Water Use Characteristics of Two Dominant Species in the Mega-Dunes of the Badain Jaran Desert
Abstract
:1. Introduction
2. Materials and Methods
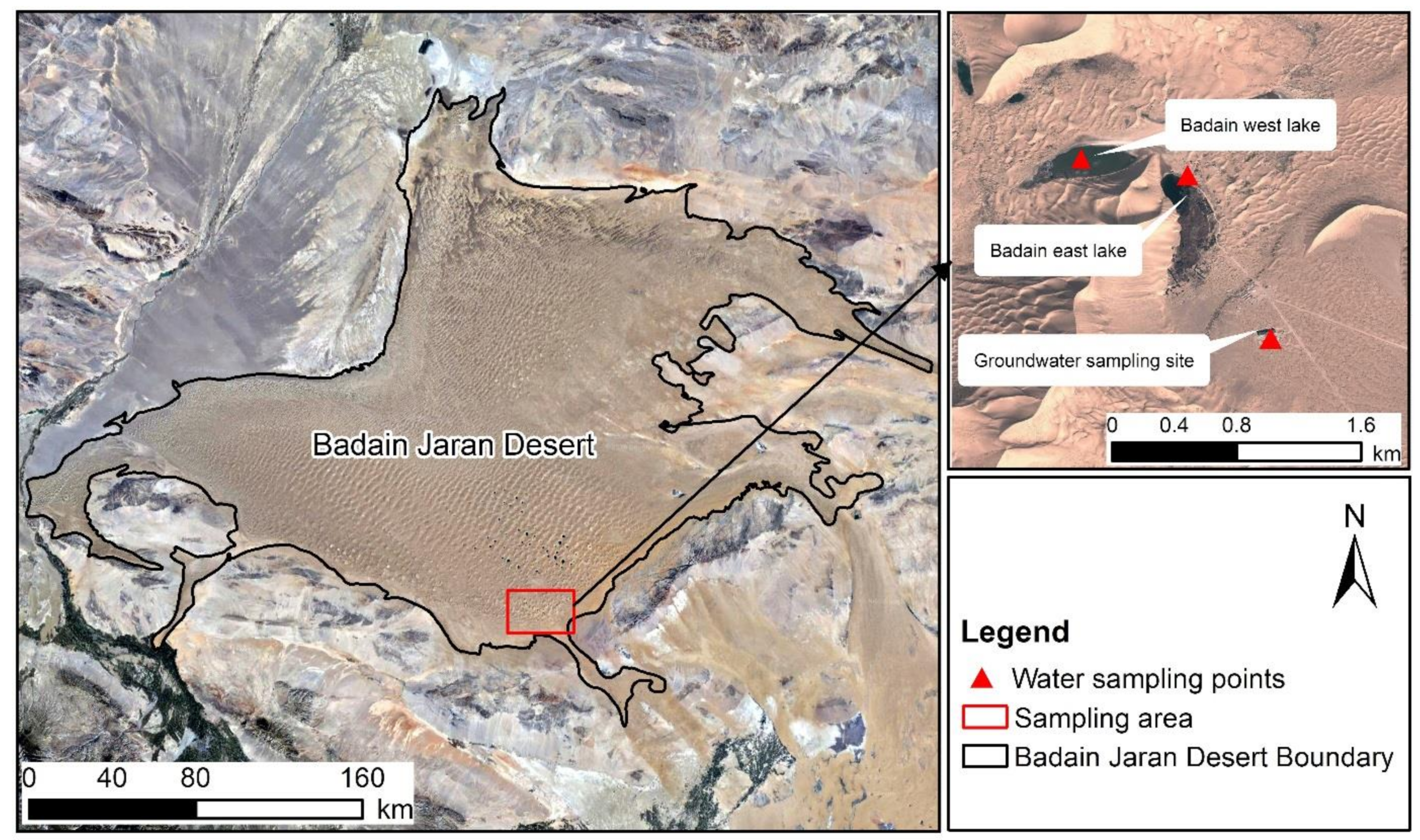
2.1. Study Area
2.2. Materials
2.3. Sample Collection
2.4. Measurement of the δ2H and δ18O of Xylem Water and Water Sources
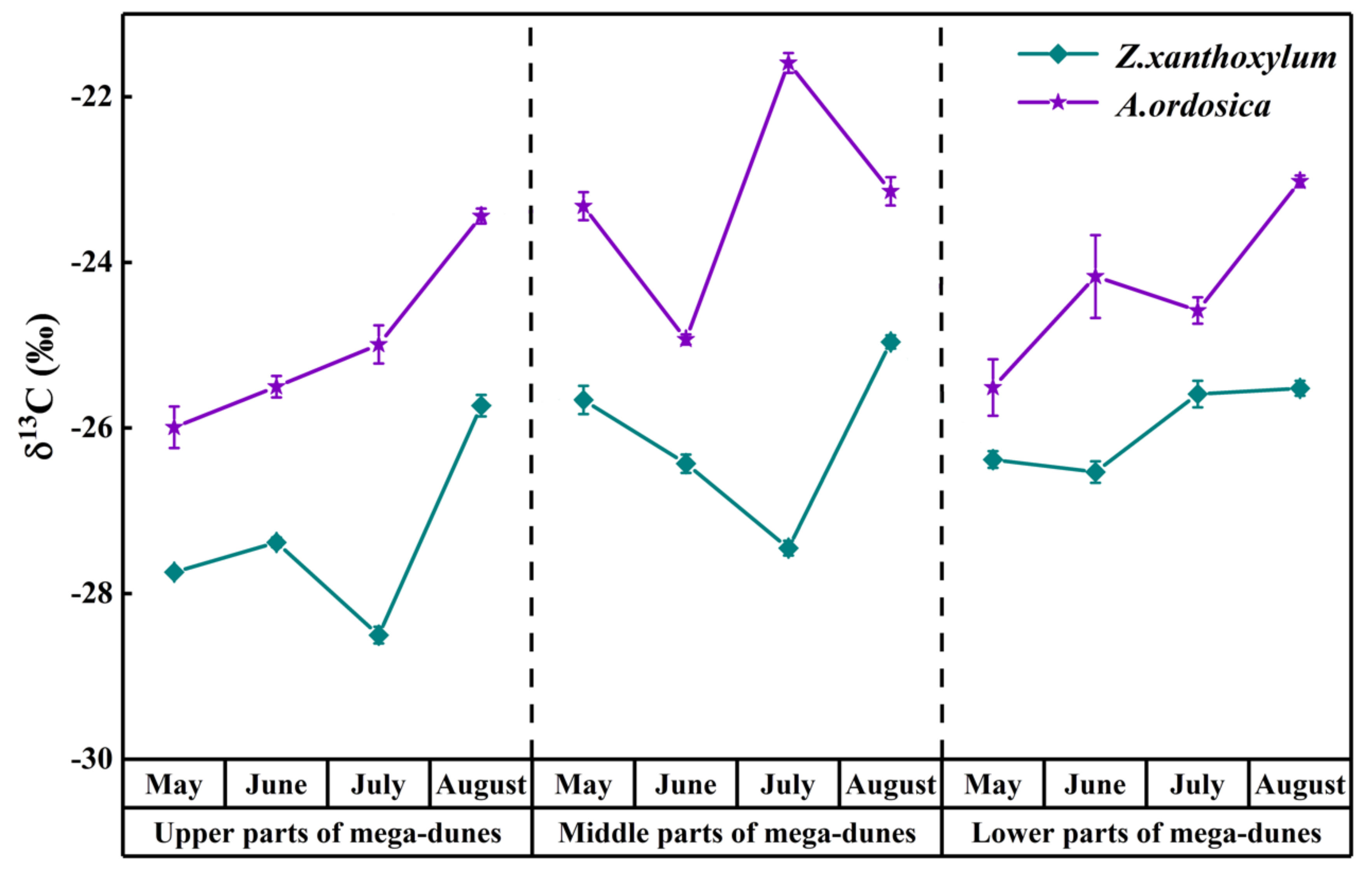
2.5. Measurement of the δ13C of Plant Leaves
2.6. Quantification of Water Sources Used by the Plants
2.7. Calculation of Plants Water Use Efficiency
2.8. Data Analysis
3. Results
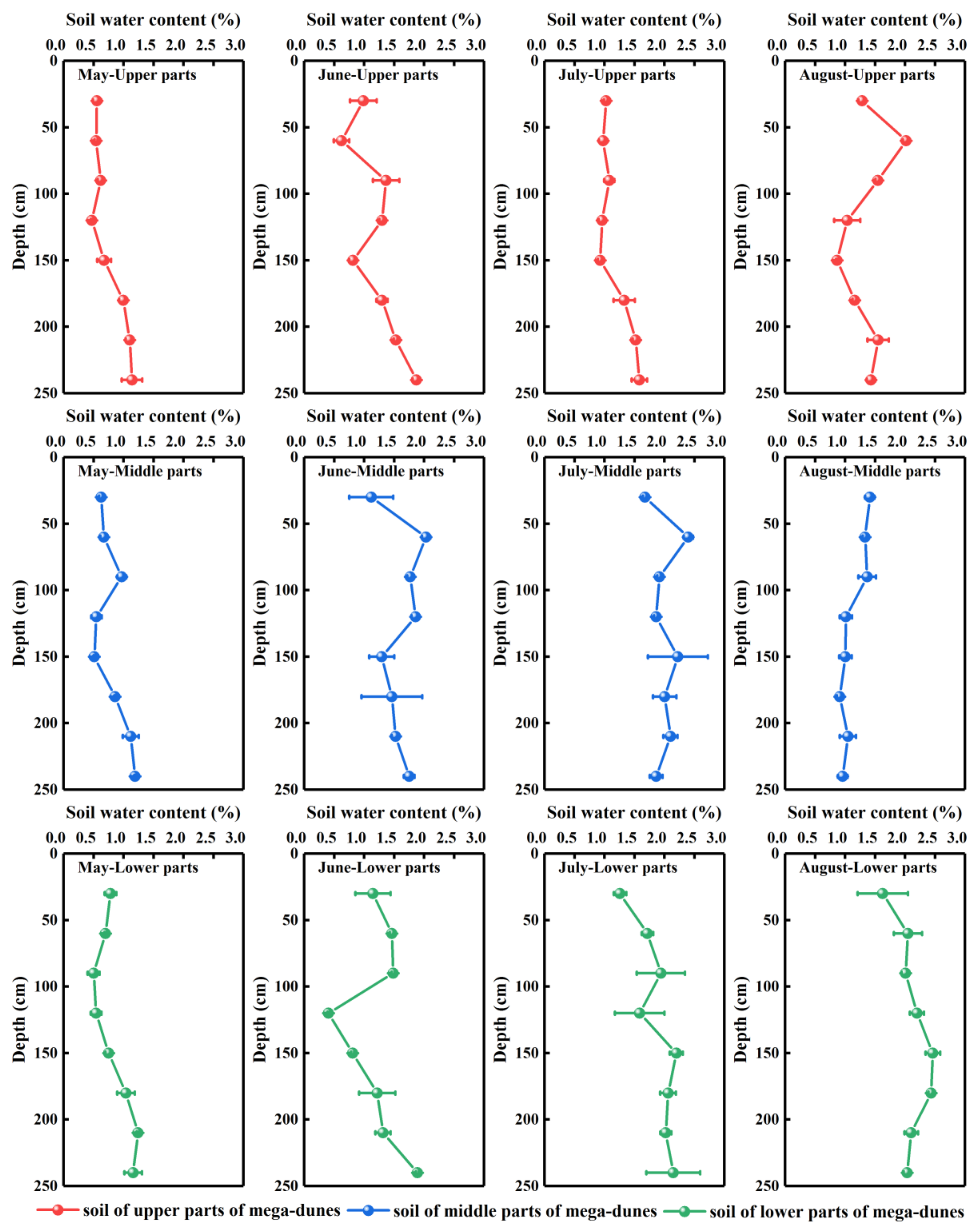
3.1. Temporal and Spatial Variations in Soil Water Content
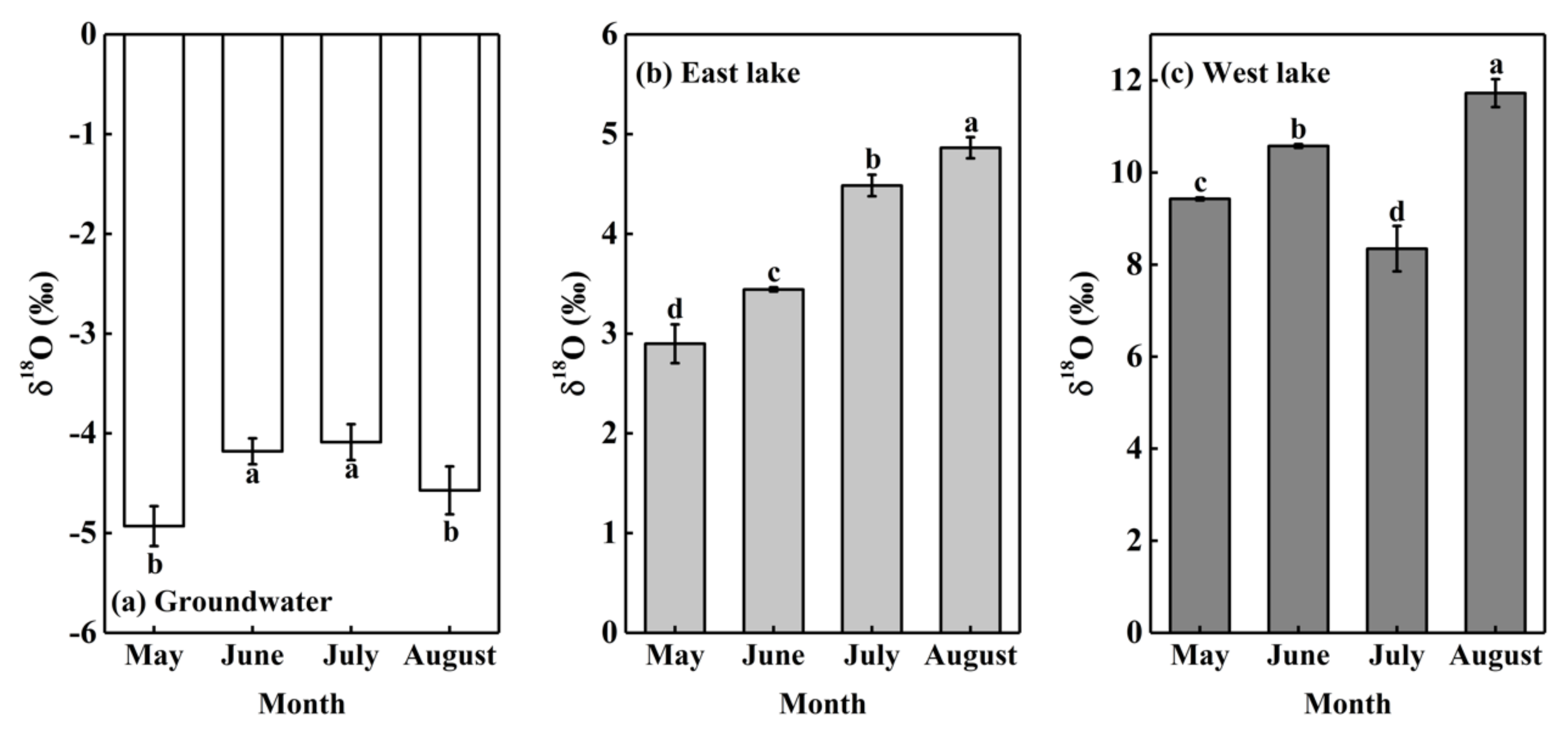
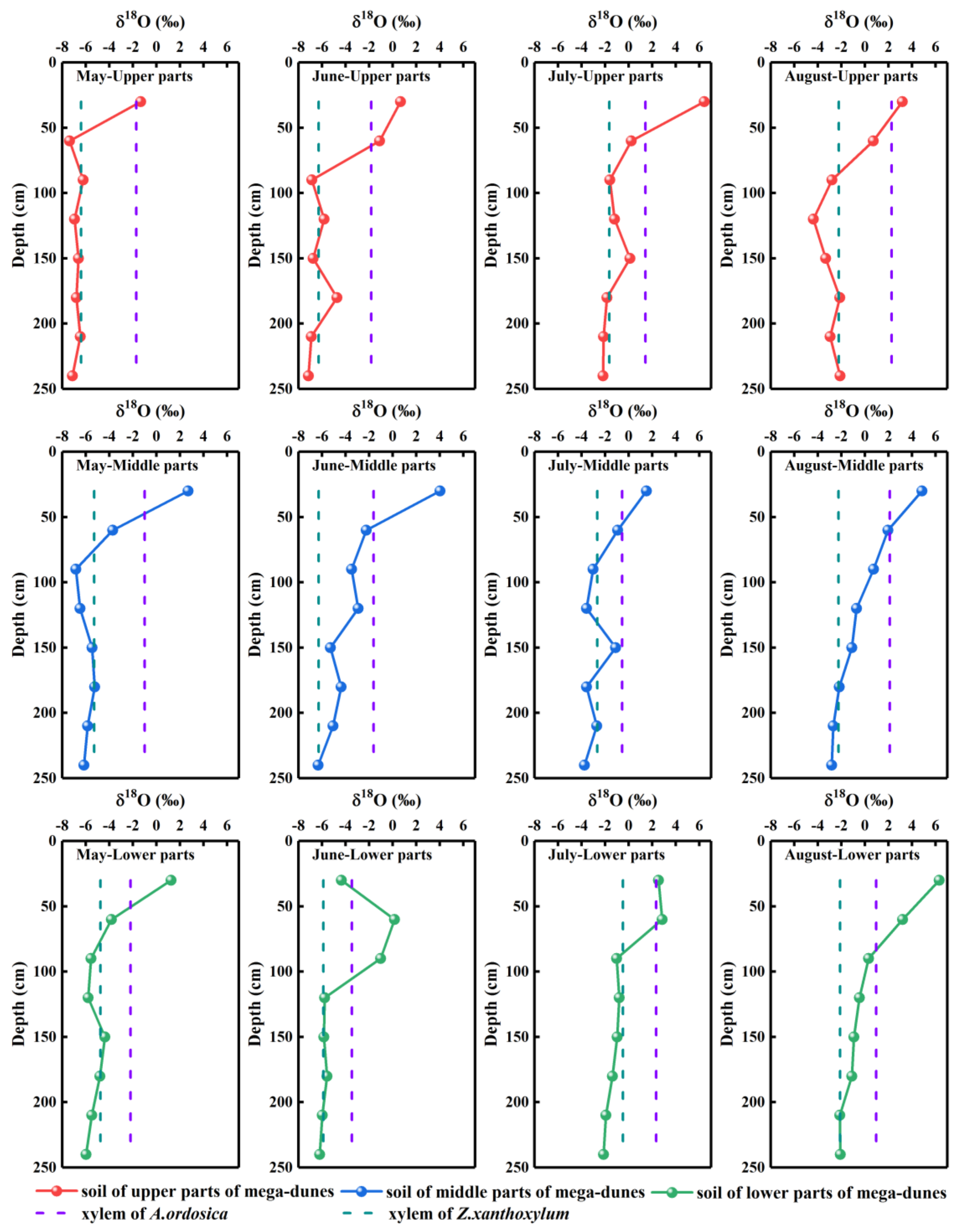
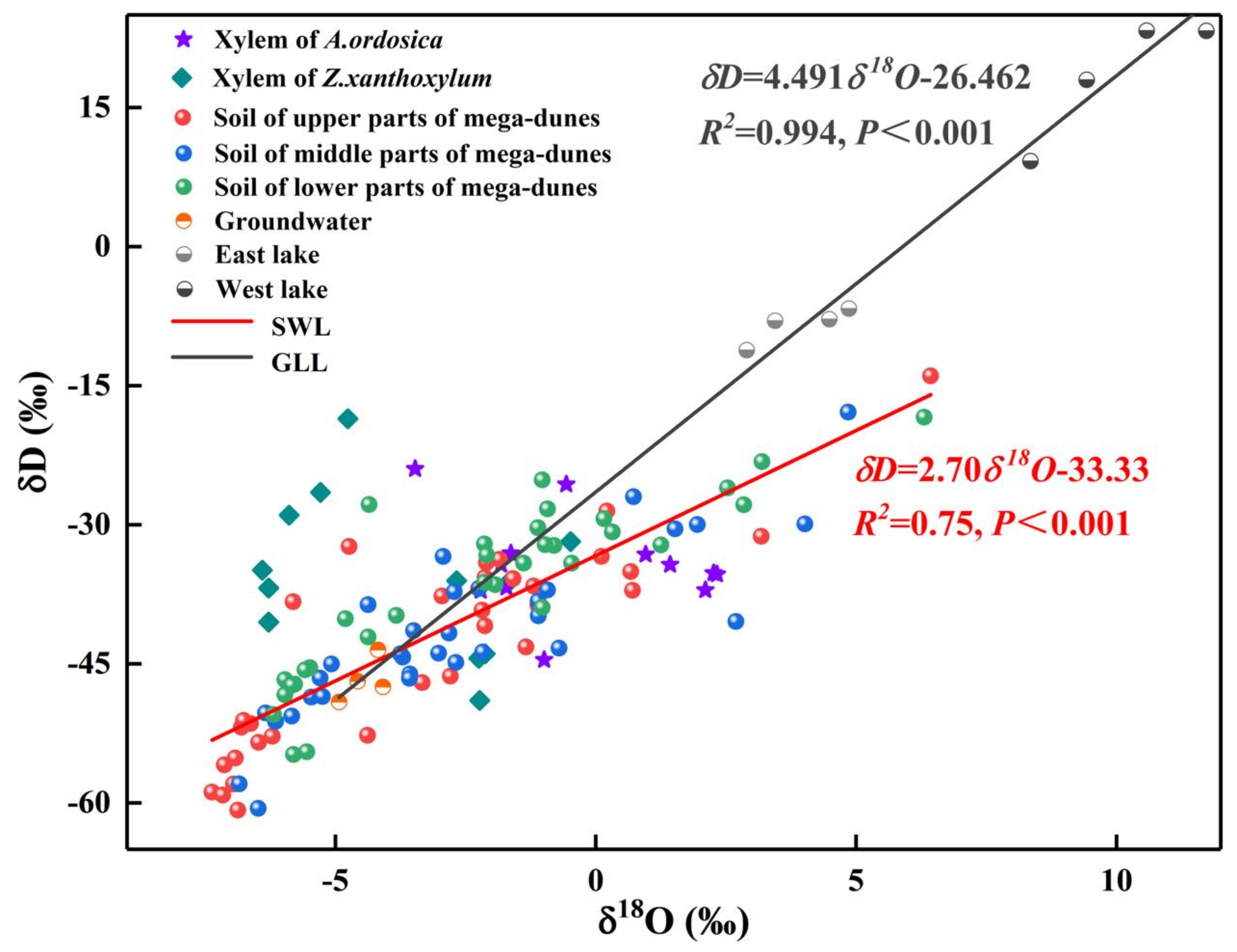
3.2. Isotopic Composition of Xylem Water and Potential Water Sources
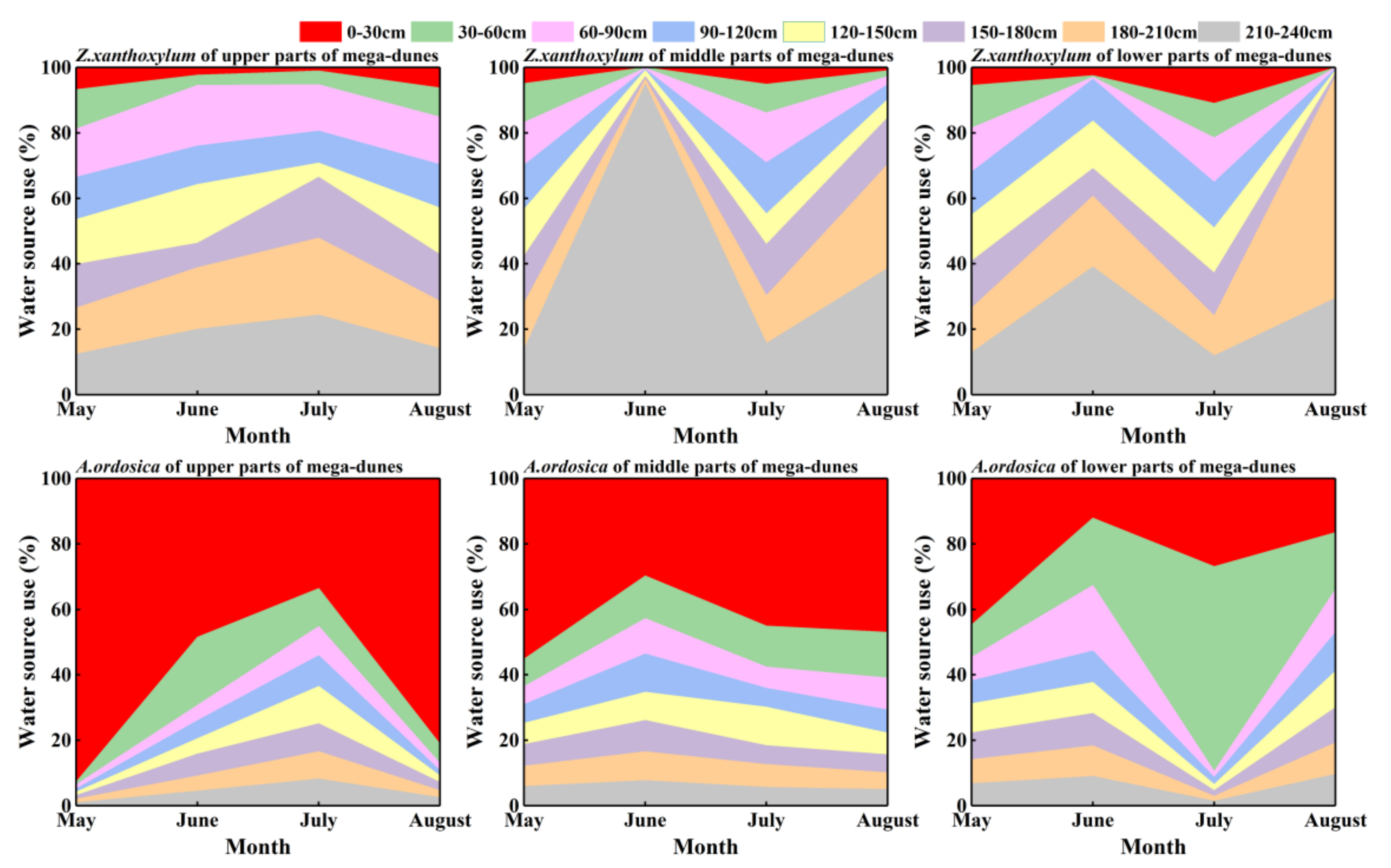
3.3. Water Use Patterns across the Growing Season
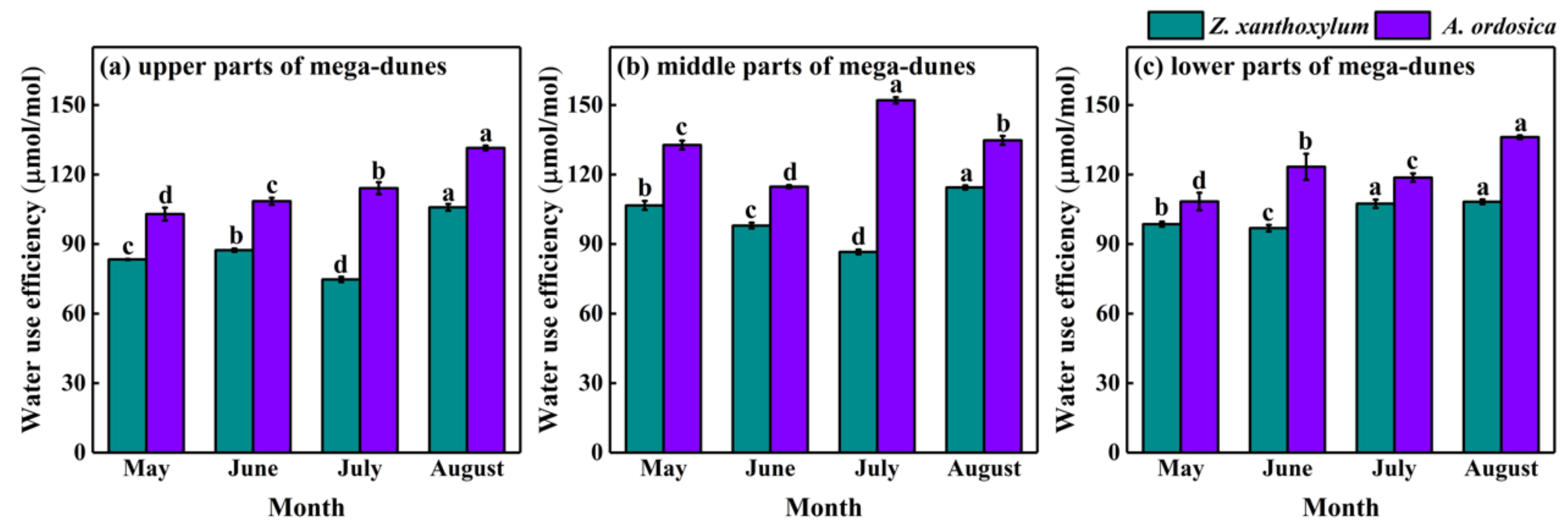
3.4. Temporal and Spatial Variations in Plant Water Use Efficiency
4. Discussion
4.1. Variations in Soil Water Content and Soil Water Isotopic Signatures
4.2. Differences in Water Use Patterns
4.3. Differences in Water Use Efficiency
4.4. Interaction between Different Plants in the Community
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fay, P.A.; Carlisle, J.D.; Knapp, A.K.; Blair, J.M.; Collins, S.L. Productivity responses to altered rainfall patterns in a C4-dominated grassland. Oecologia 2003, 137, 245–251. [Google Scholar] [CrossRef]
