Hydrogeochemical and Hydrodynamic Assessment of Tirnavos Basin, Central Greece
Abstract
:1. Introduction
- -
- Combine and test variable methodologies and tools through a specific proposed workflow, which may act a basic methodological array for assessing the hydrogeochemical and hydrodynamic conditions in similar cases.
- -
- Develop and optimize a conceptual model for the groundwater resources of the study area, based on previous literature and the newly applied combined methods/tools.
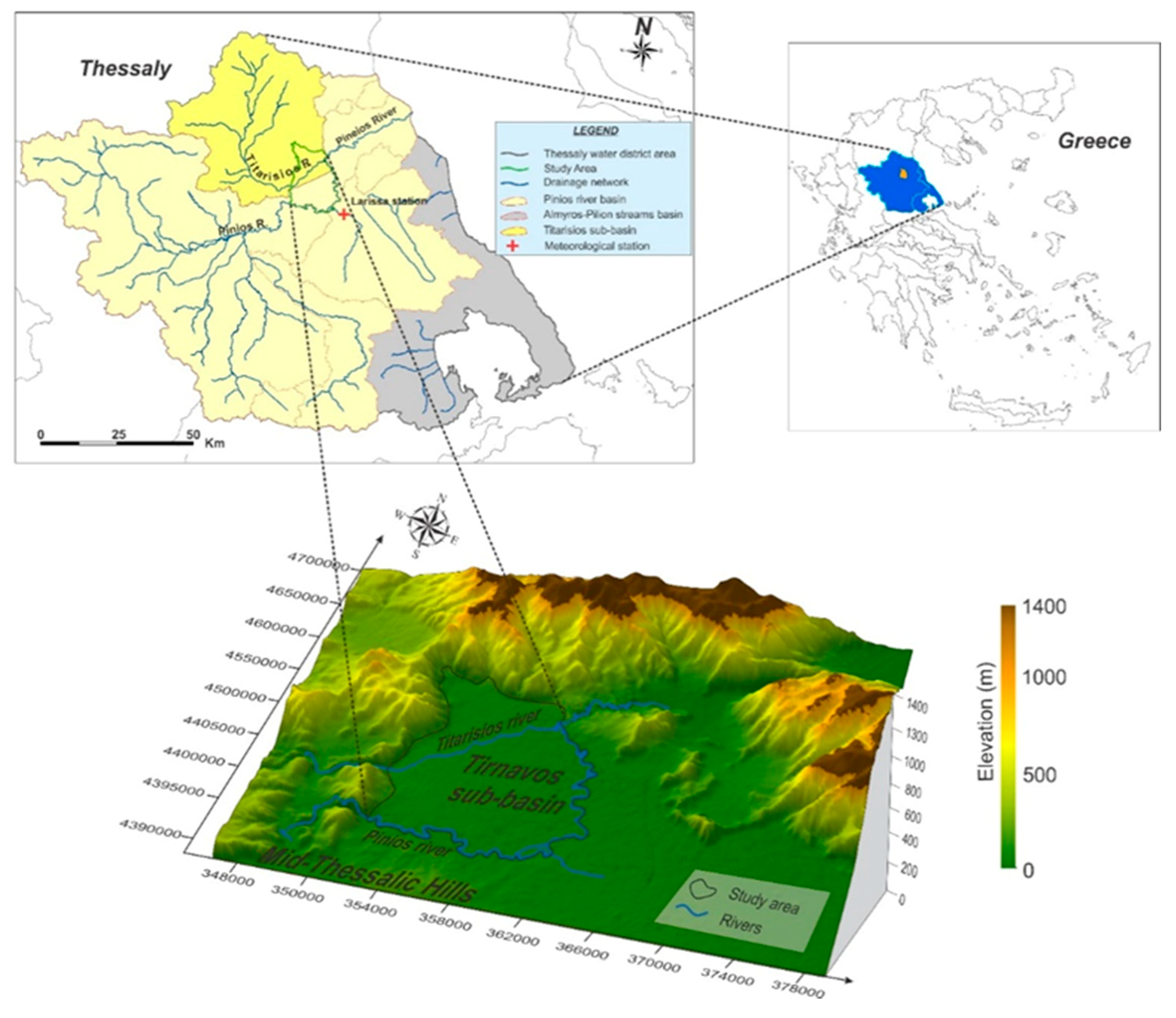
2. Study Area
3. Geology—Hydrogeology
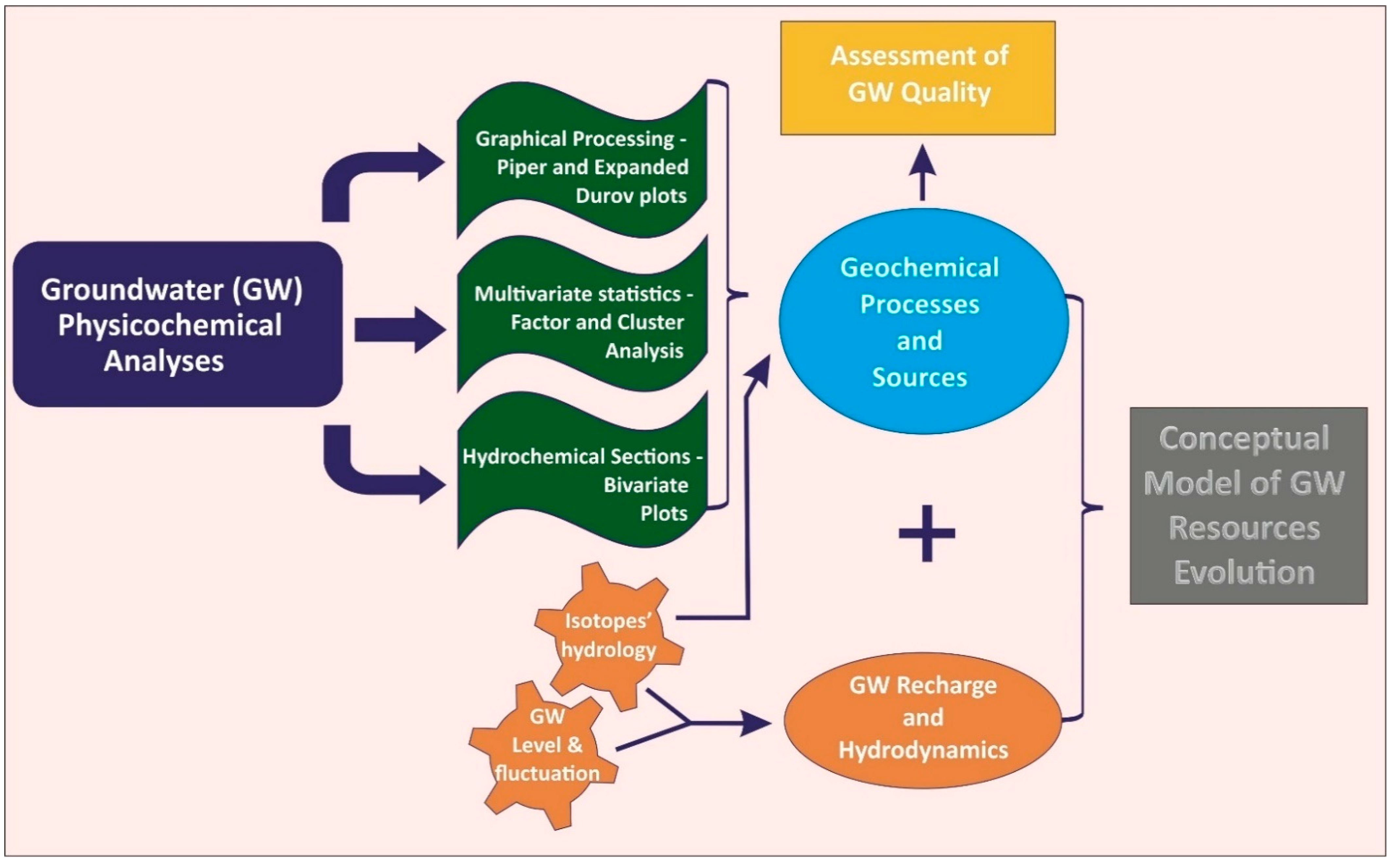
