Radiocarbon Dating of Marine Samples: Methodological Aspects, Applications and Case Studies
Abstract
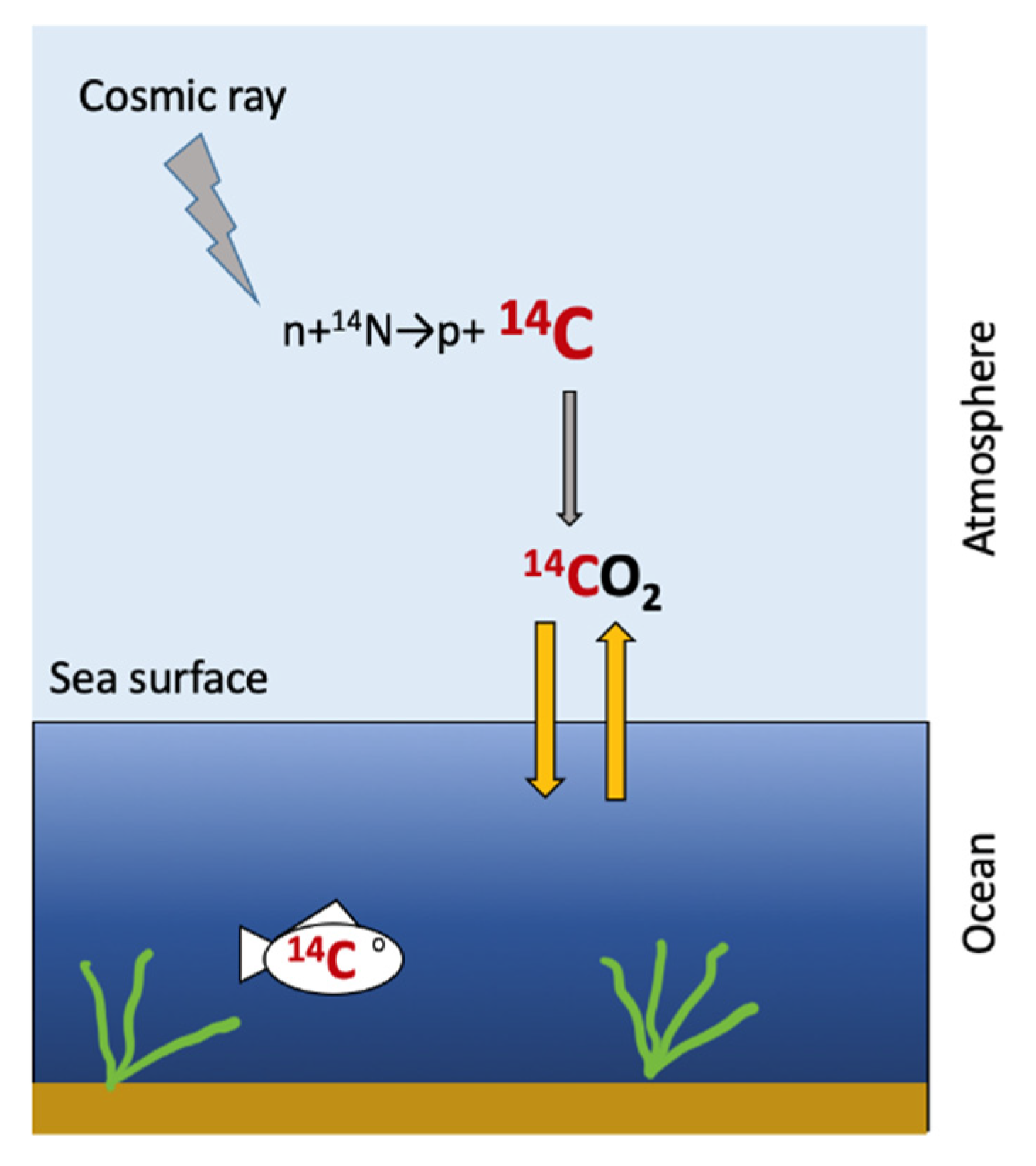
:1. Introduction
2. AMS Radiocarbon Dating
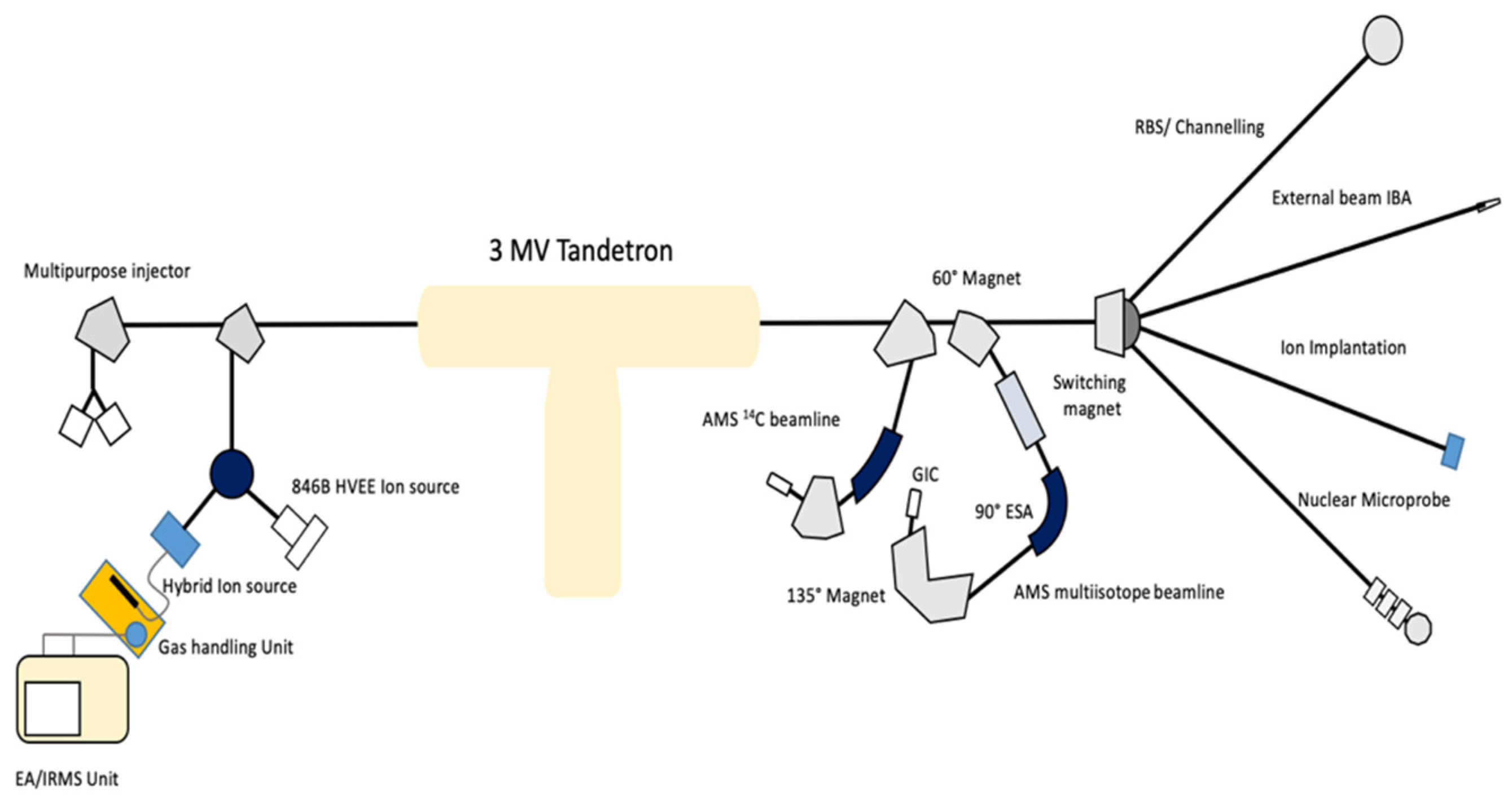
2.1. Instrumental
2.2. Sample Preparation
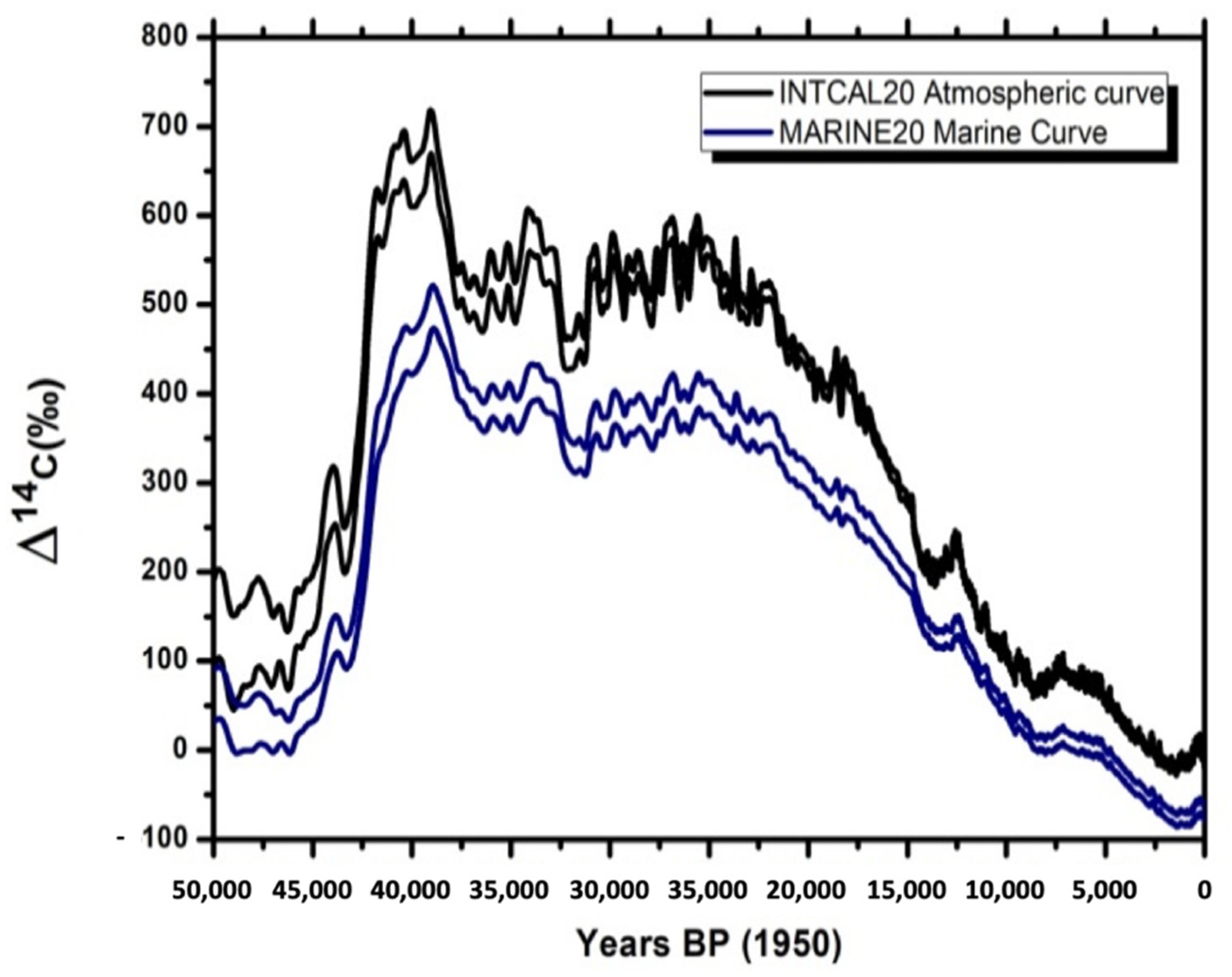
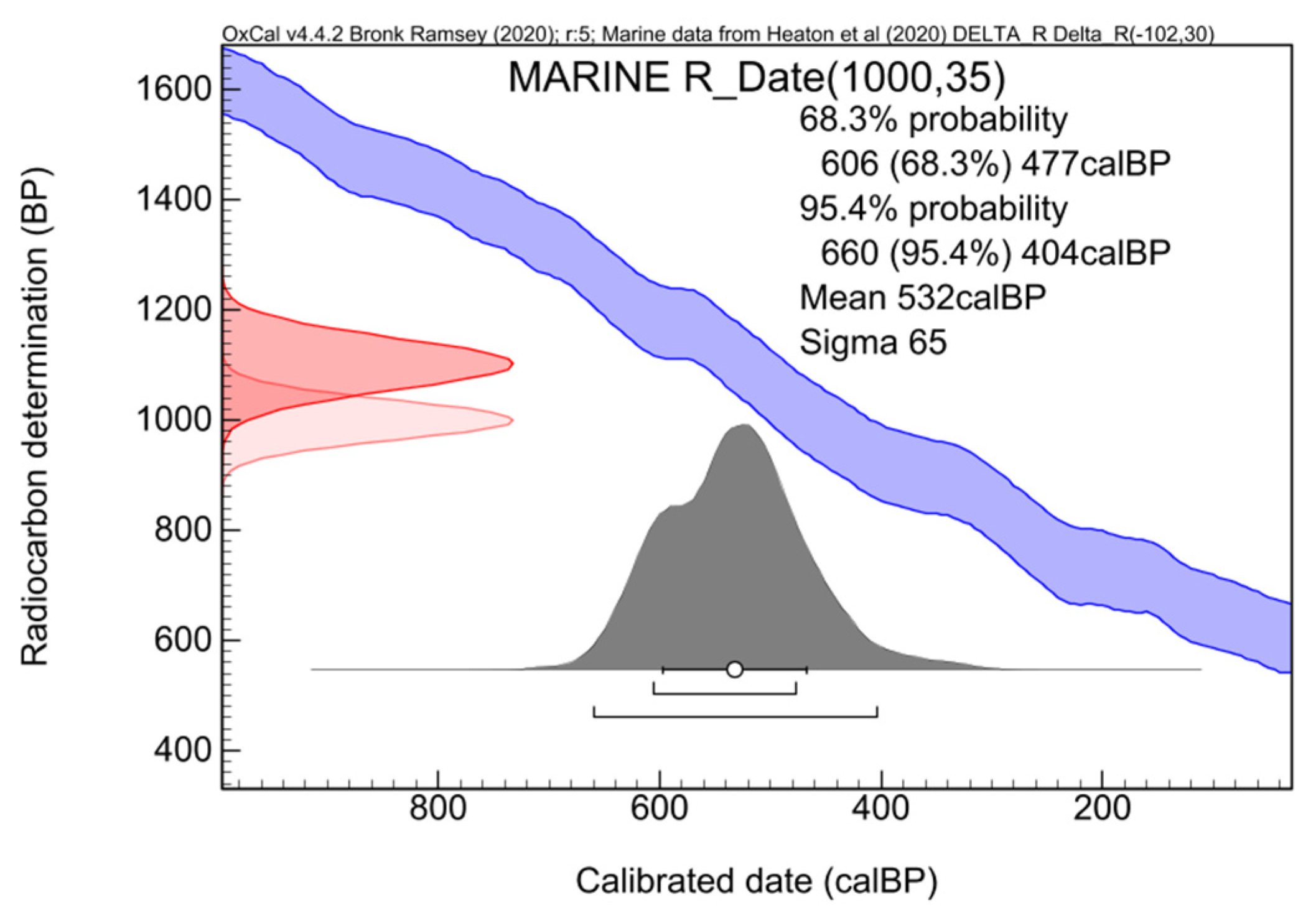
3. Calibration of “Marine Ages”
4. Applications
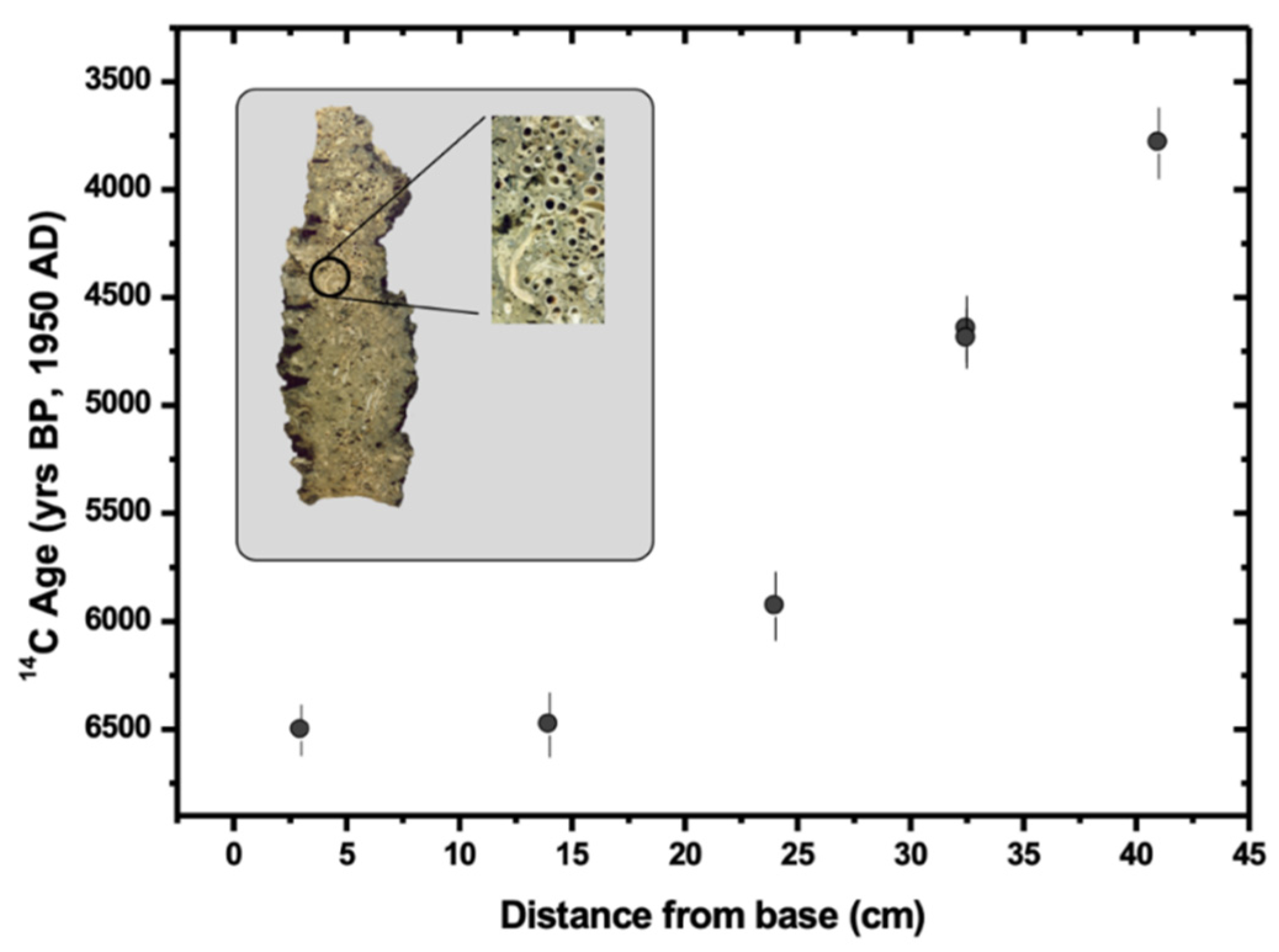
4.1. Analysis of Marine Bio-Construction
4.2. Sea Level Rise Studies
- The samples or sub-samples to be dated should be carefully identified and properly extracted from the matrix.
- Local marine reservoir correction factors should be used. This requires, when not available from literature data, its experimental estimation for instance through the measurement of paired terrestrial and marine samples from the same location or of marine samples with a known age.
- Possible local reservoir effects should be carefully evaluated, for instance those associated with 14C-depleted hard-water [34].
- Radiocarbon dates obtained on speleothems should be treated with caution and, when necessary, corrected for the possible contribution of 14C-depleted sources of carbon which can alter (making them older) 14C-ages. A detailed evaluation of the geological settings of the formation environment is mandatory as well as, when possible, paired U/Th analyses.