1. Introduction
The open ocean, in contrast to the coastline or continental shelves, is considered a low-structured habitat. Nevertheless, marine mammals, such as cetaceans and pinnipeds, often travel through the open ocean searching for places where they can forage, rest or reproduce [
1,
2,
3,
4]. Some marine mammals even return to specific haul-out or birthplaces consistently. Moreover, on their journeys, some species are even able to steer straight courses. Research on humpback whales has revealed the animals’ ability to keep a constant course with an accuracy of less than one degree over hundreds of kilometers traveled over many days [
1]. Furthermore, pinnipeds, such as elephant seals, can keep a straight course even when submerged, most likely based on visual information obtained at the water surface; the envisaged direction is even maintained after a spiraling descend [
5]. Thus, there is evidence that marine mammals are well oriented in their natural environment.
Seeing how well these animals are navigating, the question emerged which sensory information the seals and other marine mammals use for orientation and navigation. Theoretical speculation sprouted over the use of stars or the Earth’s magnetic field [
6,
7,
8]; while the first is based on good preliminary experimental evidence [
9,
10], magnetoreception has not been documented for seals [
11]. In general, previous experiments regarding orientation and navigation in seals almost exclusively focused on cues perceived by the classic sensory systems, such as visual, auditory, chemoreceptive or hydrodynamic cues [
12,
13,
14]. However, in the marine environment, these cues can be impaired or absent due to environmental factors, such as turbidity [
15], bad weather conditions or anthropogenic activities. Under these conditions, a seal could rely on navigation/orientation mechanisms that can be solely based on idiothetic cues, defined as cues derived from self-motion, such as path integration [
16,
17,
18]. Fundamental to path integration is keeping track of distances covered, and directions steered on the outbound path. This information is then integrated into a homing vector, which leads the organism back to its starting point, for example, its haul-out place. The ability to integrate paths has already been shown in many terrestrial and some semi-terrestrial species [
17,
19,
20,
21,
22,
23,
24,
25]. We consider path integration to be a navigation mechanism very promising to look at in marine mammals in line with Fuiman et al. [
26].
In this study, we analyzed if a harbor seal (
Phoca vitulina) can estimate and reproduce distances in distance reproduction tasks. In a reproduction task, the experimental animal needs to swim a specific, predetermined distance. Afterwards, the animal must continue swimming until it subjectively decides it has reproduced this distance, meaning it has swum the distance another time. The estimation of distances is interesting regarding path integration, but it might also play a role in path return or might generally assist navigation in seals. Distance information can be derived from self-motion cues or cues derived from the classic sensory systems, most notably the visual system [
18,
27]. Most of our knowledge on distance estimation stems from human subjects. These experiments were often analyzing the influence of the visual system on distance estimation. In these experiments on non-visual distance estimation and reproduction, researchers documented a high accuracy of reproduction with an absolute error of 20–35% of the path length [
27] or slightly better. While the subjects veered noticeably, the distances were accurately reproduced. Thus, distance estimation is possible in the absence of vision. In Klatzky et al. [
27], the mechanism used by the blindfolded subjects was not determined. However, since then, experiments, including normal and labyrinthine-defective human subjects, have shown that locomotor information and especially the vestibular system, can play a major role during distance estimation [
28,
29,
30].
Possible mechanisms for distance estimation were also investigated in various animals. Honeybees rely on optic flow [
31,
32,
33,
34,
35], whereas desert ants gauge distances additionally through a pedometer [
36,
37,
38,
39]. In mammals other than humans, experiments on distance estimation itself are scarce and, to our knowledge, have only been addressed in rodents, for example, in hamsters in a homing task on the basis of non-visual cues [
18,
40].
In the study at hand, we investigated distance estimation in a (semi)aquatic animal, the harbor seal. The distance reproduction task (experiment 1) involved a preset distance interval of 0.5–18.5 m length, which the subject had to reproduce subsequently by keeping the same swimming direction. A follow-up experiment (experiment 2) focused on the maximum precision the seal can achieve during a distance reproduction task. Finally (experiment 3), the influence of the visual system on distance reproduction was determined by comparing the seal’s performance with and without a blindfold. We discuss our findings in the context of orientation/navigation of seals.
2. Materials and Methods
2.1. Experimental Animal
The experiment was conducted with an adult male, captive-born harbor seal (
Phoca vitulina) named “Nick” (16 years old at the beginning of the experiments) at the Marine Science Center of the University of Rostock in Warnemünde/Hohe Düne, Germany. The seal had previously participated in many scientific experiments (see, for example, [
12,
41,
42]). Nick was housed with eight other harbor seals, two juvenile California sea lions (
Zalophus californianus), and an adult South African fur seal (
Arctocephalus pusillus pusillus) in a seawater enclosure. The seal was mainly fed freshly thawed cut herring (
Clupea harengus) and sprats (
Sprattus sprattus). During the experiment and the general training, the animal received 1–5 kg of fish a day, depending on season and motivation. We performed experiments five days a week.