4. Methodology
4.1. Methodological Array
4.2. Groundwater Sampling, Analyses and Measurements
4.3. Data Processing
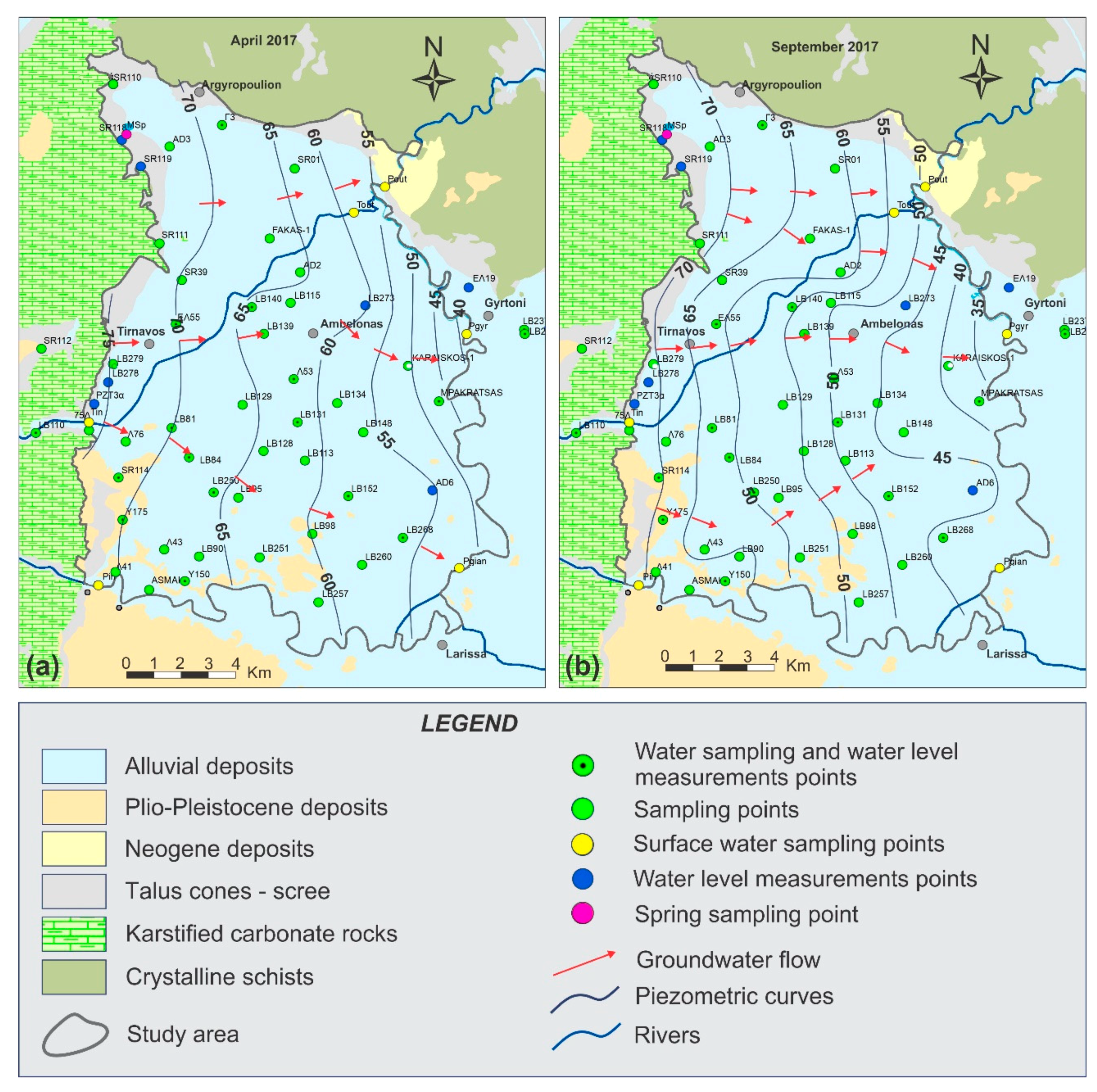
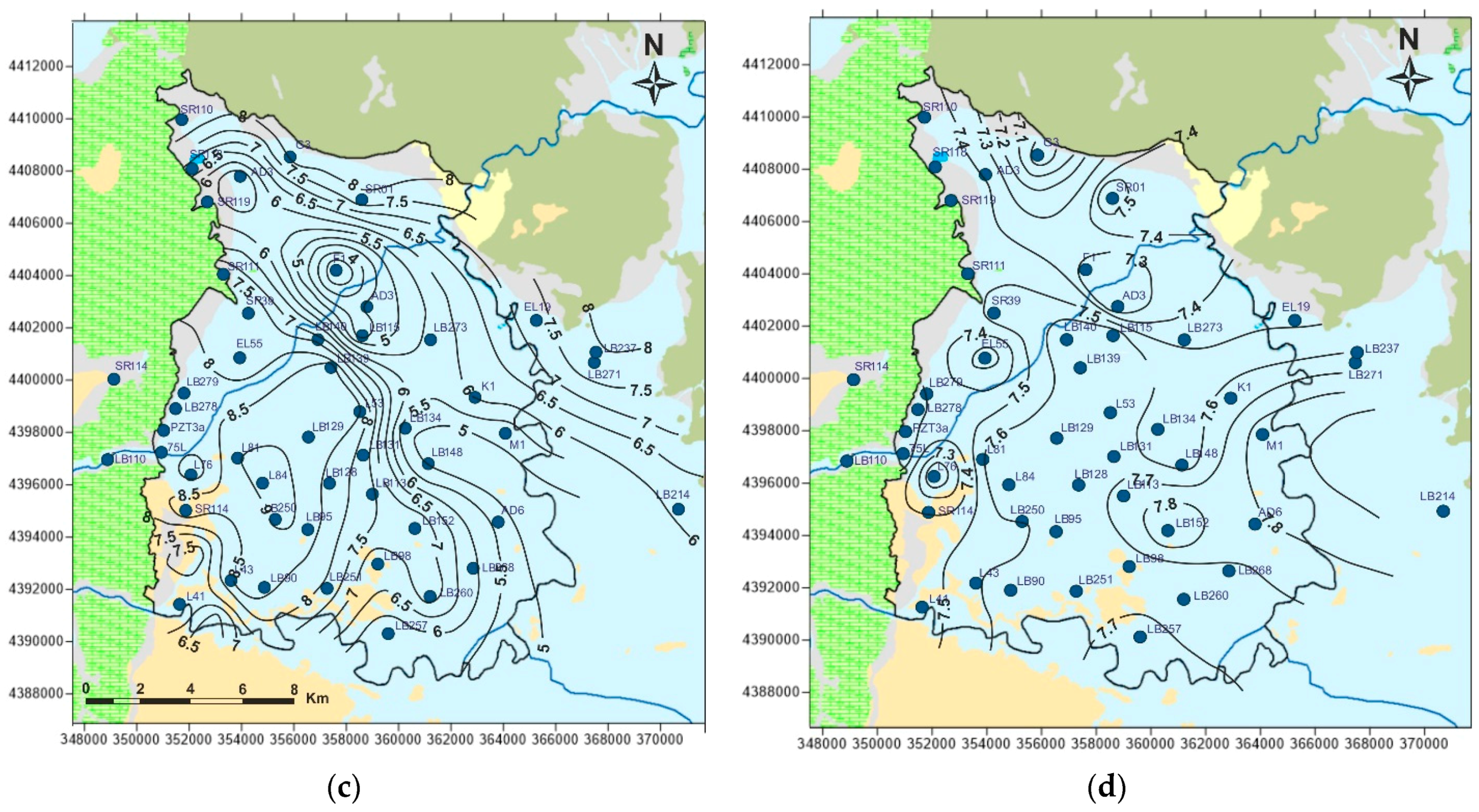
5. Results and Discussion
5.1. Physicochemcial Analyses
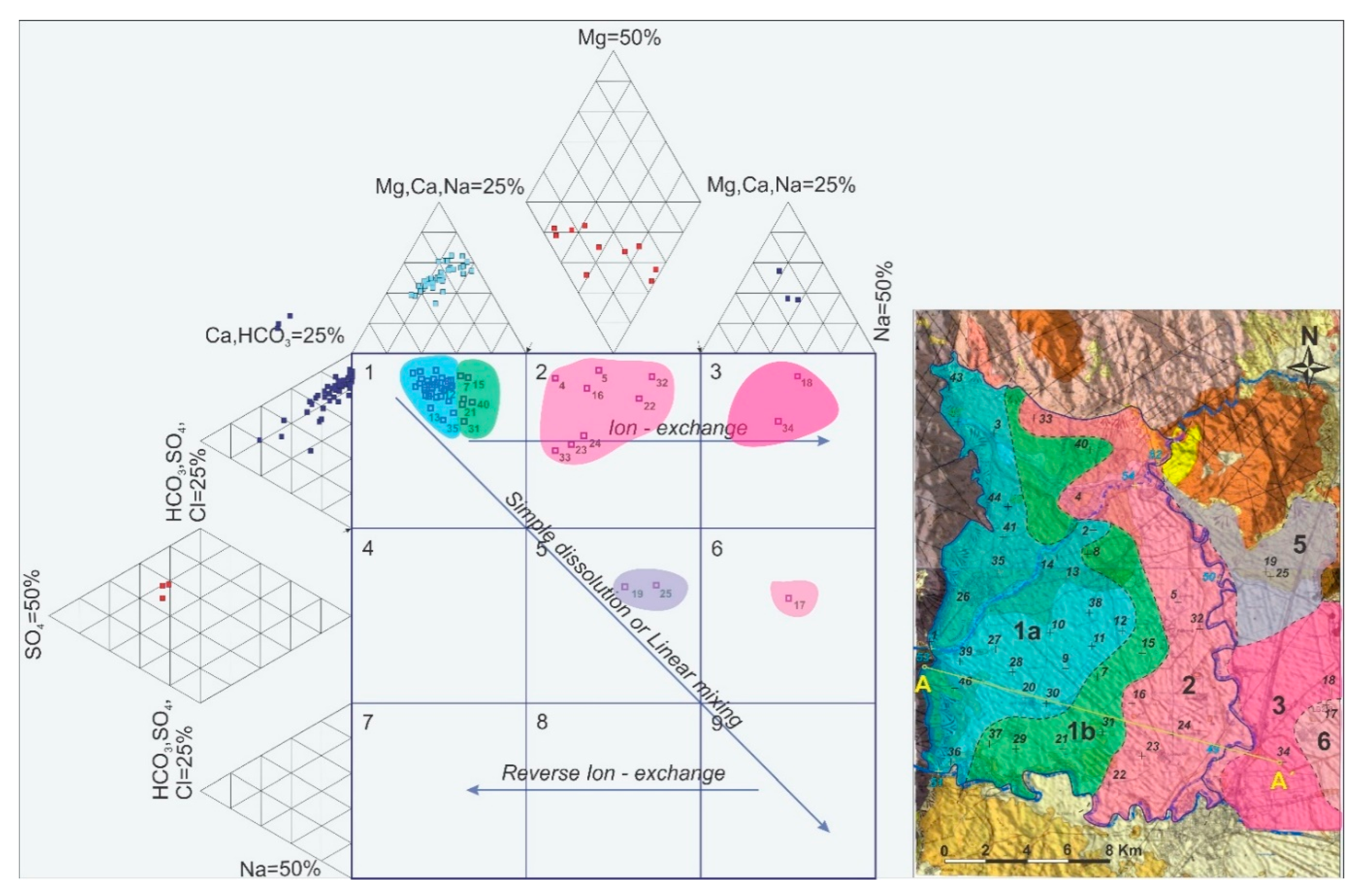
5.2. Identification of Governing Processes and Spalital Evolution of Hydrogeochemistry
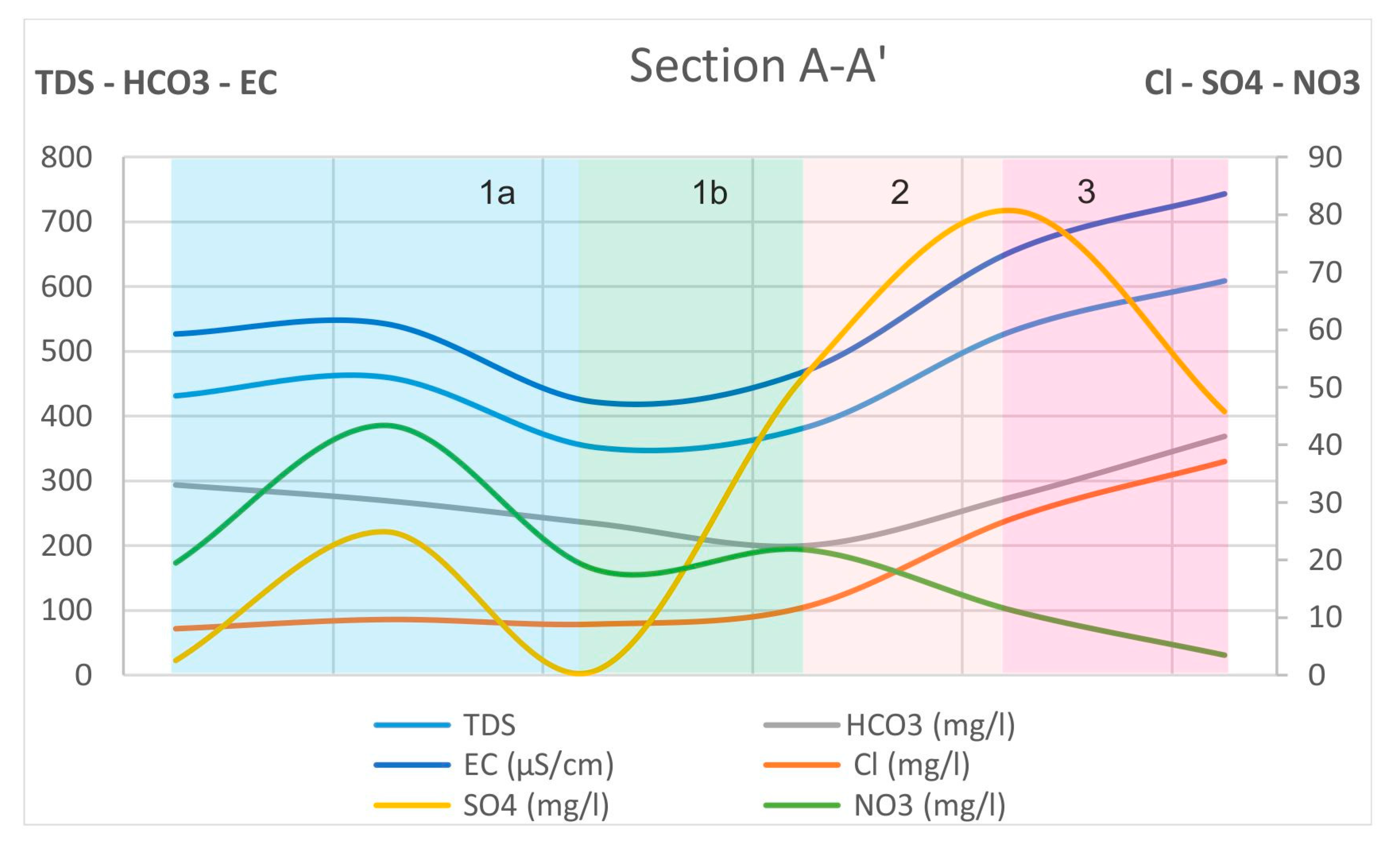
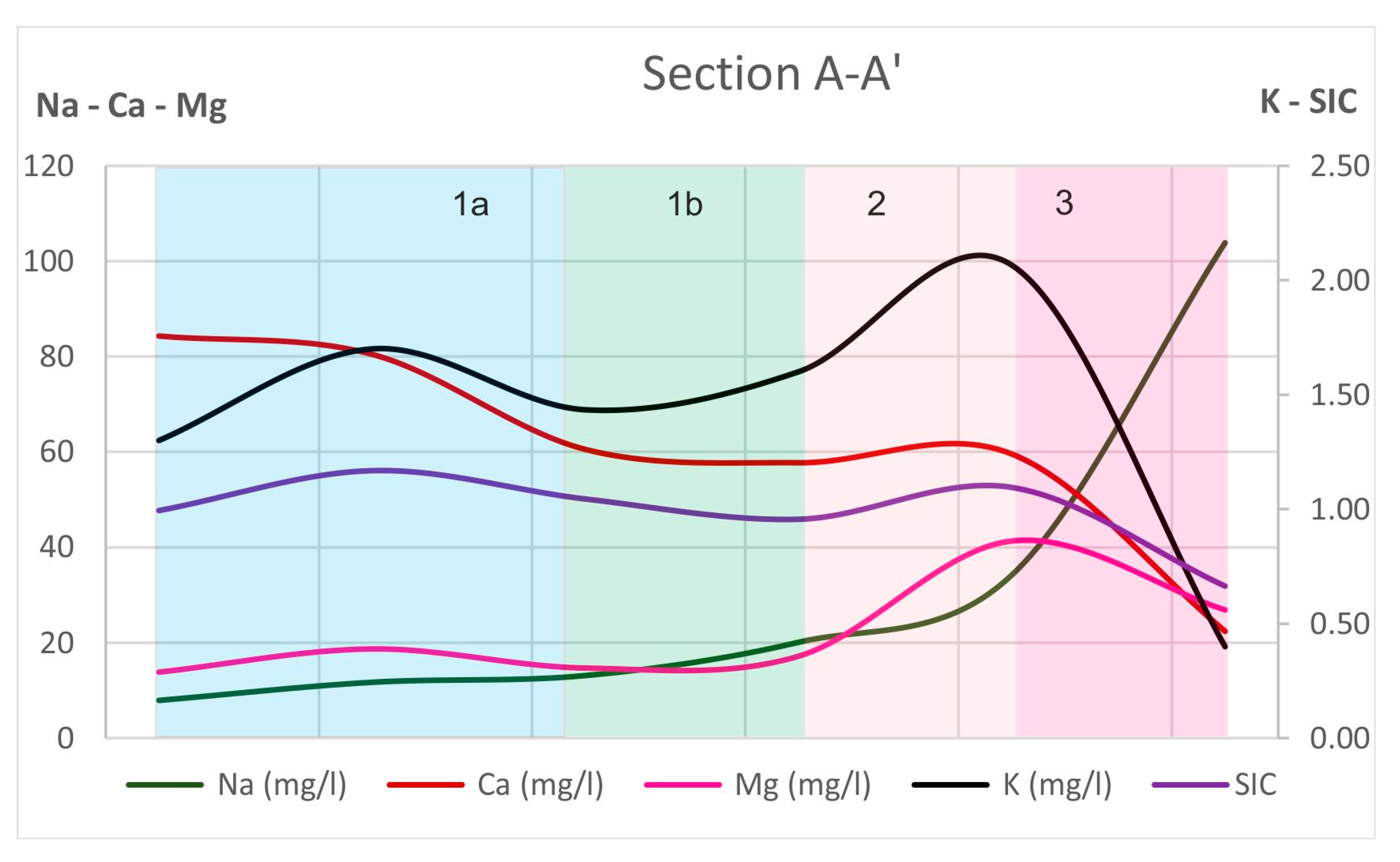
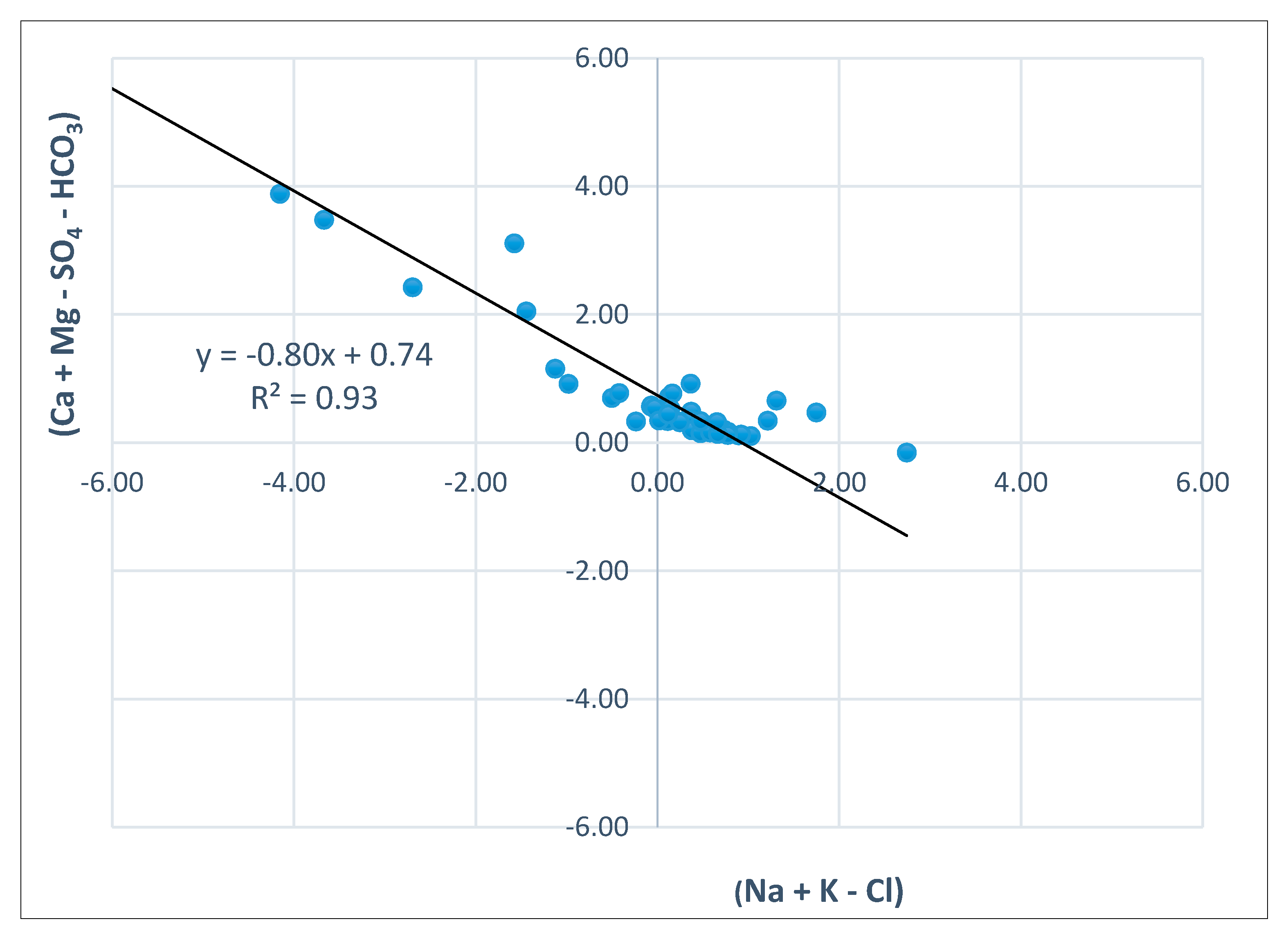
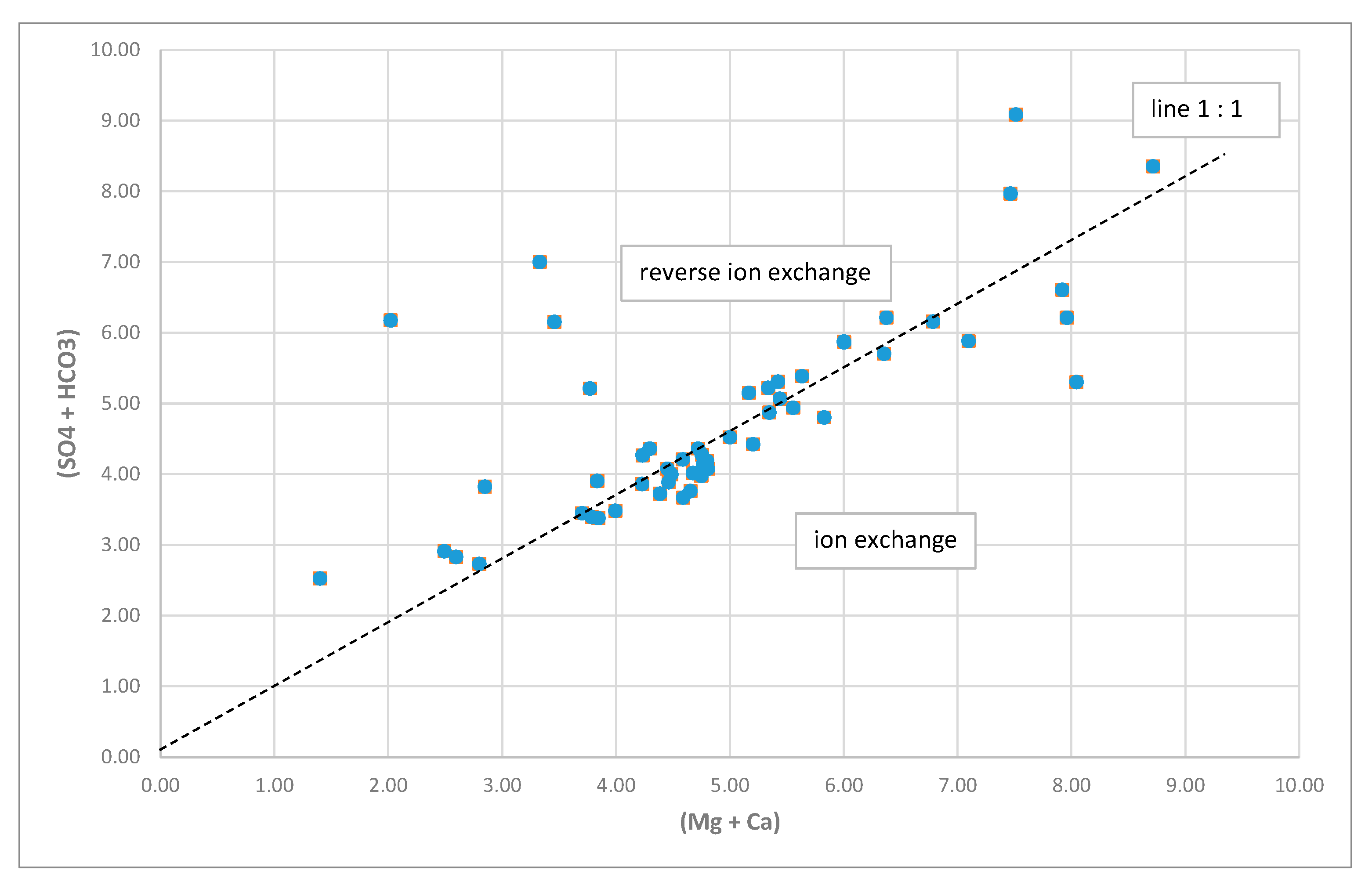