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masarik, J.; Beer, J. Simulation of particle fluxes and cosmogenic nuclide production in the Earth’s atmosphere. J. Geophys. Res. Atmos. 1999, 104, 12099–12111. [Google Scholar] [CrossRef] [Green Version]
- Kutschera, W. The Half-Life of 14C—Why Is It So Long? Radiocarbon 2019, 61, 1135–1142. [Google Scholar] [CrossRef]
- Arnold, W.F.; Libby, W. Age determinations by radiocarbon content: Checks with samples of known age. Science 1949, 110, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Beukens, R.P.; Clover, M.R.; Gove, H.E.; Liebert, R.B.; Litherland, A.E.; Purser, K.H.; Sondheim, W.E. Radiocarbon dating using electro-static accelerators. Science 1977, 198, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Purser, K.H.; Liebert, R.B.; Litherland, A.E.; Benkens, R.P.; Gove, H.E.; Bennett, C.L.; Clover, M.R.; Sondheim, W.E. An attempt to detect stable N ions from a sputter ion source and some implications of the results for the design of tandems for ultra-sensitive carbon analysis. Rev. Phys. Appl. 1977, 12, 1487–1492. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.E.; Korteling, R.G.; Stott, W.R. Carbon-14: Direct detection at natural concentrations. Science 1977, 198, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Calcagnile, L.; Quarta, G.; D’Elia, M. High resolution accelerator-based mass spectrometry: Precision, accuracy and background. Appl. Radiat. Isot. 2005, 62, 623–629. [Google Scholar] [CrossRef]