- Zhao, L.J.; Wang, X.G.; Zhang, Y.C.; Xie, C.; Liu, Q.Y.; Meng, F. Plant water use strategies in the Shapotou artificial sand-fixed vegetation of the southeastern margin of the Tengger Desert, northwestern China. J. Mt. Sci. 2019, 16, 898–908. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, B.J.; Lü, Y.H.; Gao, G.Y.; Wang, S.; Zhou, J. Linking vegetation cover patterns to hydrological responses using two process-based pattern indices at the plot scale. Sci. China-Earth Sci. 2013, 56, 1888–1898. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.M.; Chen, Y.N.; Li, W.H.; Guo, B.; Zhao, R.F. Hydraulic lift in Populus euphratica Oliv. from the desert riparian vegetation of the Tarim River Basin. J. Arid. Environ. 2010, 74, 905–911. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, X.J.; Yin, X.W.; Yue, Y.M.; Liu, R.; Xu, G.Q.; Li, Y. Seasonal variation in the groundwater dependency of two dominant woody species in a desert region of Central Asia. Plant Soil 2019, 444, 39–55. [Google Scholar] [CrossRef]
- Kolb, T.; Hart, S.; Amundson, R. Boxelder water source and physiology at perennial and ephemeral stream sites in Arizona. Tree Physiol. 1997, 17, 151–160. [Google Scholar] [CrossRef]
- Jacobs, A.F.G.; Heusinkveld, B.G.; Berkowicz, S.M. A simple model for potential dewfall in an arid region. Atmos. Res. 2002, 64, 285–295. [Google Scholar] [CrossRef]
- Cannon, W.A. The Root Habits of Desert Plants; Carnegie Inst of Washington Publ: Washington, DC, USA, 1911; p. 96. [Google Scholar]
- Nie, Y.P.; Chen, H.S.; Wang, K.L. Methods for determining plant water source in thin soil region: A review. Chin. J. Appl. Ecol. 2010, 21, 2427–2433. [Google Scholar]
- Ehleringer, J.R.; Phillips, S.L.; Schuster, W.S.F.; Sandquist, D.R. Differential utilization of summer rains by desert plants. Oecologia 1991, 88, 430–434. [Google Scholar] [CrossRef]