2.2. General Experimental Setup
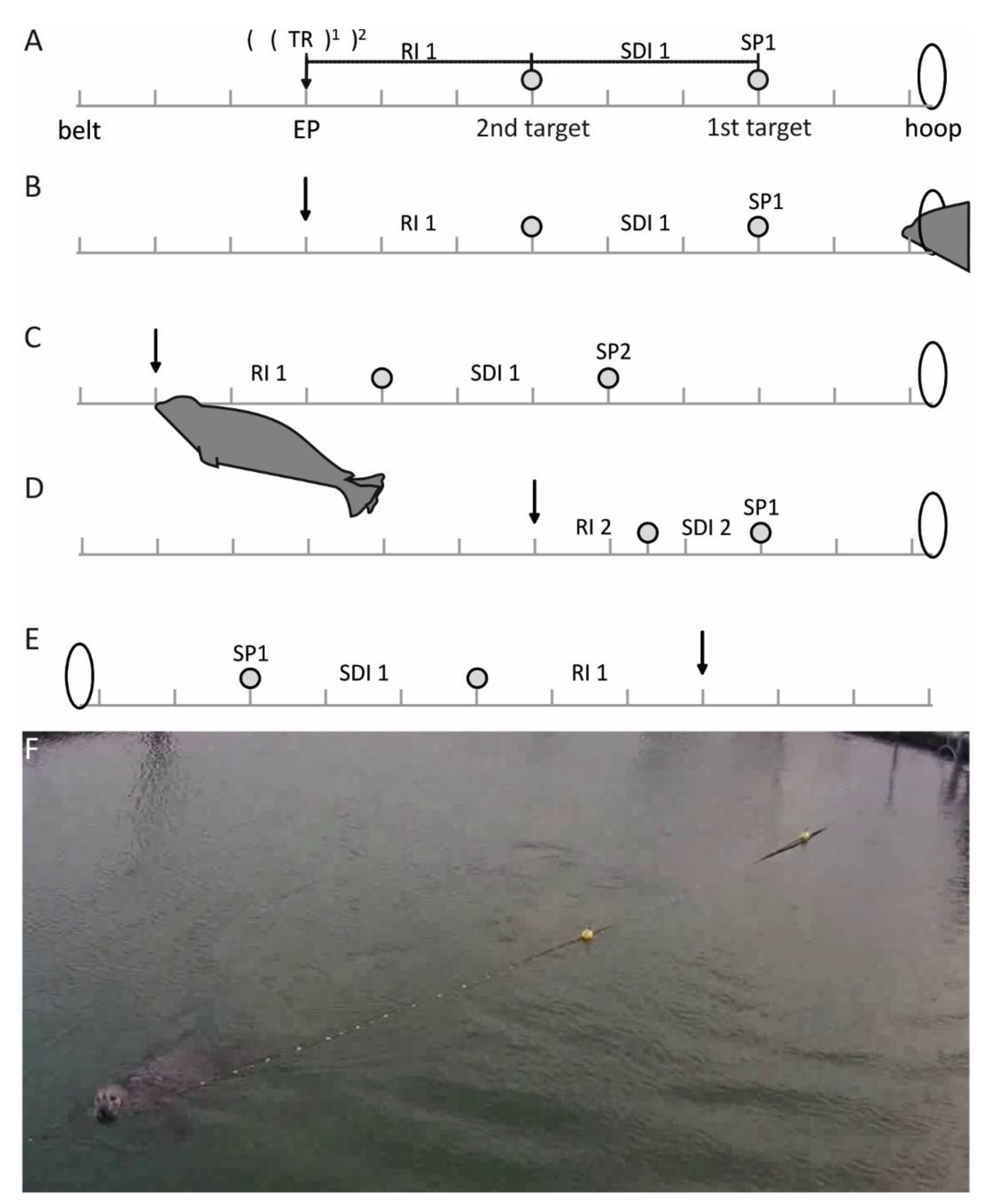
The experimental setup consisted of a belt-system stretched over 43 m (
Figure 1). This was the maximum distance that could be covered within the largest enclosure of the Marine Science Center. The belt was painted in intervals of 10 cm using water-resistant paint to be able to use the belt as measuring tape. Although the belt was clamped, the units on the belt and on a calibrated measurement tape only deviated by ±1%.