- Hydrochemical Type 1 (Ca-Mg-HCO3): In Subgroup 1a, Ca2+ and HCO3− were dominant, indicating that this was a recharge zone, which was verified by high dissolved oxygen and low water temperature values in this area (according to field measurements analyzed in previous paragraphs). Based on the dominant cations in the formation of the hydrochemical character, group 1b did not differ substantially from 1a. However, there was a slight increase in the concentrations of Na+ and Mg2+ and, with respect to the anions, higher SO42− values. Spatially, this hydrochemical type occupied two zones on either side of the Titarisios River. The first began north of it and extended to the northwestern part of the basin, while the second began from the central part of the basin (south of Titarisios) and extended to the south and southwestern part of the basin. The dominance of Ca2+ and HCO3− in this subgroup also indicated a recharge zone, possibly lower than that of group 1a. The involvement of Mg2+ in the formation of the hydrochemical type was consistent with the mild dolomitization process which characterized the carbonate system [27]. The reason for the observed differentiation between the two subgroups may have been due to the limited system recharge rate from the karst (which allows mixing of recharge water with the aquifer system and/or the development of a small degree of ion exchange processes). In particular, the development of this subgroup on its southwestern boundary may have been justified by the emergence of Neogene deposits in this area, which limited the rate of groundwater replenishment in this part of the aquifer. At the same time, in this area, according to earlier studies [53], lateral recharge from the Mid-Thessalic hills was reported—of limited extent and intensity and qualitatively inferior to that of the karst system.