- Zhao, L.J.; Wang, L.X.; Cernusak, L.A.; Liu, X.H.; Xiao, H.L.; Zhou, M.X.; Zhang, S.Q. Significant difference in hydrogen isotope composition between xylem and tissue water in Populus euphratica. Plant Cell Environ. 2016, 39, 1848–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Fu, B.J.; Lu, N.; Wang, S.; Zhang, L. Water use characteristics of native and exotic shrub species in the semi-arid Loess Plateau using an isotope technique. Agric. Ecosyst. Environ. 2019, 276, 55–63. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.J.; Lu, N.; Zhang, L. Seasonal variation in water uptake patterns of three plant species based on stable isotopes in the semi-arid Loess Plateau. Sci. Total Environ. 2017, 609, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Gries, D.; Zeng, F.; Foetzki, A.; Arndt, S.K.; Bruelheide, H.; Thomas, F.M.; Zhang, X.; Runge, M. Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant Cell Environ. 2003, 26, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Markkanen, T.; Aurela, M.; Mammarella, I.; Thum, T.; Tsuruta, A.; Yang, H.; Aalto, T. Response of water use efficiency to summer drought in a boreal Scots pine forest in Finland. Biogeosciences 2017, 14, 4409–4422. [Google Scholar] [CrossRef] [Green Version]
- Lavergne, A.; Graven, H.; De Kauwe, M.G.; Keenan, T.F.; Medlyn, B.E.; Prentice, I.C. Observed and modelled historical trends in the water-use efficiency of plants and ecosystems. Glob. Change Biol. 2019, 25, 2242–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.M.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Ehleringer, J.R.; Cooper, T.A. Correlations between carbon isotope ratio and microhabitat in desert plants. Oecologia 1988, 76, 562–566. [Google Scholar] [CrossRef]