- Calcagnile, L.; Quarta, G.; D’Elia, M.; Gottdang, A.; Klein, M.; Mous, D.J.W. Radiocarbon precision tests at the Lecce AMS facility using a sequential injection system. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2004, 215, 561–564. [Google Scholar] [CrossRef]
- Synal, H.A.; Stocker, M.; Suter, M. MICADAS: A new compact radiocarbon AMS system. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 259, 7–13. [Google Scholar] [CrossRef]
- Braione, E.; Maruccio, L.; Quarta, G.; D’Elia, M.; Calcagnile, L. A new system for the simultaneous measurement of δ13C and δ15N by IRMS and radiocarbon by AMS on gaseous samples: Design features and performances of the gas handling interface. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 361, 387–391. [Google Scholar] [CrossRef]
- D’Elia, M.; Calcagnile, L.; Quarta, G.; Rizzo, A.; Sanapo, C.; Laudisa, M.; Toma, U.; Rizzo, A. Sample preparation and blank values at the AMS radiocarbon facility of the University of Lecce. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2004, 223–224, 278–283. [Google Scholar] [CrossRef]
- Welte, C.; Wacker, L.; Hattendorf, B.; Christl, M.; Koch, J.; Synal, H.; Günther, D. Novel Laser Ablation Sampling Device for the Rapid Radiocarbon Analysis of Carbonate Samples by Accelerator Mass Spectrometry. Radiocarbon 2016, 58, 419–435. [Google Scholar] [CrossRef]
