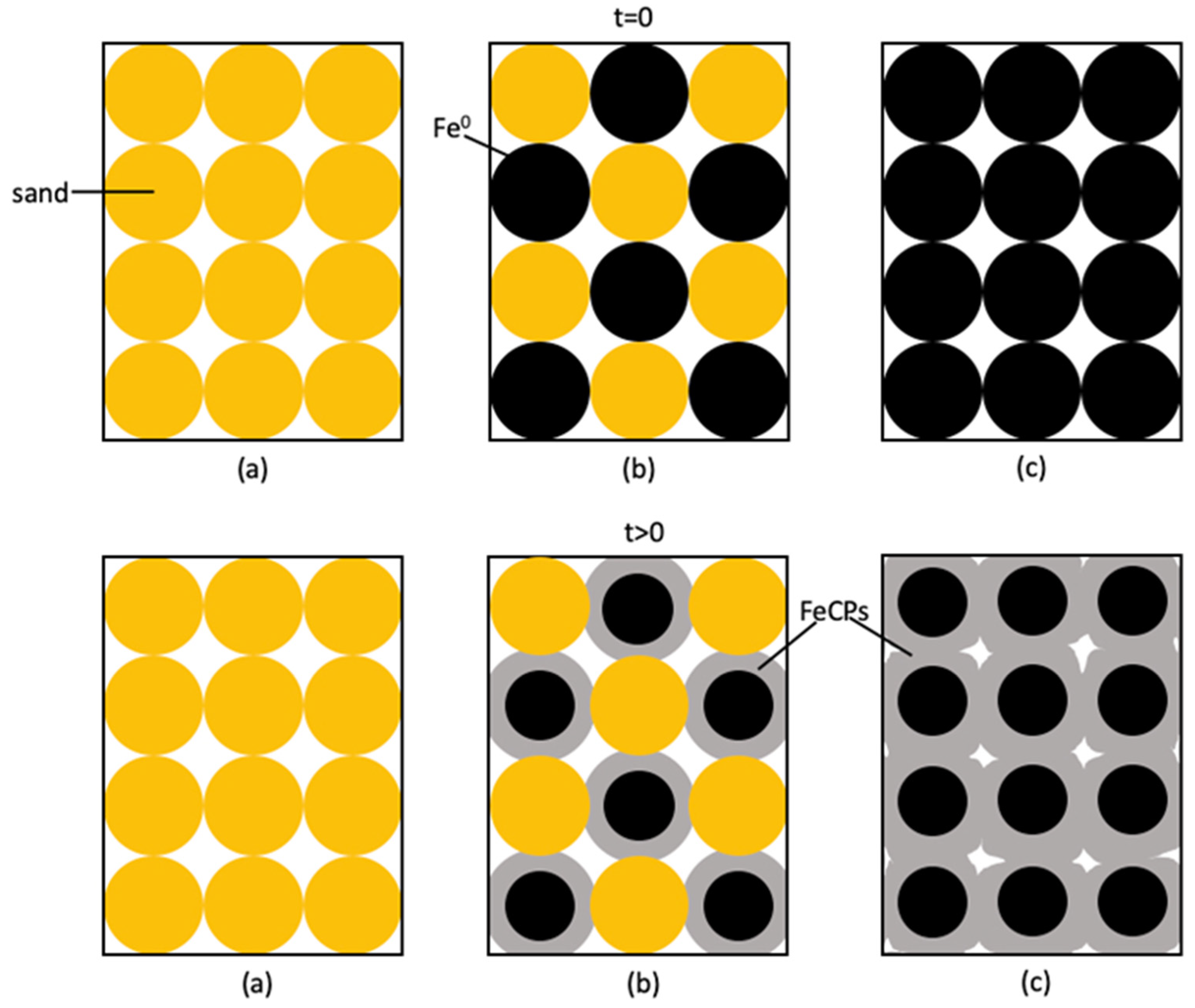
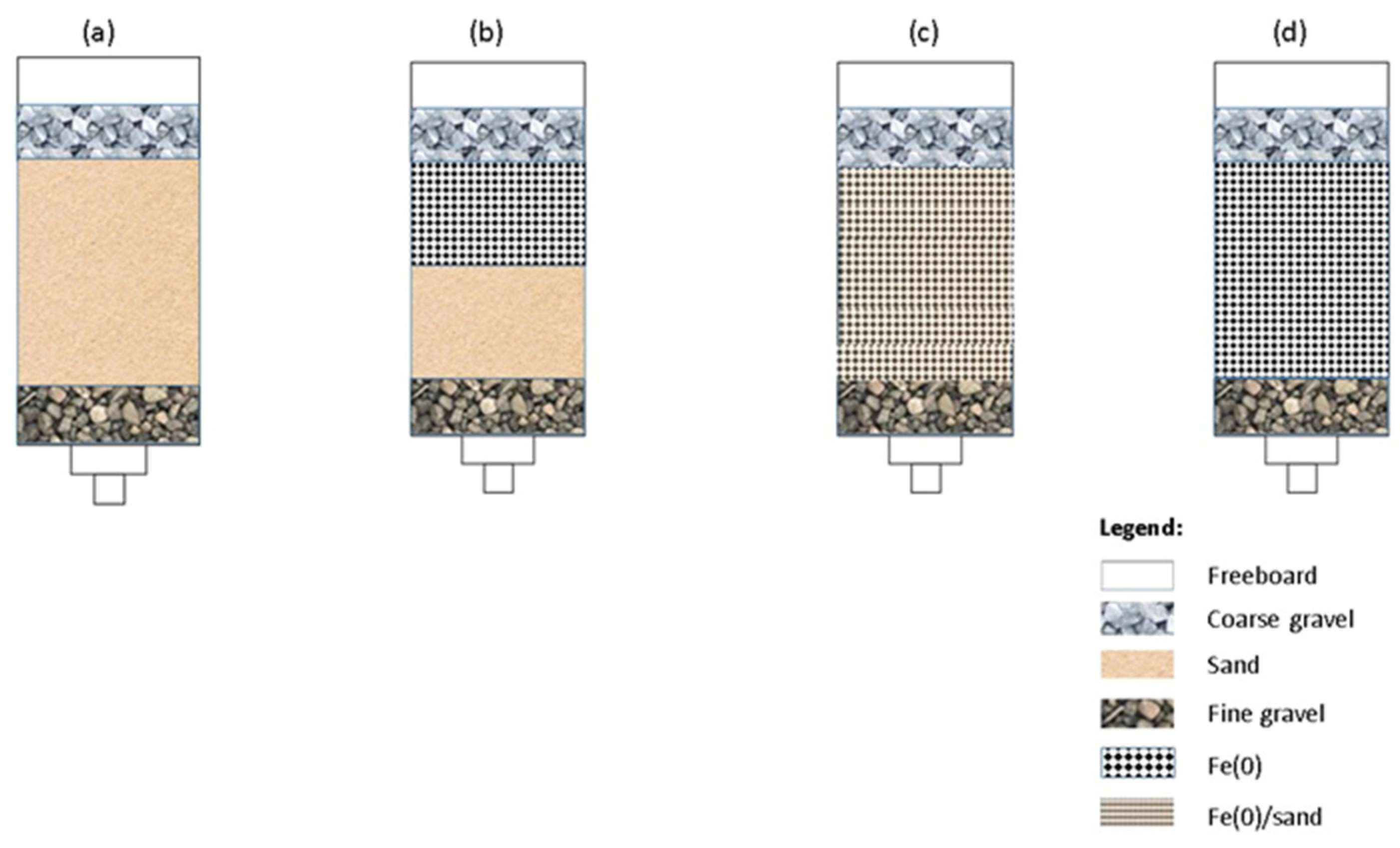
2.2. Investigating the Remediation Fe0/Aggregate Filtration System
2.3. Investigating Fe0/Aggregate Systems
3. Economics of Fe0 Filters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamlin, C. A Science of Impurity: Water Analysis in Nineteenth Century Britain; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1990. [Google Scholar]
- Howe, K.J.; Hand, D.W.; Crittenden, J.C.; Trussell, R.R.; Tchobanoglous, G. Principles of Water Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. 674. [Google Scholar]
- Baker, M.N. The Quest for Pure Water: The History of Water Purification from the Earliest Records to the Twentieth Century; American Water Works Assn.: New York, NY, USA, 1948; p. 527. [Google Scholar]
- UN SDGs. Transforming Our World: The 2030 Agenda for Sustainable Development. Resolution Adopted by the UN General Assembly. 2015. Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 4 December 2021).
- He, Z.; Bishwajit, G.; Zou, D.; Yaya, S.; Cheng, Z.; Zhou, Y. Burden of Common Childhood Diseases in Relation to Improved Water, Sanitation, and Hygiene (WASH) among Nigerian Children. Int. J. Environ. Res. Public Health 2018, 15, 1241. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wu, J. Drinking water quality and public health. Expo. Health 2019, 11, 73–79. [Google Scholar] [CrossRef]
- Hope, R.; Ballon, P. Global water policy and local payment choices in rural Africa. NPJ Clean Water 2019, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Hope, R.; Thomson, P.; Koehler, J.; Foster, T. Rethinking the economics of rural water in Africa. Oxford Rev. Econ. 2020, 36, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Domènech, L. Rethinking water management: From centralised to decentralised water supply and sanitation models. Doc. D’anàlisi Geogràfica 2011, 57, 293–310. [Google Scholar] [CrossRef]
- Schumacher, E.F. Small is Beautiful: Economics as if People Mattered; Harper & Row: New York, NY, USA, 1973; p. 324. [Google Scholar]
- Moropeng, R.C.; Budeli, P.; Mpenyana-Monyatsi, L.; Momba, M.N.B. Dramatic reduction in diarrhoeal diseases through implementation of cost-effective household drinking water treatment systems in Makwane Village, Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 410. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Nya, E.L.; Cao, V.; Gwenzi, W.; Rahman, M.A.; Noubactep, C. Universal access to safe drinking water: Escaping the traps of non-frugal technologies. Sustainability 2021, 13, 9645. [Google Scholar] [CrossRef]
- Kearns, J.; Dickenson, E.; Aung, M.T.; Joseph, S.M.; Summers, S.R.; Knappe, D. Biochar water treatment for control of organic micropollutants with UVA surrogate monitoring. Environ. Eng. Sci. 2021, 38, 298–309. [Google Scholar] [CrossRef]