Two balls were clipped onto the belt that served as the starting point (SP) and endpoint (EP) of the sample distance interval (SDI, see
Figure 1). The SP was defined as the distance from the hoop station, in which the seal was resting during the inter-trial interval, to the first target ball. The second target ball also indicated the start of the reproduction interval (RI). Both target balls could be shifted in position along the belt (
Figure 1) according to a preset schedule (
Table 1). This way, the SDI could be presented at different positions in space defined by the SP. The variation of the position of the SDI and RI along the belt achieved by the variation of the SPs served to minimize the possibility that the seal was learning EPs defined absolutely in space to solve the task instead of estimating/reproducing the distance in the SDI. For the control session, the belt system was clamped from the other side of the enclosure (
Figure 1E).
2.3. General Experimental Procedure
Each trial began with the experimenter asking the animal to station in the hoop station (
Figure 1). Then the experimenter positioned the target balls and thus laid out the SP/SDI combination of the respective trial with the help of an observer. The observer was situated at an elevated position, from which she/he could oversee the whole experiment.
On verbal command by the experimenter, the seal left the hoop station and started to move alongside the belt to the SP. The seal then proceeded to the second target ball at the end of the SDI. It was then required to reproduce the distance presented in the SDI by swimming further along the belt. A trial ended when the seal stationed at the belt.
The accuracy of reproduction was determined by the observer and was communicated to the experimenter, who could then reward the animal according to its performance. When reading the belt during the experiment, the error of the observer was determined as ±2%. Exact reproduction, meaning that SDI and RI were equal in distance, led to a reward of five pieces of fish. For the purpose of differential rewarding, we introduced two tolerance ranges (TRs;
Table 1). Reproduction within the predefined first tolerance range (TR1) led to a reward of three pieces of fish. If the animal reproduced the distance within the predefined second tolerance range (TR2), it received a reward of one piece of fish. In stage 1, the TR1 was set to 1 m, approximately half the body length of the animal, and the TR2 was 10% of the distance (50 cm). In the following experimental stages (stage 2–5), we chose relative percentile TRs (
Table 1). The values we picked for both TRs were set in line with values from the literature [
27,
43]. No reward was given if the seal stopped outside the respective TRs.
The observer filmed the trials with a camera (Rollei Actioncamera 7S Wi-Fi, Hamburg, Germany) for further offline analysis of the animal’s movements and behavior.
2.4. Experiment 1
Experiment 1 was divided into five experimental stages. Stages 1–4 of the experiment were subdivided into a phase of acquisition, in which the seal was familiarized with (a) distance(s), a phase of testing, in which the precision of the seal was determined with familiar and unfamiliar SPs, and a control session, in which the experimental procedure was performed in reversed swimming direction. Stage 5 consisted of a phase of testing only.
In the acquisition phase, the seal had to reproduce the distance(s) from various preset SPs. The sessions were composed of 24–36 trials in which we presented distance/SP combinations following a pseudorandom schedule [
44].
The acquisition phase was considered to be finished once the animal achieved the learning criterion. To reach the learning criterion, the seal’s average precision of reproduction had to fall within TR1 (criterion 1). In addition, the standard deviation had to be smaller than 1 m (criterion 2). These criteria need to be met in two consecutive sessions for the acquisition phase to be completed and for the phase of testing to begin. When the seal had reached the learning criterion, its average precision of reproduction during the session was determined.
During the testing phase, the number of SPs was increased, further avoiding that the animal relied its responses on memorized absolute EPs. The general experimental procedure remained as in the acquisition phase. Each session consisted of 18 to 36 trials, and each distance was tested 25 times from each SP, totaling up to 150 trials per distance.
After each testing phase, except for phase 5, a control session was run. During this control session, the seal performed the experiment from the opposite side of the enclosure. Thus, the animal was in a familiar environment but experienced a new setting/panorama that it had never reproduced the distances from and consequently could not have gathered experience with in the first trial. In the control session, the distance(s) presented and the SPs, as well as the general experimental procedure, were the same as in the respective testing phase (
Table 1).
2.4.1. Pretraining
During pretraining (P;
Table 1), the seal was familiarized with the reproduction task. It was taught to swim towards the target balls with a 5 m distance presented in the SDI. After passing the second target ball, it had to proceed to a third target ball positioned 5 m away from the second target ball, marking the end of the RI. In one session, 10–23 trials were run. After a total of 280 trials, in which the seal learned this procedure, the third target ball was removed, and stage 1 could be started.