- Hydrochemical Type 2 (Ca-Mg-Na-HCO3-SO4): The second hydrochemical type was characterized by higher Na+ and SO42− concentrations. The involvement of Ca2+ and HCO3− in the formation of the hydrochemical type of subgroup 2 (Mg-Ca-HCO3-SO4) was still significant; however, it was less than that of subgroups 1a and 1b. The projection position of subgroup 2 in the expanded Durov diagram indicated progressive involvement of ion exchange and perhaps also mixing mechanisms. At least locally and seasonally [27], the mixing of Pinios River water with aquifer water through filtration along its bed in the formation of the observed hydrochemical type cannot be excluded. It was also characterized by low analogues with subgroups 1a and 1b at Ca2+ and HCO3− concentrations. Subgroup 2 (Ca-Mg-Na-HCO3) can therefore be considered as representative of a transition zone from intense recharge zones (subgroups 1a and 1b) to a restricted recharge zone and longer groundwater residence time in the aquifer (subgroups 3, 5, 6). In subgroup 3 (Na-HCO3), HCO3− and Na+ dominated, and the ion exchange phenomenon was fully evolved, as indicated by the projection of its representative samples (wells 18 and 34) on the expanded Durov diagram. Subgroup 5 (Na-Mg-Ca-SO4-HCO3) represented mixing or dissolution waters, where Mg2+ (but mainly Na+ from cations) and Cl- (but mainly SO42− and HCO3−, to a lesser extent from anions) dominated, thus suggesting a contributing recharge mechanism in the form of lateral influx from surrounding formations that contributed to the aquifer balance and obviously affected its hydrochemical identity. Last, subgroup 6 (Na-SO4-HCO3), which was only represented by a single but distinct sample, plotted on an uncommon part of the graph for groundwaters, which is often a product of mixing.
5.3. Multivariate Statistics
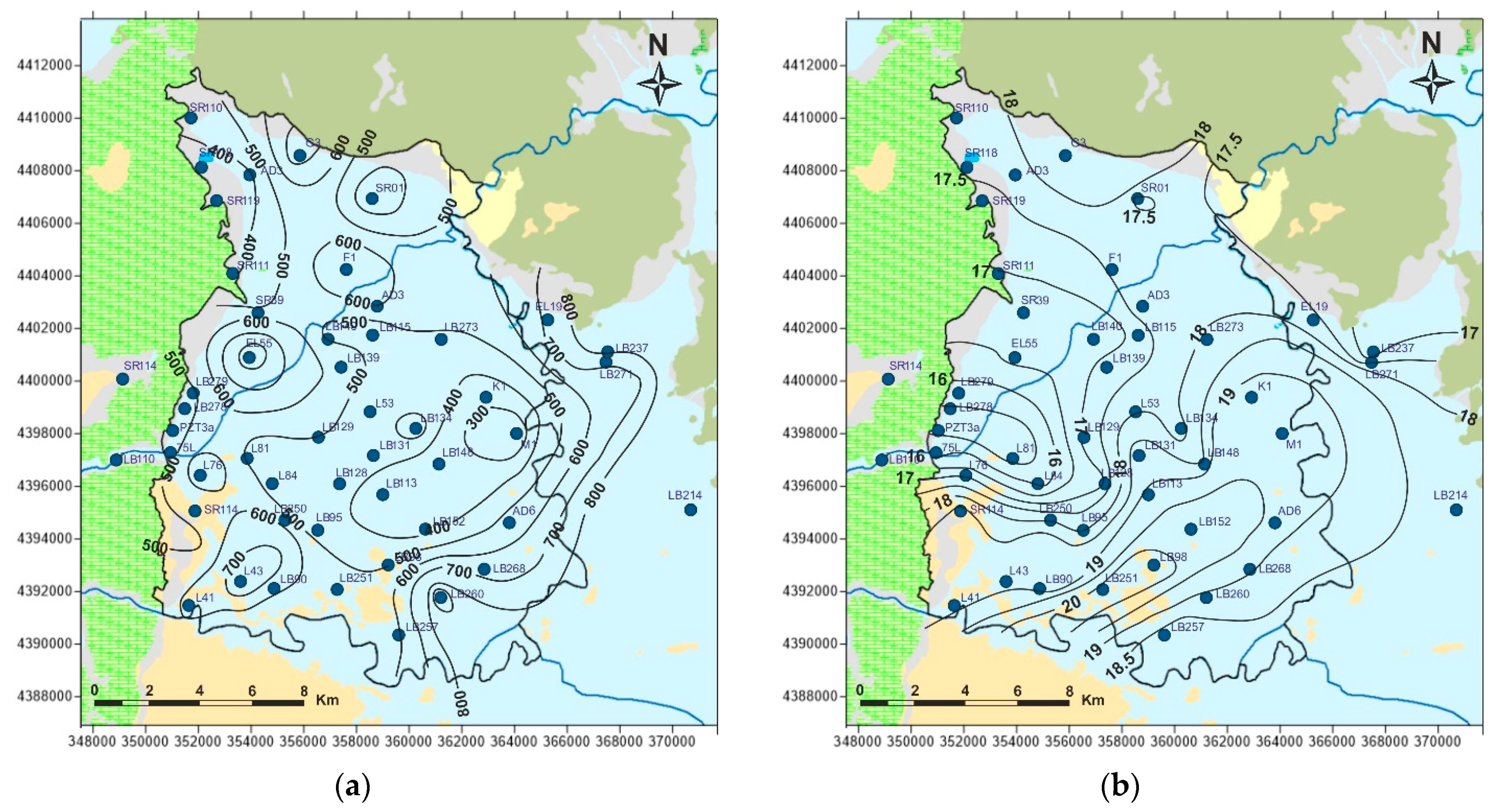
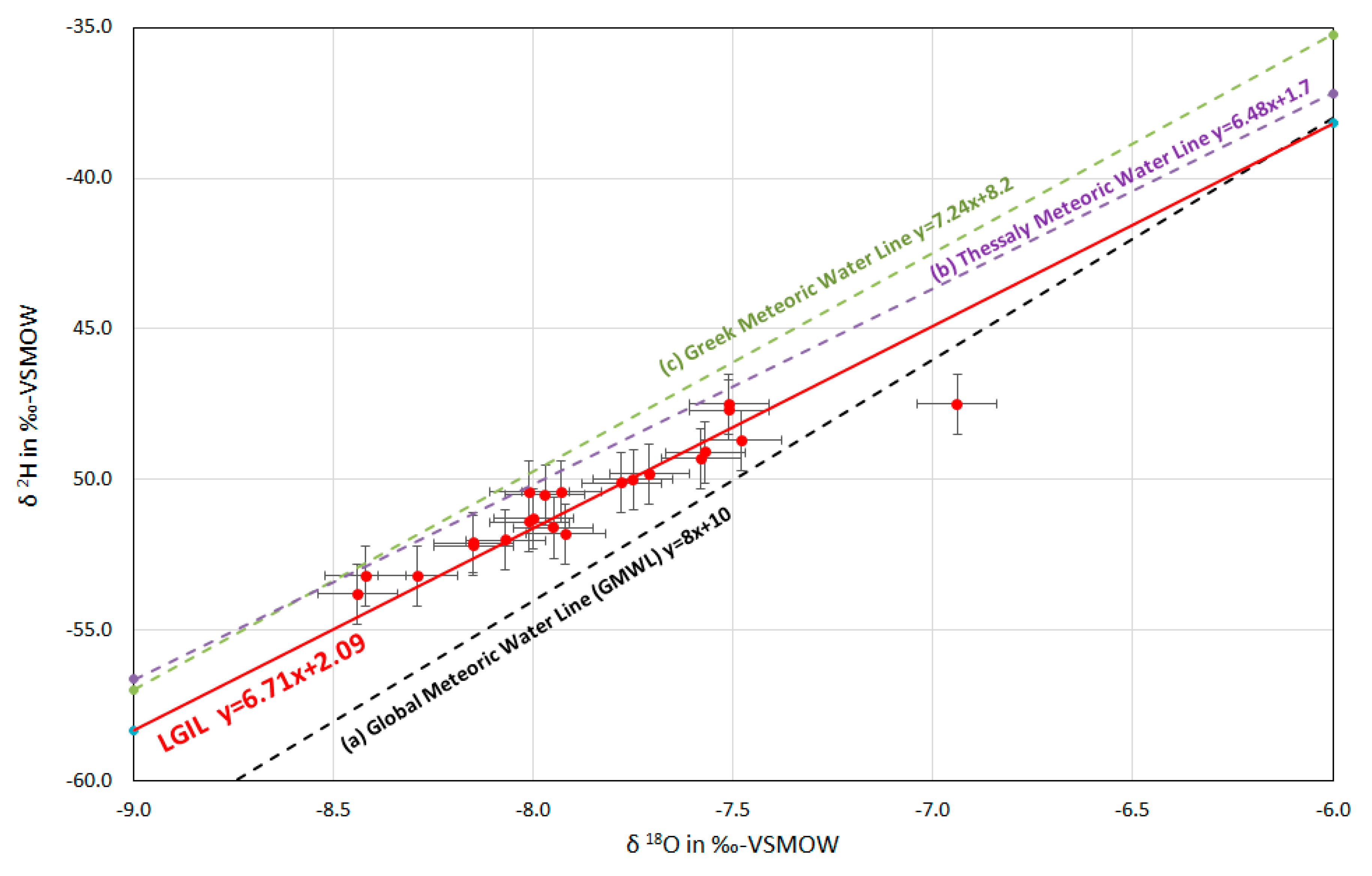
5.4. Stable Isotopes
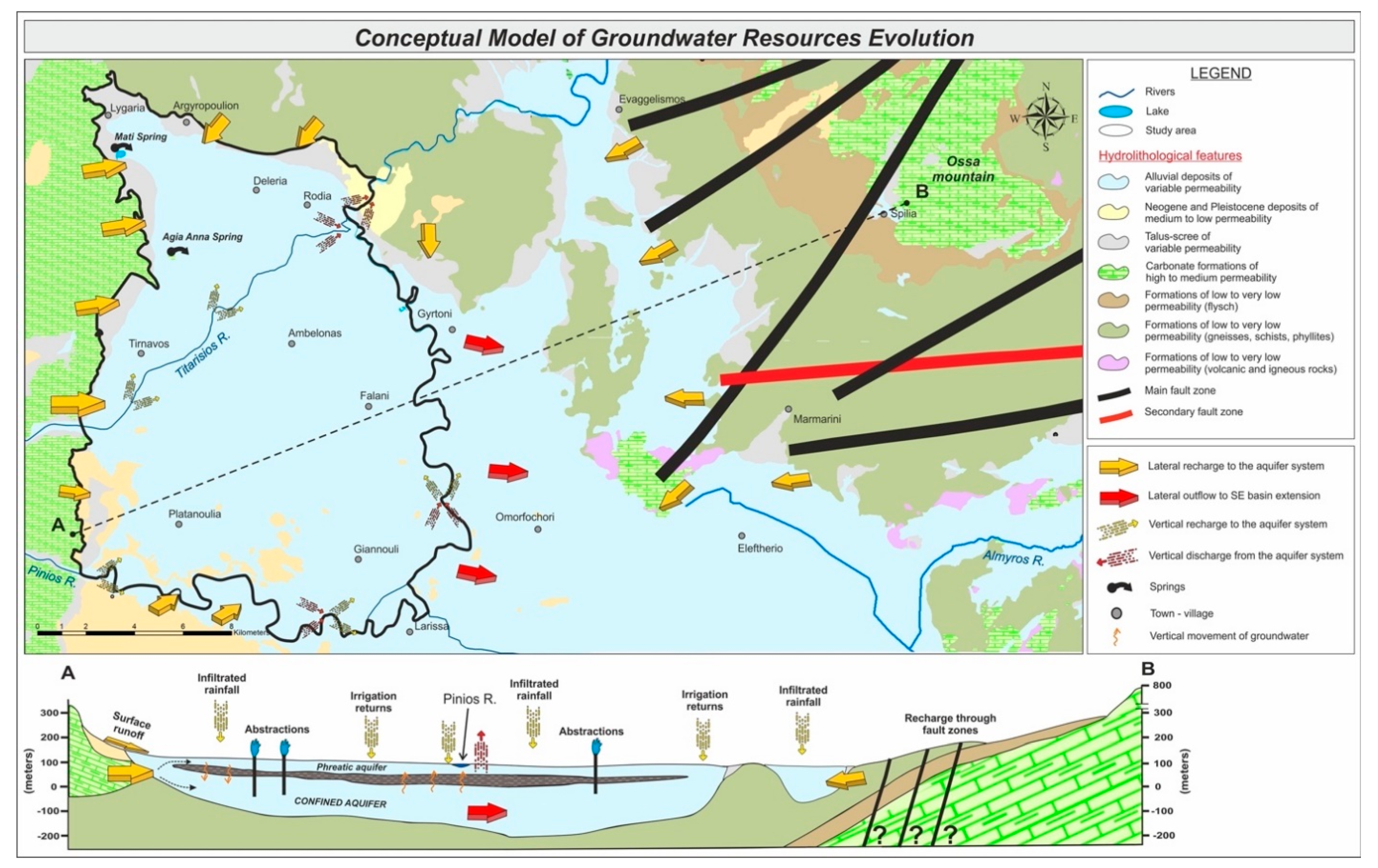
5.5. Conceptual Model of Groundwater Resources
6. Conclusions
- -
- The dominant ions (Ca2+ and HCO3−) of groundwater were indicative for the main recharge mechanisms—which were related to the karstic substrate.