- Nya, E.L.; Feumba, R.; Fotsing-Kwetché, P.R.; Gwenzi, W.; Noubactep, C. A hybrid model for achieving universal safe drinking water in the medium-sized city of Bangangté (Cameroon). Water 2021, 13, 3177. [Google Scholar] [CrossRef]
- Roy, J. Economic benefits of arsenic removal from ground water—A case study from West Bengal, India. Sci. Total Environ. 2008, 397, 1–12. [Google Scholar] [CrossRef]
- Roy, B.; Hartigan, J. Empowering the rural poor to develop themselves: The barefoot approach. Innovations 2008, 3, 67–93. [Google Scholar] [CrossRef]
- Banerji, T.; Chaudhari, S. A cost-effective technology for arsenic removal: Case study of zerovalent iron-based IIT Bombay arsenic filter in West Bengal. In Water and Sanitation in the New Millennium; Nath, K., Sharma, V., Eds.; Springer: New Delhi, India, 2017; pp. 127–137. [Google Scholar]
- Hering, J.G.; Maag, S.; Schnoor, J.L. A call for synthesis of water research to achieve the sustainable development goals by 2030. Environ. Sci. Technol. 2016, 50, 6122–6123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, M.N. Sketch of the history of water treatment. J. Am. Water Works Assoc. 1934, 26, 902–938. [Google Scholar] [CrossRef]
- Van Craenenbroeck, W. Easton & Anderson and the water supply of Antwerp (Belgium). Ind. Archaeol. Rev. 1998, 20, 105–116. [Google Scholar]
- Mwakabona, H.T.; Ndé-Tchoupé, A.I.; Njau, K.N.; Noubactep, C.; Wydra, K.D. Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res. 2017, 117, 127–142. [Google Scholar] [CrossRef]
- Antia, D.D.J. Water treatment and desalination using the eco-materials n-Fe0 (ZVI), n-Fe3O4, n-FexOyHz[mH2O], and n-Fex[Cation]nOyHz[Anion]m [rH2O]. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Ed.; Springer Nature: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Wolfe, N.L.; Cipollone, M.G. Remediation of environmental contaminants using a metal and a sulfur-containing compound. Int. J. Occup. Med. Environ. Health 2001, 14, 241–248. [Google Scholar]
- Zhang, P.; Simunek, J.; Bowman, R.S. Nonideal transport of solute and colloidal tracers through reactive zeolite/iron pellets. Water Resour. Res. 2004, 40, W04207. [Google Scholar] [CrossRef] [Green Version]
- Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freiberg Online Geosci. 2012, 32, 1–60. [Google Scholar]
- Miyajima, K.; Noubactep, C. Effects of mixing granular iron with sand on the efficiency of methyleneblue discoloration. Chem. Eng. J. 2012, 200–202, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Miyajima, K.; Noubactep, C. Impact of Fe0 amendment on methylene blue discoloration by sand columns. Chem. Eng. J. 2013, 217, 310–319. [Google Scholar] [CrossRef] [Green Version]
- Miyajima, K.; Noubactep, C. Characterizing the impact of sand addition on the efficiency of granular iron for water treatment. Chem. Eng. J. 2015, 262, 891–896. [Google Scholar] [CrossRef]