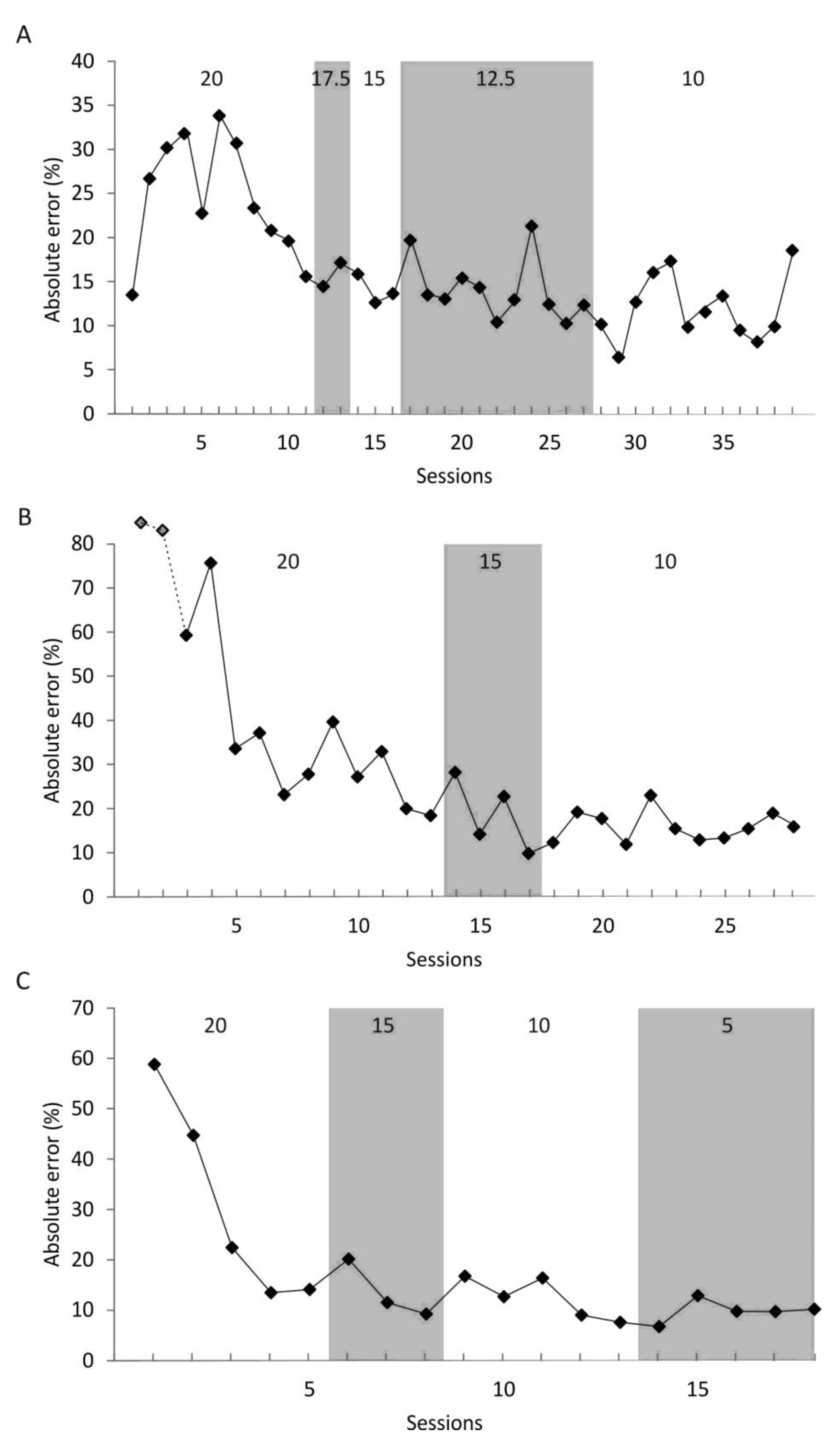
2.4.2. Stage 1
The acquisition phase of stage 1 was started with a 10 m distance presented from an SP at 3 m (
Table 1). In each session, 30 trials were conducted. The TR1 was chosen as ±0.5 m. In addition, the TR2 was set at ±1 m from the exact distance of 10 m. In the acquisition phase, the seal did not show any signs of learning for the 10 m distance. We then chose to switch to the 5 m distance the animal had experienced during pretraining in an attempt to facilitate learning; the TRs remained as described. With this distance, we could complete the acquisition phase.
In each session during the testing phase, the seal was asked to reproduce the 5 m distance from six SPs, out of which the seal had only experienced the 3 m-SP previously (
Table 1).
Additionally, the seal was asked to reproduce the 5 m distance from these six SPs, however, with reversed swimming direction in a control session consisting of 30 trials.