- Ebdon, J.S.; Petrovic, A.M.; Dawson, T.E. Relationship between carbon isotope discrimination, water use efficiency, and evapotranspiration in Kentucky Bluegrass. Crop Sci. 1998, 38, 157–162. [Google Scholar] [CrossRef]
- Zhu, J.F.; Wang, N.A.; Chen, H.B.; Dong, C.Y.; Zhang, H.A. Study on the boundary and the area of Badain Jaran Desert based on remote sensing imagery. Prog. Geogr. 2010, 29, 1087–1094. [Google Scholar]
- Ma, N.; Wang, N.A.; Zhu, J.F.; Chen, X.L.; Chen, H.B.; Dong, C.Y. Climate change around the Badain Jaran Desert in recent 50 years. J. Desert Res. 2011, 31, 1541–1547. [Google Scholar]
- Qin, J.; Si, J.H.; Jia, B.; Zhao, C.Y.; Li, D.; Luo, H.; Ren, L.X. Study on the relationship between vegetation community characteristics and soil moisture in Badain Jaran Desert. Arid. Zone Res. 2021, 38, 207–222. [Google Scholar]
- Zhang, J.; Wang, X.S.; Hu, X.N.; Lu, H.T.; Gong, Y.P.; Wan, L. The macro-characteristics of Groundwater flow in the Badain Jaran Desert. J. Desert Res. 2015, 35, 774–782. [Google Scholar]
- Querejeta, J.I.; Estrada-Medina, H.; Allen, M.F.; Jiménez-Osornio, J.J. Water source partitioning among trees growing on shallow karst soils in a seasonally dry tropical climate. Oecologia 2007, 152, 26–36. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Roden, J.; Dawson, T.E. Assessing Ecosystem-Level Water Relations through Stable Isotope Ratio Analyses. In Methods in Ecosystem Science; Springer: New York, NY, USA, 2000; pp. 181–198. [Google Scholar]
- West, A.G.; Patrickson, S.J.; Ehleringer, J.R. Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Commun. Mass Spectrom. 2006, 20, 1317–1321. [Google Scholar] [CrossRef]