- Yang, H.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Ruppert, H.; Noubactep, C. Designing the next generation of Fe0-based filters for decentralized safe drinking water treatment. Processes 2020, 8, 745. [Google Scholar] [CrossRef]
- Yang, H.; Hu, R.; Ruppert, H.; Noubactep, C. Modeling porosity loss in Fe0-based permeable reactive barriers with Faraday’s law. Sci. Rep. 2021, 11, 16998. [Google Scholar] [CrossRef]
- Noubactep C: Should the term ‘metallic iron’ appear in the title of a research paper? Chemosphere 2022, 287, 132314. [CrossRef] [PubMed]
- Anderson, W. On the purification of water by agitation with iron and by sand filtration. J. Soc. Arts 1886, 35, 29–38. [Google Scholar] [CrossRef]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- Njaramba, L.K.; Park, J.-B.; Lee, C.-S.; Nzioka, A.M.; Kim, Y.-J. Permeable reactive barriers with zero-valent iron and pumice for remediation of groundwater contaminated with multiple heavy metals. Environ. Eng. Sci. 2021, 38, 245–255. [Google Scholar] [CrossRef]
- Mackenzie, P.D.; Horney, D.P.; Sivavec, T.M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 1999, 68, 1–17. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef] [Green Version]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 1994, 32, 958–967. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “iron wall” for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Bi, E.; Devlin, J.F.; Huang, B. Effects of mixing granular iron with sand on the kinetics of trichloroethylene reduction. Ground Water Monit. Remed. 2009, 29, 56–62. [Google Scholar] [CrossRef]
- Kaplan, D.I.; Gilmore, T.J. Zero-valent iron removal rates of aqueous Cr(VI) Measured under flow conditions. Water Air Soil Pollut. 2004, 155, 21–33. [Google Scholar] [CrossRef]
- Ulsamer, S. A Model to Characterize the Kinetics of Dechlorination of Tetrachloroethylene and Trichloroethylene by a Zero Valent Iron Permeable Reactive Barrier. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2011; p. 73. [Google Scholar]
- Song, D.-I.; Kim, Y.H.; Shin, W.S. A simple mathematical analysis on the effect of sand in Cr(VI) reduction using zero valent iron. Korean J. Chem. Eng. 2005, 22, 67–69. [Google Scholar] [CrossRef]
- Konadu-Amoah, B.; Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Noubactep, C. Investigating the Fe0/H2O systems using the methylene blue method: Validity, applications and future directions. Chemosphere 2021, 132913, in press. [Google Scholar] [CrossRef] [PubMed]
- Sarr, D. Zero-valent-iron permeable reactive barriers—How long will they last? Remediation 2001, 11, 1–18. [Google Scholar]
- Whitney, W.R. The corrosion of iron. J. Am. Chem. Soc. 1903, 25, 394–406. [Google Scholar] [CrossRef]
- ITRC (Interstate Technology & Regulatory Council). Permeable Reactive Barrier: Technology Update. PRB-5; PRB: Technology Update Team; Interstate Technology & Regulatory Council: Washington, DC, USA, 2011; Available online: www.itrcweb.org (accessed on 5 December 2021).