2.4.3. Stage 2
To test whether the seal could discriminate between distances, the distances 3 m, 7 m, and 12 m were presented during stage 2 of experiment 1. During the acquisition phase of stage 2, the distances were presented from three different SPs (
Table 1). The TR1 was set at ±10% of the distance; the TR2 was defined as ±1 m for the 3 m distance, ±1.5 m for the 7 m distance and ±2.4 m for the 12 m distance. In the acquisition phase, the seal easily learned to reproduce the 3 m and 7 m distance but had difficulties in learning to reproduce the 12 m distance. Consequently, we excluded the 12 m distance enabling us to proceed with training.
Once the learning criterion (see stage 1) was fulfilled, the number of SPs was increased to six SPs for the testing phase; four of these SPs were new to the seal.
2.4.4. Stage 3
In the third stage, the question was addressed whether the seal was capable of estimating and reproducing particularly small and long distances. Therefore, the distances of 1 m and 11 m were chosen (
Table 1). The TRs in this session were set to ±10% and ±20% of the respective distance. SPs, learning criteria, and testing procedure were the same as in the previous stages.
2.4.5. Stage 4
In stage 4, the seal’s ability to reproduce four of the distances (1 m, 3 m, 7 m, 11 m) presented in stages 2 and 3 within one session was assessed. Together with three SPs, 12 different distance/SP-combinations were trained in the acquisition phase. Each combination was tested twice in a session of 24 trials until reaching the learning criterion. During the testing phase, the distances needed to be reproduced from four new and two old SPs as in the previous phases. The TRs in this session were set as in stage 3. Each distance/SP combination was tested once in 24 trials-sessions.
2.4.6. Stage 5
In stage 5 of the experiment, the seal was confronted with one session, in which a new distance was presented in every trial. Moreover, these unknown distances were presented from unknown SPs. Altogether 29 unfamiliar combinations of distances and SPs (see
Appendix A) were tested. Here, TRs were set as in stage 3 (
Table 1).
2.4.7. Data Analysis
To assess the precision of the animal during the session, we analyzed medium averages of signed and absolute error with Excel 2016 (Microsoft Corporation, Redmond, Washington WA, USA) in line with the analysis of Bigel and Ellard [
45]. Additionally, we used IBM SPSS (v.26; International Business Machines Corporation Armonk, New York, NY, USA) for the analysis of variances.
Saliences in the performance in both swimming directions were also studied. In a first-trial-analysis, we compared the performance during the first trials during the control session swum for each distance and SP in reversed swimming direction with the average performance of the animal in the testing phase. This analysis was informative regarding the influence of memorized EPs on the performance in the previous stages of the experiment. The learning of EPs could have influenced the performance of the animal during the testing and acquisition phases, although we drastically reduced this possibility by varying the intervals along the belt, but could not influence its performance in the first trial in reversed swimming direction, as the animal had no previous memory of the EP.
All videos made during data collection by the observer were analyzed with Avidemux (v.2.6;
http://fixounet.free.fr/avidemux/ (accessed on 15 May 2017)). We analyzed the swimming speed in the RI by calculating the time elapsing between the animal leaving the second target ball and the animal stopping at the belt at its chosen EP. Since it was not always unambiguous at which frame the seal touched the second target ball or stopped at the belt, we calculated the resulting error in swimming speed, which amounted to ±2%.
In stage 4, with four distances presented, we investigated if the seal used specific motion patterns for reproducing a specific distance. We chose to investigate the movement patterns in stage 4 only since this was the sole stage in which the animal experienced more than two distances over a longer period. After reviewing the videos, we could classify the movements of the seals in every single trial and analyzed how often a specific movement pattern occurred during the reproduction of a specific distance. We performed a Chi2 test to test if the seal performed better if it moved with a specific motion pattern.
The ethogram consisted of the following movement patterns:
“touch–dive–drift”—the animal casually strived the second target ball, performed a flap with its fin and began diving. After diving, the seal surfaced, drifted, and then stopped;
“touch–dive–stop”—the animal quickly touched the second target ball with full snout contact and started a dive. After resurfacing, it immediately stopped;
“touch–drift”—the animal touched the second target ball with full snout contact and started gliding. During gliding, it performed a turn until it stopped;
“touch–stop”—after touching the second target ball, the seal swam continuously at the water surface until stopping entirely;
“other”—the seal showed a movement pattern different from points 1–4.
2.5. Experiment 2
In experiment 1, predefined TRs mostly adapted from the literature were used to train the animal. In experiment 2, we wanted to test the precision of reproduction the seal can maximally achieve. Therefore, the seal was trained to reproduce a new distance, which was kept constant during the experiment. It was presented from 24 SPs in a 24 trials-session; these 24 SPs were used throughout experiment 2 in randomized order.