- Reimer, P.; Austin, W.; Bard, E.; Bayliss, A.; Blackwell, P.; Bronk Ramsey, C.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Heaton, T.; Köhler, P.; Butzin, M.; Bard, E.; Reimer, R.; Austin, W.; Skinner, L.; Ramsey, C.B.; Grootes, P.M.; Hughen, K.A.; et al. Marine20—The Marine Radiocarbon Age Calibration Curve (0–55,000 cal BP). Radiocarbon 2020, 62, 779–820. [Google Scholar] [CrossRef]
- Stuiver, M.; Polach, H.A. Discussion: Reporting of 14C data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Hammer, S.; Levin, I. Monthly mean atmospheric D14CO2 at Jungfraujoch and Schauinsland from 1986 to 2016. Tellus B 2017. [Google Scholar] [CrossRef]
- Macchia, M.; D’Elia, M.; Quarta, G.; Gaballo, V.; Braione, E.; Maruccio, L.; Calcagnile, L.; Ciceri, G.; Martinotti, V.; Wacker, L. Extraction of dissolved inorganic carbon (DIC) from seawater samples at CEDAD: Results of an intercomparison exercise on samples from Adriatic sea shallow water. Radiocarbon 2013, 55, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Alves, E.Q.; Macario, K.; Ascough, P.; Bronk Ramsey, C. The worldwide marine radiocarbon reservoir effect: Definitions, mechanisms, and prospects. Rev. Geophys. 2018, 56, 278–305. [Google Scholar] [CrossRef]
- Bronk Ramsey, C. Development of the Radiocarbon Calibration Program. Radiocarbon 2001, 43, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar] [PubMed]