- Hu, R.; Cui, X.; Gwenzi, W.; Wu, S.; Noubactep, C. Fe0/H2O systems for environmental remediation: The scientific history and future research directions. Water 2018, 10, 1739. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water treatment using metallic iron: A tutorial review. Processes 2019, 7, 622. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Ndé-Tchoupé, A.I.; Cao, V.; Gwenzi, W.; Noubactep, C. Metallic iron for environmental remediation: The fallacy of the electron efficiency concept. Front. Environ. Chem. 2021, 2, 677813. [Google Scholar] [CrossRef]
- Moraci, N.; Lelo, D.; Bilardi, S.; Calabrò, P.S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Can. Geotech. J. 2016, 53, 946–961. [Google Scholar] [CrossRef]
- Florea, A.F.; Lu, C.; Hansen, H.C.B. A zero-valent iron and zeolite filter for nitrate recycling from agricultural drainage water. Chemosphere 2022, 287, 131993. [Google Scholar] [CrossRef]
- Notter, J.L. The purification of water by filtration. Br. Med. J. 1878, 2, 556–557. [Google Scholar] [CrossRef] [Green Version]
- Tucker, W.G. The purification of water by chemical treatment. Science 1892, 20, 34–38. [Google Scholar] [CrossRef]
- Oldright, G.L.; Keyes, H.E.; Miller, V.; Sloan, W.A. Precipitation of lead and copper from solution on sponge iron. BuMines B 1928, 281, 131. [Google Scholar]
- Ali, I. Water treatment by adsorption columns: Evaluation at ground level. Sep. Purif. Rev. 2014, 43, 175–205. [Google Scholar] [CrossRef]
- Siwila, S.; Brink, I.C. A small-scale low-cost water treatment system for removal of selected heavy metals, bacteria and particles. Water Pract. Technol. 2018, 13, 446–459. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R. Designing and Piloting a household filter for the peri-urban population of Douala (Cameroon). Freiberg Online Geosci. 2021, 61, 1–90. [Google Scholar]
- Sasaki, K.; Nukina, S.; Wilopo, W.; Hirajima, T. Removal of arsenate in acid mine drainage by a permeable reactive barrier bearing granulated blast furnace slag: Column study. Mater. Trans. 2008, 49, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Komnitsas, K.; Bazdanis, G.; Bartzas, G.; Sahinkaya, E.; Zaharaki, D. Removal of heavy metals from leachates using organic/inorganic permeable reactive barriers. Desalination Water Treat. 2013, 51, 3052–3059. [Google Scholar] [CrossRef]
- Westholm, L.J.; Repo, E.; Sillanpää, M. Filter materials for metal removal from mine drainage—A review. Environ. Sci. Pollut. Res. 2014, 21, 9109–9128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandra, H.; McCarthy, D.T.; Fletcher, T.D.; Deletic, A. Assessment of clogging phenomena in granular filter media used for stormwater treatment. J. Hydrol. 2014, 512, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Kandra, H.S.; Deletic, A.; McCarthy, D. Assessment of impact of filter design variables on clogging in stormwater filters. Water Resour. Manag. 2014, 28, 1873–1885. [Google Scholar] [CrossRef]
- Kandra, H.; Callaghan, J.; Deletic, A.; McCarthy, D. Investigation of biological clogging in stormwater filters. J. Environ. Eng. ASCE 2015, 141, 1–8. [Google Scholar] [CrossRef]
- Yao, K.-M.; Habibian, M.T.; O’Melia, C.R. Water and waste water filtration: Concepts and applications. Environ. Sci. Technol. 1971, 5, 1105–1112. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Impact of solids formation and gas production on the permeability of ZVI PRBs. J. Environ. Eng. 2011, 137, 689–696. [Google Scholar] [CrossRef]
- Reddy, K.R.; Dastgheibi, S.; Cameselle, C. Mixed versus layered multi-media filter for simultaneous removal of nutrients and heavy metals from urban stormwater runoff. Environ. Sci. Pollut. Res. 2021, 28, 7574–7585. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for water treatment: A critical review. Clean—Soil Air Water 2013, 41, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Noubactep, C. Predicting the hydraulic conductivity of metallic iron filters: Modeling gone astray. Water 2016, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Caré, S.; Crane, R.; Calabrò, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modeling the permeability loss of metallic iron water filtration systems. Clean—Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Metals. 1923, 29, 529–591. [Google Scholar]
- Lauderdale, R.A.; Emmons, A.H. A method for decontaminating small volumes of radioactive water. J. Am. Water Work. Assoc. 1951, 43, 327–331. [Google Scholar] [CrossRef]
- Anderson, W. Purification of water by iron on a large scale. J. Soc. Arts 1883, 32, 963. [Google Scholar] [CrossRef]