Training started with a preset TR. After reaching the learning criterion, defined as a performance with an absolute error of ≤20% achieved in two consecutive sessions, the TR was reduced by 2.5% (9 m distance) or 5% (2 m and 13 m distance). This way, the TR was continuously reduced until the seal was unable to reach the learning criterion within 5–10 sessions. We finally decided to run only five sessions as the seal’s performance did not increase over ten sessions; thus, no learning seemed to have taken place neither over five sessions nor over ten sessions. The maximum precision of reproduction was determined for three distances in the following order: 9 m, 2 m, and 13 m.
We used IBM SPSS for the statistical analysis of the data.
2.6. Experiment 3
In experiment 3, the influence of the visual system on the performance of the animal was determined. Hereby, the general procedure was as in the previous experiments. However, now every session included baseline and masked trials. In the baseline trials, the seal was presented with a 4 m distance, which had never been presented to the animal before, from four different SPs: 3 m, 4 m, 5 m, and 6 m. In each session, the distance was presented six times from each SP. Thus, a session included 24 baseline trials.
Within all sessions, masked trials were interspersed in which the seal had to complete the task with a blindfold, a latex mask over its eyes. The animal was highly experienced wearing masks and thus easily adapted to perform the task with a blindfold. With the interspersed masked trials, we could evaluate and compare the precision of reproduction in the baseline trials, in which the seal could rely on all available cues, and the masked trials, in which vision was occluded.
At the beginning of training for experiment 3, the number of masked trials was increased within three sessions from four to the maximum number of eight masked trials within a session. Training continued until the learning criterion was met. The learning criterion was defined as the seal reproducing all baseline trials with an absolute error of ≤20% in two consecutive sessions. Thereafter, testing started in which the number of SPs was increased to six: 2 m, 3 m, 4 m, 5 m, 7 m, and 8 m. During testing, seven sessions were conducted with 18–24 trials. This cumulated in a total of 162 trials, including 108 baseline trials and 54 masked trials.
The performance of the animal was analyzed as in the previous experiment with IBM SPSS. Additionally, we also compared masked and unmasked trials in terms of precision of reproduction.
4. Discussion
In this study, we analyzed the distance estimation ability of one captive harbor seal, which was available for the extended behavioral experiments. Consequently, the conclusions drawn always need to be considered taking this small sample size as well as the laboratory conditions of our large seawater enclosure, under which the data were obtained, into account and will remain speculative regarding the behavior of wild seals. However, the experimental animal behaves normally and does not have any general deficits, which makes us confident about the reliability of the data concerning this seal individual.
In experiment 1, in phases 1–4, we could show that a harbor seal can learn to estimate and reproduce distances between 1 and 13 m within a predefined range of accuracy. This conclusion is based on the fact that, when presented with two or more distances within one session, the seal could only solve the task if it had derived distance information from the SDI. The use of memorized EPs to solve the task can largely be excluded, as (1) we asked the seal to perform from numerous SPs, (2) the seal was able to perform successfully in most first trials when asked to run the experiment with inversed swimming direction in the control sessions, and (3), over the course of the experiment, one point in space could have been the EP of two SP/SDI combinations, such as 12 m/3 m and 14 m/1 m. Thus, we present the first evidence for distance estimation and reproduction in a marine mammal, the harbor seal. Previously, distance estimation has only been demonstrated in terrestrial animals, such as in insects, gerbils, and humans (see, for example, [
18,
45,
46,
47,
48]). However, as aquatic mammals, harbor seals may also profit from this ability when navigating their environment using, for example, path integration or path return (see Introduction) to find their way back to a location, such as a haul-out place.
In the first stages of experiment 1, the question was addressed, if a harbor seal could learn to reproduce distances in a reproduction task typically used with human subjects. The seal here showed an absolute error between 12% and 31%. This means that not only can the seal learn to reproduce single and multiple distances, but the resulting precision from the experiment also implies that seals could use distance estimation for navigation purposes. Short distances, when seals stay close to shore, could thus be estimated effectively. However, even on a journey covering kilometers, the precision shown in the first phases of experiment 1 would bring the animal close enough towards its goal to then to pinpoint the exact location using, for example, visual cues; for visual cues to be effectively used to, for example, spot a haul-out place rising only 0.1 m above the water surface under good visibility at sea [
49,
50], the seal would need to get as close as 1.2 km. Additionally, information obtained by other sensory systems can reduce the errors that might accumulate when large distances are traveled or during path integration.