- Guido, A.; Heindel, K.; Birgel, D.; Rosso, A.; Mastandrea, A.; Sanfilippo, R.; Russo, F.; Peckmann, J. Pendant bioconstructions cemented by microbial carbonate in submerged marine caves (Holocene, SE Sicily). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 388, 166–180. [Google Scholar] [CrossRef]
- Guido, A.; Gerovasileiou, V.; Russo, F.; Rosso, A.; Sanfilippo, R.; Voultsiadou, E.; Mastandrea, A. Composition and biostratinomy of sponge-rich biogenic crusts in submarine caves (Aegean Sea, Eastern Mediterranean). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 534, 109338. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Rosso, A.; Guido, A.; Mastandrea, A.; Russo, F.; Riding, R.; Taddei Ruggiero, E. Metazoan/microbial biostalactites from modern submarine caves in the Mediterranean Sea. Mar. Ecol. 2015, 36, 1277–1293. [Google Scholar] [CrossRef]
- Rosso, A.; Sanfilippo, R.; Guido, A.; Gerovasileiou, V.; Taddei Ruggiero, E.; Belmonte, G. Coloniser of the dark: Biostalactite-associated metazoans from “lu Lampiùne” submarine cave (Apulia, Mediterranean Sea). Mar. Ecol. 2021, 42, e12634. [Google Scholar]
- D’Elia, M.; Quarta, G.; Calcagnile, L.; Belmonte, G. Study of the formation of biogenic speleothems found in submarine caves at the cape of Otranto, Italy, by 14C AMS. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 259, 395–397. [Google Scholar] [CrossRef]