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ. Res. 1992, 64, 699–705. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Esumi, H.; Omura, S. High performance and N, P removable on-site domestic wastewater treatment system by multi-soil-layering method. Water Sci. Technol. 1993, 27, 31–40. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Enhanced sand filtration for storm water phosphorus removal. J. Environ. Eng. 2007, 133, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Hussam, A. Contending with a development disaster: Sono filters remove arsenic from well water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Kenneke, J.F.; McCutcheon, S.C. Use of pretreatment zones and zero-valent iron for the remediation of chloroalkenes in an oxic aquifer. Environ. Sci. Technol. 2003, 37, 2829–2835. [Google Scholar] [CrossRef]
- McMahon, P.; Dennehy, K.; Sandstrom, M. Hydraulic and geochemical performance of a permeable reactive barrier containing zero-valent iron. Ground Water 1999, 37, 396–404. [Google Scholar] [CrossRef]
- Phillips, D.; Gu, B.; Watson, D.; Roh, Y.; Liang, L.; Lee, S. Performance evaluation of a zerovalent iron reactive barrier: Mineralogical characteristics. Environ. Sci. Technol. 2000, 34, 4169–4176. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S. Dimensioning metallic iron beds for efficient contaminant removal. Chem. Eng. J. 2010, 163, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C.; Caré, S. Enhancing sustainability of household water filters by mixing metallic iron with porous materials. Chem. Eng. J. 2010, 162, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Karmakar, S.; Salama, H.; Gactha-Bandjun, N.; Btatkeu, K.B.D.; Noubactep, C. Optimising the design of Fe0-based filtration systems for water treatment: The suitability of porous iron composites. J. Appl. Solution Chem. Model. 2013, 2, 165–177. [Google Scholar]
- Noubactep, C.; Schöner, A.; Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean Soil Air Water 2009, 37, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C.; Schöner, A. Metallic iron: Dawn of a new era of drinking water treatment research? Fresenius Environ. Bull. 2010, 19, 1661–1668. [Google Scholar]
- Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; Noubactep, C.; Nassi, A.; Ruppert, H. Characterizing a newly designed steel-wool-based household filter for safe drinking water provision: Hydraulic conductivity and efficiency for pathogen removal. Processes 2019, 7, 966. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C. The fundamental mechanism of aqueous contaminant removal by metallic iron. Water SA 2010, 36, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C. Aqueous contaminant removal by metallic iron: Is the paradigm shifting? Water SA 2011, 37, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C. Metallic iron for environmental remediation: A review of reviews. Water Res. 2015, 85, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y. Application of coupled zero-valent iron/biochar system for degradation of chlorobenzene-contaminated groundwater. Water Sci. Technol. 2017, 75, 571–580. [Google Scholar] [CrossRef]
- Huang, Y.H.; Tang, C.; Zeng, H. Removing molybdate from water using a hybridized zero-valent iron/magnetite/Fe(II) treatment system. Chem. Eng. J. 2012, 200–202, 257–263. [Google Scholar] [CrossRef]
- Lü, Y.; Li, Z.; Li, J.; Chen, K.; Dong, H.; Shou, J.; Li, Y. Synergetic effect of pyrite on Cr(VI) removal by zero valent iron in column experiments: An investigation of mechanisms. Chem. Eng. J. 2018, 349, 522–529. [Google Scholar] [CrossRef]
- Tseng, H.H.; Su, J.G.; Liang, C. Synthesis of granular activated carbon/zero valent iron composites for simultaneous adsorption/dechlorination of trichloroethylene. J. Hazard Mater. 2011, 192, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Bilardi, S.; Calabrò, P.S.; Moraci, N. The removal efficiency and long-term hydraulic behaviour of zero valent iron/lapillus mixtures for the simultaneous removal of Cu2+, Ni2+ and Zn2+. Sci. Tot. Environ. 2019, 675, 490–500. [Google Scholar] [CrossRef]
- Dong, G.; Huang, L.; Wu, X.; Wang, C.; Liu, Y.; Liu, G.; Wang, L.; Liu, X.; Xia, H. Effect and mechanism analysis of MnO2 on permeable reactive barrier (PRB) system for the removal of tetracycline. Chemosphere 2018, 193, 702–710. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I.; Makota, S.; Nassi, A.; Hu, R.; Noubactep, C. The suitability of pozzolan as admixing aggregate for Fe0-based filters. Water 2018, 10, 417. [Google Scholar] [CrossRef] [Green Version]
- Moraci, N.; Calabrò, P.S. Heavy metals removal and hydraulic performance in zero-valent iron/pumice permeable reactive barriers. J. Environ. Manag. 2010, 91, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Wilopo, W.; Sasaki, K.; Hirajima, T. Contribution of sheep manurebacteria in the immobilization ofarsenic from groundwater usingzero-valent iron. J. SE Asian Appl. Geol. 2010, 2, 1–11. [Google Scholar]