- West, J.B.; Bowen, G.J.; Cerling, T.E.; Ehleringer, J.R. Stable isotopes as one of nature’s ecological recorders. Trends Ecol. Evol. 2006, 21, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, P.Z.; Williams, D.G. Hydrogen isotope fractionation during water uptake by woody xerophytes. Plant Soil 2007, 291, 93–107. [Google Scholar] [CrossRef]
- Phillips, D.L.; Gregg, J.W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 1982, 9, 281–292. [Google Scholar] [CrossRef]
- Chen, L.; Wang, N.A.; Wang, H.; Dong, C.Y.; Lu, Y.; Lu, J.W. Spatial patterns of chemical parameters of lakes and groundwater in Badain Jaran Desert. J. Desert Res. 2012, 32, 531–538. [Google Scholar]
- Penna, D.; Brocca, L.; Borga, M.; Dalla, F.G. Soil moisture temporal stability at different depths on two alpine hillslopes during wet and dry periods. J. Hydrol. 2013, 477, 55–71. [Google Scholar] [CrossRef]
- Yang, L.; Wei, W.; Chen, L.D.; Chen, W.L.; Wang, J.L. Response of temporal variation of soil moisture to vegetation restoration in semi-arid Loess Plateau, China. Catena 2014, 115, 123–133. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Corti, T.; Davin, E.L.; Hirschi, M.; Jaeger, E.B.; Lehner, I.; Orlowsky, B.; Teuling, A.J. Investigating soil moisture–climate interactions in a changing climate: A review. Earth-Sci. Rev. 2010, 99, 125–161. [Google Scholar] [CrossRef]