- Belmonte, G.; Ingrosso, G.; Poto, M.; Quarta, G.; D’Elia, M.; Onorato, R.; Calcagnile, L. Biogenic stalactites in submarine caves at the Cape of Otranto (SE Italy): Dating and hypothesis on their formation. Mar. Ecol. 2009, 30, 376–382. [Google Scholar] [CrossRef]
- Quarta, G.; D’Elia, M.; Calcagnile, L.; Belmonte, G.; Ingrosso, G. Reconstructing the formation mechanism of submarine biogenic stalactites: The contribution of AMS. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 1244–1247. [Google Scholar] [CrossRef]
- Pepe, F.; Corradino, M.; Parrino, N.; Besio, G.; Lo Presti, V.; Renda, P.; Calcagnile, L.; Quarta, G.; Sulli, A.; Antonioli, F. Boulder coastal deposits at Favignana Island rocky coast (Sicily, Italy): Litho-structural and hydrodynamic control. Geomorphology 2018, 303, 191–209. [Google Scholar] [CrossRef]
- Antonioli, F.; De Falco, G.; Lo Presti, V.; Moretti, L.; Scardino, G.; Anzidei, M.; Bonaldo, D.; Carniel, S.; Leoni, G.; Furlani, S.; et al. Relative Sea-Level Rise and Potential Submersion Risk for 2100 on 16 Coastal Plains of the Mediterranean Sea. Water 2020, 12, 2173. [Google Scholar] [CrossRef]
- Rovere, A.; Stocchi, P.; Vacchi, M. Eustatic and Relative Sea Level Changes. Curr. Clim. Chang. Rep. 2016, 2, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Lambeck, K.; Antonioli, F.; Purcell, A.; Silenzi, S. Sea-level change along the Italian coast for the past 10,000 yr. Quat. Sci. Rev. 2004, 23, 1567–1598. [Google Scholar] [CrossRef]