- Fronczyk, J. Properties of reactive materials for application in runoff water treatment systems. J. Ecol. Eng. 2020, 21, 185–197. [Google Scholar] [CrossRef]
- Reardon, J.E. Anaerobic corrosion of granular iron: Measurement and interpretation of hydrogen evolution rates. Environ. Sci. Technol. 1995, 29, 2936–2945. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Sauter, M.; Merkel, B. Testing the suitability of zerovalent iron materials for reactive Walls. Environ. Chem. 2005, 2, 71–76. [Google Scholar] [CrossRef]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A novel and facile method to characterize the suitability of metallic iron for water treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef] [Green Version]
- Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Nassi, A.; Noubactep, C. Characterizing the reactivity of metallic iron for water treatment: H2 evolution in H2SO4 and uranium removal efficiency. Water 2020, 12, 1523. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E.; Harms, S.; Milburn, R.; Sprah, G.; Nadarajah, N. Degradation of carbon tetrachloride in the presence of iron and sulphur containing compounds. Chemosphere 1994, 29, 1477–1489. [Google Scholar] [CrossRef]
- Butler, C.E.; Hayes, F.K. Factors influencing rates and products in the transformation of trichloroethylene by iron sulfide and iron metal. Environ. Sci. Technol. 2001, 35, 3884–3891. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Merkel, B. Mitigating uranium in groundwater: Prospects and limitations. Environ. Sci. Technol. 2003, 37, 4304–4308. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C.; Meinrath, G.; Merkel, J.B. Investigating the mechanism of uranium removal by zerovalent iron materials. Environ. Chem. 2005, 2, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C.; Schöner, A.; Meinrath, G. Mechanism of uranium (VI) fixation by elemental iron. J. Hazard Mater. 2006, 132, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, A.D.; Demond, A.H. Permeability of iron sulfide (FeS)-based materials for groundwater remediation. Water Res. 2013, 47, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, Y.; Hussain, I.; Du, X.; Huang, S.; Wen, W. Effect of pyrite on enhancement of zero-valent iron corrosion for arsenic removal in water: A mechanistic study. Chemosphere 2019, 233, 744–753. [Google Scholar] [CrossRef]
- Lü, Y.; Li, J.; Li, Y.; Liang, L.; Dong, H.; Chen, K.; Yao, C.; Li, Z.; Li, J.; Guan, X. The roles of pyrite for enhancing reductive removal of nitrobenzene by zero-valent iron. Appl. Catal. B Environ. 2019, 242, 9–18. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Zeng, X.; Kuang, H.; Huang, S. Enhancement of ballmiling on pyrite/zero-valent iron for arsenic removal in water: A mechanistic study. Chemosphere 2020, 249, 126130. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Cui, X.; Xiao, M.; Gwenzi, W.; Noubactep, C. Characterizing the impact of pyrite addition on the efficiency of Fe0/H2O systems. Sci. Rep. 2021, 11, 2326. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, R.; Cui, X.; Gwenzi, W.; Noubactep, C. Understanding the operating mode of Fe0/Fe-sulfide/H2O systems for water treatment. Processes 2020, 8, 409. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.; Cui, X.; Hu, R.; Gwenzi, W.; Noubactep, C. Validating the efficiency of the FeS2 method for elucidating the mechanisms of contaminant removal using Fe0/H2O systems. Processes 2020, 8, 1162. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Kojima, M.; Hiroyoshi, N.; Ito, M. Galvanic microencapsulation (GME) using zero-valent aluminum and zero-valent iron to suppress pyrite oxidation. Mater. Trans. 2019, 60, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Tabelin, C.B.; Park, I.; Li, X.; Seng, S.; Villacorte-Tabelin, M.; Igarashi, T.; Ito, M.; Hiroyoshi, N. Development of advanced pyrite passivation strategies towards sustainable management of acid mine drainage. IOP Conf. Ser. Earth Environ. Sci. 2019. [Google Scholar] [CrossRef]
- Naseri, E.; Ndé-Tchoupé, A.I.; Mwakabona, H.T.; Nanseu-Njiki, C.P.; Noubactep, C.; Njau, K.N.; Wydra, K.D. Making Fe0-based filters a universal solution for safe drinking water provision. Sustainability 2017, 9, 1224. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C. Processes of contaminant removal in “Fe0–H2O” systems revisited. The importance of co-precipitation. Open Environ. Sci. 2007, 1, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Btatkeu, K.B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Determining the optimum Fe0 ratio for sustainable granular Fe0/sand water filters. Chem. Eng. J. 2014, 247, 265–274. [Google Scholar] [CrossRef]