Two interesting phenomena were documented that provide evidence regarding the mechanism of distance estimation in our reproduction tasks. First, the seal used motoric cues to reproduce a distance. All distances, except for one distance, were reproduced with a specific motion pattern, and the accuracy of reproduction was highest if the seal used a specific motion pattern for the reproduction of a specific distance. We consider it unlikely that seals are using specific motion patterns for every single distance traveled in the wild. However, seals might nevertheless use motoric cues to reproduce and maybe to estimate distances in general. Harbor seals could, for example, apply a pedometer comparable to desert ants [
37,
51]. In seals, this would correspond to counting the number of tail fin flaps, which might be revealed when seals are asked to estimate larger distances. A detailed analysis of GPS recordings in combination with accelerometer tags [
52,
53] might even enable researchers to determine whether the number of tail fin flaps correlates with the distance swum in wild seals. Second, the seal’s performance was highest if it was reproducing a distance with a specific swimming speed. As previously it was documented that harbor seals have an accurate sense of time [
54,
55], the seal might have kept swimming speed constant, allowing it to use time as a measure for distance. Future studies are required to further elucidate the mechanism of distance estimation in harbor seals. These studies also need to consider ecological factors, such as waves or ocean currents, determining whether seals can perceive and compensate for them or whether these present challenges for using motoric cues or for the determination of swimming speed.
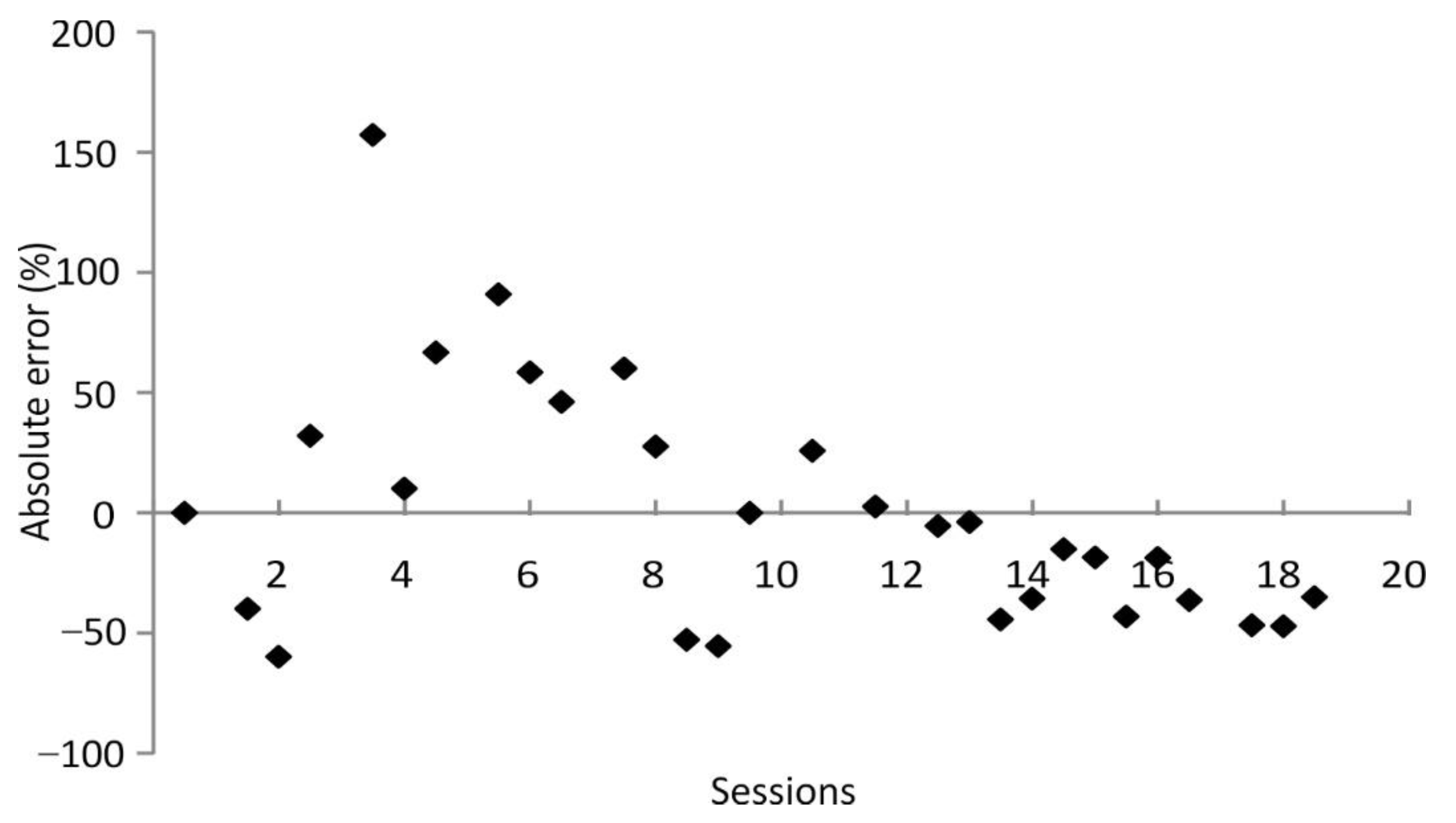
In phase 5 of experiment 1, we asked the seal to reproduce new distances without prior acquisition/learning phase for these distances. The seal’s precision noticeably dropped in this stage to an overall error of 39.2%. However, its accuracy was best, with a mean absolute error of 27.1%, when asked to reproduce distances ≥10 m, which is comparable to the previous stages that included acquisition phases. On the one hand, the seal might have performed better for distances ≥10 m as these are most likely of higher ecological relevance. Seals in their natural habitat are reported to travel distances of dozens of kilometers per day [
56]. However, a fine-scale analysis of these trips needs to be done to unravel if these long trips consist of short-distance legs in which distances in the range of the distances or slightly beyond those presented in this study are covered. On the other hand, the seal might have performed worse for distances <10 m as it had already gained extensive experience for distances in this short-range in stages 1–4 of experiment 1. Its previous experience might have negatively affected his performance for distances <10 m in the final phase as it might have tried to fit a solution, such as a motion pattern, for a previously presented distance to a new distance of comparable length. In conclusion, comparing the results of phases 1–4 to the results of phase 5 of experiment 1, repeatedly swimming a specific path/distance increases accuracy, but nevertheless, even when asked to reproduce unknown distances, the seal achieved accuracy in the same range when presented with distances longer than 10 m.
The maximum precision a seal can reach during the reproduction of a repeatedly swum distance was assessed in experiment 2. We found that with specific training to the highest possible precision, the harbor seal’s absolute error for distance reproduction could decrease to a precision lower than 10%. The highest accuracy, an absolute error as small as 6.4%, was documented for the 9 m distance. We can thus further stress that the seal’s precision when repeatedly swimming a specific distance can be very high. Tagging wild seals has indeed revealed that some seals occasionally choose a specific route repeatedly [