- Auriemma, R.; Solinas, E. Archaeological remains as sea level change markers: A review. Quat. Int. 2009, 206, 134–146. [Google Scholar] [CrossRef]
- Antonioli, F.; Furlani, S.; Montagna, P.; Stocchi, P. The Use of Submerged Speleothems for Sea Level Studies in the Mediterranean Sea: A New Perspective Using Glacial Isostatic Adjustment (GIA). Geosciences 2021, 11, 77. [Google Scholar] [CrossRef]
- Quarta, G.; Fago, P.; Calcagnile, L.; Cipriano, G.; D’Elia, M.; Moretti, M.; Scardino, G.; Valenzano, E.; Mastronuzzi, G. 14C Age Offset in the Mar Piccolo Sea Basin in Taranto (Southern Italy) Estimated on Cerastoderma Glaucum (Poiret, 1789). Radiocarbon 2019, 61, 1387–1401. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarta, G.; Maruccio, L.; D’Elia, M.; Calcagnile, L. Radiocarbon Dating of Marine Samples: Methodological Aspects, Applications and Case Studies. Water 2021, 13, 986. https://doi.org/10.3390/w13070986
Quarta G, Maruccio L, D’Elia M, Calcagnile L. Radiocarbon Dating of Marine Samples: Methodological Aspects, Applications and Case Studies. Water. 2021; 13(7):986. https://doi.org/10.3390/w13070986
Chicago/Turabian StyleQuarta, Gianluca, Lucio Maruccio, Marisa D’Elia, and Lucio Calcagnile. 2021. "Radiocarbon Dating of Marine Samples: Methodological Aspects, Applications and Case Studies" Water 13, no. 7: 986. https://doi.org/10.3390/w13070986