- Btatkeu, K.B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Characterizing the impact of MnO2 on the efficiency of Fe0-based filtration systems. Chem. Eng. J. 2014, 250, 416–422. [Google Scholar] [CrossRef]
- Cao, V.; Alyoussef, G.; Gatcha-Bandjun, N.; Gwenzi, W.; Noubactep, C. The key role of contact time in elucidating the mechanism of enhanced decontamination by Fe0/MnO2/sand systems. Sci. Rep. 2021, 11, 12069. [Google Scholar] [CrossRef]
- Cao, V.; Alyoussef, G.; Gatcha-Bandjun, N.; Gwenzi, W.; Noubactep, C. The suitability of methylene blue discoloration (MB method) to investigate the Fe0/MnO2 system. Processes 2021, 9, 548. [Google Scholar] [CrossRef]
- Attia, A.A.; Girgis, B.S.; Fathy, N.A. Removal of methylene blue by carbons derived from peach stones by H3PO4 activation: Batch and column studies. Dye. Pigment. 2008, 76, 282–289. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Murcott, S.; Shrestha, R.R.; Dangol, B.; Maharjan, M. Development and dissemination of Kanchan™ Arsenic Filter in rural Nepal. Water Sci. Technol. Water Supply 2006, 6, 137–146. [Google Scholar] [CrossRef]
- Ogata, R.; Dangol, B.; Sakamoto, M. Sustainability assessment of long-term, widely used household Kanchan Arsenic Filters in Nepal. J. Environ. Sci. Health Part A Tox Hazard Subst. Environ. Eng. 2020, 55, 517–527. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, V.; Nya, E.L.; Gwenzi, W.; Noubactep, C. Kanchan arsenic filters and the future of Fe0-based filtration systems for single household drinking water supply. Processes 2021, 9, 58. [Google Scholar] [CrossRef]
- Mueller, B.; Dangol, B.; Ngai, T.K.K.; Hug, S.J. Kanchan arsenic filters in the lowlands of Nepal: Mode of operation, arsenic removal, and future improvements. Environ. Geochem. Health 2021, 43, 375–389. [Google Scholar] [CrossRef]
- Huang, J.; Ye, Y.; Fu, Z.; Dun, W.J.; Wang, Y.; Fang, L.; Ye, S.; Ye, X.; Jin, J.; Hu, Q.; et al. Synergetic effects of zero-valent iron and Morganella morganii on the removal of Cr(VI) from Wastewater. Nat. Environ. Poll. Tech. 2019, 18, 871–877. [Google Scholar]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorgani, A.E.; Cibati, A.; Trois, C. Assessment of a natural iron-based sand for the removal of nitrate from water. Water Air Soil Pollut. 2016, 227, 1–11. [Google Scholar]
- Al-Hashimi, O.; Hashim, K.; Loffill, E.; Cebašek, T.M.; Nakouti, I.; Faisal, A.A.H.; Al-Ansari, N. A Comprehensive Review for Groundwater Contamination and Remediation: Occurrence, Migration and Adsorption Modelling. Molecules 2021, 26, 5913. [Google Scholar] [CrossRef]
- Amoako-Nimako, G.K.; Yang, X.; Chen, F. Denitrification using permeable reactive barriers with organic substrate or zero-valent iron fillers: Controlling mechanisms, challenges, and future perspectives. Environ. Sci. Pollut. Res. 2021, 28, 21045–21064. [Google Scholar] [CrossRef]
- Cao, V.; Alyoussef, G.; Gatcha-Bandjun, N.; Gwenzi, W.; Noubactep, C. Characterizing the impact of MnO2 addition on the efficiency of Fe0/H2O systems. Sci. Rep. 2021, 11, 9814. [Google Scholar] [CrossRef]
- Gong, L.; Qiu, X.; Cheng, D.; Hu, Y.; Zhang, Z.; Yuan, Q.; Yang, D.; Liu, C.; Liang, L.; He, F. Coincorporation of N and S into zero-valent iron to enhance TCE dechlorination: Kinetics, electron efficiency, and dechlorination capacity. Environ. Sci. Technol. 2021, 55, 16088–16098. [Google Scholar] [CrossRef]
- Huang, J.; Jones, A.; Waite, T.D.; Chen, Y.; Huang, X.; Rosso, K.M.; Kappler, A.; Mansor, M.; Tratnyek, P.G.; Zhang, H. Fe(II) redox chemistry in the environment. Chem. Rev. 2021, 121, 8161–8233. [Google Scholar] [CrossRef]
- Natarajan, P.; Gulliver, J.S.; Arnold, W.A. Iron filings application to reduce lake sediment phosphorus release. Lake Reserv. Manag. 2021, 27, 143–159. [Google Scholar] [CrossRef]
- Berthod, A.; Armstrong, D.W. Future perspectives for ionic liquids. In Ionic Liquids in Analytical Chemistry; American Chemical Society: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Coughlin, B.L.; Litt, P.K.; Kim, S.; Craighead, S.; Kelly, A.J.; Chiu, P.; Sharma, M.; Kniel, K.E. Zero-valent iron filtration reduces microbial contaminants in irrigation water and transfer to raw agricultural commodities. Microorganisms 2021, 9, 2009. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tian, A.; Feng, Z.; Zhang, Y.; Jiang, F.; Tang, Q. Evaluation of zero-valent iron for Pb(II) contaminated soil remediation: From the analysis of experimental mechanism hybird with carbon emission assessment. Sustainability 2021, 13, 452. [Google Scholar] [CrossRef]