- Cao, L.; Shen, J.M.; Nie, Z.L.; Meng, L.Q.; Liu, M.; Wang, Z. Stable isotopic characteristics of precipitation and moisture recycling in Badain Jaran Desert. Earth Sci. 2021, 46, 2973–2983. [Google Scholar]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Schenk, H.J. Soil depth, plant rooting strategies and species’ niches. New Phytol. 2008, 178, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.J.; Barbour, M.G. Root systems, spatial patterns, and competition for soil moisture between two desert subshrubs. Am. J. Bot. 1988, 75, 885–893. [Google Scholar] [CrossRef]
- Dawson, T.E.; Ehleringer, J.R. Streamside trees that do not use stream water. Nature 1991, 350, 335–337. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Gao, R.H.; Jin, H. Studies on the ecological characteristics of four bushes roots in desert of West Erdos. J. Inn. Mong. Agric. Univ. 2005, 26, 39–43. [Google Scholar]
- Chen, J.; Xu, Q.; Gao, D.Q.; Ma, Y.B. Water use of Helianthemum songaricum and co-occurring plant species Sarcozygium xanthoxylum in Western Ordos. Sci. Silvae Sin. 2016, 52, 47–56. [Google Scholar]
- Zhou, H.; Zhao, W.Z.; He, Z.B. Water sources of Nitraria sibirica and response to precipitation in two desert habitats. Chin. J. Appl. Ecol. 2017, 28, 2083–2092. [Google Scholar]
- Donovan, L.A.; Ehleringer, J.R. Water stress and use of summer precipitation in a Great Basin shrub community. Funct. Ecol. 1994, 8, 289–297. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.S.; Li, X.R. Sap flow of Artemisia ordosica and the influence of environmental factors in a revegetated desert area: Tengger Desert, China. Hydrol. Processes 2010, 24, 1248–1253. [Google Scholar]
- Fu, X.; Wu, Y.S.; Zhang, Y.J. Analysis of water sources utilized by four shrubs during the growing season in the southern margin of Mu Us Sandy Land. J. Inn. Mong. Norm. Univ. 2020, 49, 245–250. [Google Scholar]