57]. Consequently, under these circumstances, they will be able to precisely locate their goal.
The final experiment 3 revealed that the occlusion of vision does not have a major effect on distance reproduction. Instead, (1) the seal quickly transferred the paradigm to the non-visual procedure, (2) even when blindfolded, the distance was reproduced with an error comparable to the errors documented in studies involving human subjects in similar tasks [
27,
45], and (3) the difference in accuracy when reproducing a distance without a blindfold and with the blindfold was, although significantly different, only 4%. These results most likely mimic that seals frequently encounter conditions in which vision is reduced, such as when active at night or when diving in dark or turbid waters. Future experiments can address the role of other senses during distance estimation. Moreover, in line with our original thought, it would be interesting to examine the seal’s ability to estimate and reproduce distances solely based on idiothetic cues and then to put it into the larger context of path integration and orientation/navigation in general.
Throughout the experiment, the seal did not show the same motion pattern in the SDI and the RI; whereas it swam parallel to the belt in the SDI, it included curves or diving phases into the RI. The distance swam during the RI thus deviated from the distance of the SDI. However, the horizontal distance of both intervals matched. Documentation of the horizontal distance to compute a homing vector has been shown for the desert ant, if the ant was foraging in uneven terrain [
38,
58]. It may be highly adaptive for a harbor seal to also neglect the third dimension, meaning to not include the diving profile into the estimation of traveled distance as its diving pattern and thus distance traveled most likely deviate between the out- and inbound trip. Instead, it would be advantageous to keep track of the horizontal distance, which the seal did during our experiment.
In our study, we also found an influence of the distance on the precision of the animal. The seal was, in general, overshooting short distances and undershooting the reproduction of long distances as previously reported, for example, in humans [
59]. The underestimation of long distances has been explained by the amount of information intake exceeding the processing capabilities of the brain [
59]. This overflow of information results in a less accurate estimation of the distance; the animal is coming short [
60]. An underestimation of a distance can be highly adaptive and has been observed, for example, during path integration [
21]. If the animal underestimates the distance of its homing vector, it is not directly brought back to its goal, but its homing vector ends close to its goal. In the familiar terrain close to the goal, the animal can then use, for example, landmarks or the panorama to locate its goal precisely [
61]. An interesting future experiment, besides experiments on path integration or path return, could be a visual distance estimation experiment [
30], in which the seal must approach a goal, at which a target was presented, before the animal starts to move towards it. Such an experiment would mimic a natural situation, in which the seal was viewing a landmark at the water surface and was approaching it underwater without directly seeing the landmark anymore. These experiments could then be complemented by studies on the use of landmarks.