- Kundu, D.K.; Mol, A.P.J.; Gupta, A. Failing arsenic mitigation technology in rural Bangladesh: Explaining stagnation in niche formation of the Sono filter. Water Pol. 2016, 18, 1490–1507. [Google Scholar] [CrossRef]
- Smith, K.; Li, Z.; Chen, B.; Liang, H.; Zhang, X.; Xu, R.; Li, Z.; Dai, H.; Wei, C.; Liu, S. Comparison of sand-based water filters for point-of-use arsenic removal in China. Chemosphere 2017, 168, 155–162. [Google Scholar] [CrossRef]
- Bretzler, A.; Nikiema, J.; Lalanne, F.; Hoffmann, L.; Biswakarma, J.; Siebenaller, L.; Demange, D.; Schirmer, M.; Hug, S.J. Arsenic removal with zero-valent iron filters in Burkina Faso: Field and laboratory insights. Sci. Tot. Environ. 2020, 737, 139466. [Google Scholar] [CrossRef]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health A 2007, 42, 1869–1878. [Google Scholar] [CrossRef]
- Singh, S.K. An analysis of the cost-effectiveness of arsenic mitigation technologies: Implications for public policy. Int. J. Sust. Built Environ. 2017, 6, 522–535. [Google Scholar] [CrossRef]
| Year | Main Results | References |
|---|---|---|
| 1882 | Spongy iron filters are clogged at the water works of Antwerp (Belgium) because of cementation of iron and gravel. | [71] |
| 1903 | Whitney reported on the expansive volumetric nature of iron corrosion. | [45] |
| 1923 | Pilling and Bedworth established the rule of the volumetric expansive nature of iron corrosion. | [72] |
| 1928 | Bed clogging is attributed to Fe replacement by Pb. | [54] |
| 1951 | It is observed that column clogging occurs rapidly if fine iron filings are used in place of the steel wool. Using extremely fine grade of steel wool should also be avoided. | [73] |
| 1986 | Filtration beds containing 100% iron filings are very efficient at removing selenium from drainage water, but clogging occurs very rapidly. | [74] |
| 1992 | Using steel wool as Fe0 source for phosphate removal, it is demonstrated that Fe0/peat performed better than Fe0/sand. | [75] |
| 1993 | Filtration systems containing 10% to 25% Fe0 particles (iron fillings) mixed with pelletized jute do not experience any permeability loss. | [76] |
| 2001 | Fe0/pyrite filters are essentially more efficient for water treatment than pure Fe0 filters. | [24] |
| 2007 | Filtration systems containing less than 5% Fe0 (steel wool) do not experience any permeability loss. | [77] |
| 2000–2009 | Household arsenic filters with pure Fe0 layers are mostly efficient but not sustainable due to clogging. Only filters using porous materials (CIM = composite iron material) were sustainable. | [78] |
| 2009 | TCE removal rates are higher in an 85% Fe0 filter than in the 100% system. | [39] |
| Aggregate | Rationale for Use | Comments | Reference |
|---|---|---|---|
| Biochar | Adsorbs and accumulates contaminants | Also used as support for nano-Fe0 | [93] |
| Fe oxides | Adsorbs and accumulates contaminants | Fe3O4 is used the most | [94] |
| Fe sulfides | Shifts pH to lower values | FeS2 is used the most | [95] |
| GAC | Builds galvanic cells with Fe0 | GAC coating with FeCPs will hinder electron transfer | [96] |
| Lapillus | Stores FeCPs | Pores are not interconnected | [97] |
| Mn oxides | Sustains Fe0 corrosion | Extends Fe0 filter’s service life | [98] |
| Peat | Accumulates contaminants | More efficient than sand | [75] |
| Pozzolan | Stores FeCPs | More efficient than sand | [99] |
| Pumice | Stores FeCPs | Pores are not interconnected | [100] |
| Sand | Reduces the Fe0 cost | Reference additive | [39] |
| Wood chips | Accumulates contaminants | Mostly used in PO43− removal | [101] |
| Zeolite | Accumulates contaminants | Also used as support for nano-Fe0 | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, R.; Yang, H.; Cui, X.; Xiao, M.; Gatcha-Bandjun, N.; Kenmogne-Tchidjo, J.F.; Lufingo, M.; Konadu Amoah, B.; Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; et al. The Suitability of Hybrid Fe0/Aggregate Filtration Systems for Water Treatment. Water 2022, 14, 260. https://doi.org/10.3390/w14020260
Tao R, Yang H, Cui X, Xiao M, Gatcha-Bandjun N, Kenmogne-Tchidjo JF, Lufingo M, Konadu Amoah B, Tepong-Tsindé R, Ndé-Tchoupé AI, et al. The Suitability of Hybrid Fe0/Aggregate Filtration Systems for Water Treatment. Water. 2022; 14(2):260. https://doi.org/10.3390/w14020260
Chicago/Turabian StyleTao, Ran, Huichen Yang, Xuesong Cui, Minhui Xiao, Nadège Gatcha-Bandjun, Joseline Flore Kenmogne-Tchidjo, Mesia Lufingo, Bernard Konadu Amoah, Raoul Tepong-Tsindé, Arnaud Igor Ndé-Tchoupé, and et al. 2022. "The Suitability of Hybrid Fe0/Aggregate Filtration Systems for Water Treatment" Water 14, no. 2: 260. https://doi.org/10.3390/w14020260