- Dawson, T.E.; Pate, J.S. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia 1996, 107, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.Y.; Wang, Y.; Jia, L.M.; Bloomberg, M.; Li, G.D.; Di, N. Characteristics of fine root system and water uptake in a triploid Populus tomentosa plantation in the North China Plain: Implications for irrigation water management. Agric. Water Manag. 2013, 117, 83–92. [Google Scholar] [CrossRef]
- Xing, X.; Chen, H.; Zhu, J.J.; Chen, T.T. Water sources of five dominant desert plant species in Nuomuhong area of Qaidam Basin. Acta Ecol. Sin. 2014, 34, 6277–6286. [Google Scholar]
- Wang, J.; Zhang, R.; Yan, Y.T.; Dong, X.Q.; Li, J.M. Locating hazardous gas leaks in the atmosphere via modified genetic, MCMC and particle swarm optimization algorithms. Atmos. Environ. 2017, 157, 27–37. [Google Scholar] [CrossRef]
- Feng, Q.; Cheng, G.D. Moisture distribution and movement in sandy lands of China. Acta Pedol. Sin. 1999, 225–236. [Google Scholar]
- Gong, X.W.; Lü, G.H.; Ran, Q.Y.; Yang, X.D. Response of vegetative growth and biomass allocation of Lappula semiglabra seedlings to dew gradient. Chin. J. Appl. Ecol. 2016, 27, 2257–2263. [Google Scholar]
- Hill, A.J.; Dawson, T.E.; Shelef, O.; Rachmilevitch, S. The role of dew in Negev Desert plants. Oecologia 2015, 178, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.C.; Rao, R.C.; Farquhar, G.D. Water-use efficiency and carbon isotope discrimination in peanut under water deficit conditions. Crop Sci. 1994, 34, 92–97. [Google Scholar] [CrossRef]
- Chen, H.S.; Shao, M.G.; Li, Y.Y. Soil desiccation in the Loess Plateau of China. Geoderma 2008, 143, 91–100. [Google Scholar] [CrossRef]
- Noymeir, I. Desert ecosystems: Environment and producers. Annu. Rev. Ecol. Syst. 1973, 4, 25–51. [Google Scholar] [CrossRef]
- Darrouzet-Nardi, A.; D’ Antonio, C.M.; Dawson, T.E. Depth of water acquisition by invading shrubs and resident herbs in a Sierra Nevada meadow. Plant Soil 2006, 285, 31–43. [Google Scholar] [CrossRef]
- Wu, K.S. Study of Hydraulic Lift in Zygophyllum xanthoxylum of Eremophytes. Master’s Thesis, Lanzhou University, Lanzhou, China, 2010. [Google Scholar]
- Zhu, Y.J.; Jia, Z.Q. Water source of Haloxylon ammodendron plantations in autumn at the southeast edge of Badain Jaran Desert. Sci. Silvae Sin. 2012, 48, 1–5. [Google Scholar]

| Organic Matter (g/kg) | Total Nitrogen (g/kg) | Total Carbon (g/kg) | Total Phosphorus (g/kg) | Total Salt (g/kg) | pH | Conductivity (s/m) |
|---|---|---|---|---|---|---|
| 3.91 ± 0.46 | 0.71 ± 0.04 | 7.83 ± 0.51 | 0.75 ± 0.03 | 10.61 ± 1.87 | 8.15 ± 0.06 | 0.63 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Si, J.; Jia, B.; Zhao, C.; Zhou, D.; He, X.; Wang, C.; Zhu, X. Water Use Characteristics of Two Dominant Species in the Mega-Dunes of the Badain Jaran Desert. Water 2022, 14, 53. https://doi.org/10.3390/w14010053
Qin J, Si J, Jia B, Zhao C, Zhou D, He X, Wang C, Zhu X. Water Use Characteristics of Two Dominant Species in the Mega-Dunes of the Badain Jaran Desert. Water. 2022; 14(1):53. https://doi.org/10.3390/w14010053
Chicago/Turabian StyleQin, Jie, Jianhua Si, Bing Jia, Chunyan Zhao, Dongmeng Zhou, Xiaohui He, Chunlin Wang, and Xinglin Zhu. 2022. "Water Use Characteristics of Two Dominant Species in the Mega-Dunes of the Badain Jaran Desert" Water 14, no. 1: 53. https://doi.org/10.3390/w14010053