- -
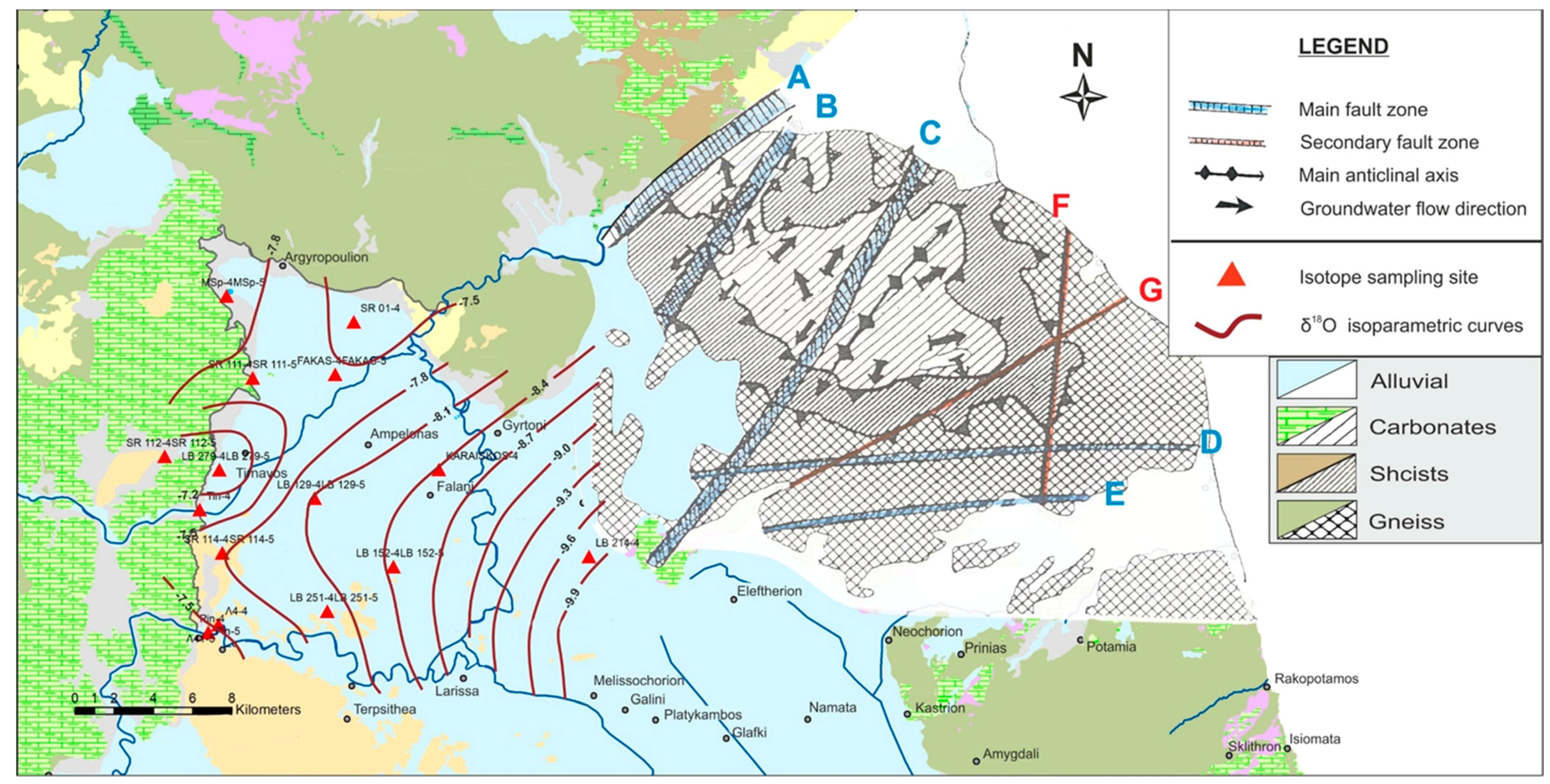
- The recharge areas were delineated with the joint use of variable tools and concluded in two major axes in an E–W direction. The western direction was related to the recharge from the karstic system of Titarisios and the eastern one was related to the recharge through preferential flow paths from the karstic massif of Mt. Ossa.
- -
- The prevalence of carbonate formations was also reflected in the dominant hydrochemical type (Ca-Mg-HCO3) which also encompassed the Mg content from relevant Mg-rich carbonate formations (dolostones).
- -
- Groundwater quality in the Tirnavos subbasin was in a generally good state, with few exceptions (elevated values of NO3 and Cu) which indicate local impact due to anthropogenic activities.
- -
- Nitrates can be considered the main adverse environmental aspect, occurring locally in hot spots at the area, due to irrational agricultural activities.
- -
- The groundwater quality was also impacted by salinization due to the combined use of irrigation water returns (agricultural leachates) and evaporitic mineral leaching.
- -
- The governing hydrogeochemical process identified was ion exchange, which progressively alters the chemical composition of groundwater from west to east.
- -
- A secondary process which seems to affect hydrogeochemistry was redox, which locally controls speciation of groundwater solute parameters.
- -
- The physicochemical evolution mechanisms were jointly assessed and verified by the hydrochemical sections of major ions and saturation indices of critical mineralogical phases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| pH | EC (μS/cm) | K (mg/L) | Na (mg/L) | Ca (mg/L) | Mg (mg/L) | Tot. Hardness (mg CaCO3/L) | Cl (mg/L) | HCO3 (mg/L) | CO3 (mg/L) | SO4 (mg/L) | |
| MIN | 6.92 | 253.00 | 0.40 | 6.86 | 14.66 | 8.10 | 69.96 | 4.77 | 118.81 | 0.00 | 0.11 |
| MAX | 7.96 | 1821.00 | 7.21 | 286.69 | 120.15 | 52.98 | 436.05 | 88.62 | 453.87 | 0.00 | 604.34 |
| MEDIAN | 7.59 | 473.04 | 1.68 | 13.60 | 67.69 | 16.67 | 234.53 | 10.82 | 238.37 | 0.00 | 12.21 |
| STDEV | 0.21 | 246.39 | 1.11 | 44.50 | 23.27 | 10.84 | 83.51 | 14.06 | 64.37 | 0.00 | 99.21 |
| NO3 (mg/L) | NH4 (mg/L) | B (mg/L) | Cu (μg/L) | Fe (μg/L) | Mn (μg/L) | Pb (μg/L) | Cd (μg/L) | As (μg/L) | SAR | TDS | |
| MIN | 0.22 | 0.01 | 0.00 | 0.00 | 0.81 | 0.07 | 0.00 | 0.00 | 0.00 | 0.20 | 216.00 |
| MAX | 145.37 | 5.76 | 1.25 | 24.22 | 100.85 | 150.83 | 3.12 | 0.13 | 4.22 | 6.76 | 1374.87 |
| MEDIAN | 19.08 | 0.12 | 0.02 | 1.65 | 22.26 | 1.27 | 0.08 | 0.04 | 1.62 | 0.39 | 390.39 |
| STDEV | 27.89 | 0.98 | 0.23 | 5.14 | 17.70 | 28.32 | 0.72 | 0.03 | 1.06 | 1.19 | 187.85 |
References
- Tziritis, E.P.; Datta, P.S.; Barzegar, R. Characterization and assessment of groundwater resources in a complex hydrological basin of central Greece (Kopaida basin) with the joint use of hydrogeochemical analysis, multivariate statistics and stable isotopes. Aquat. Geochem. 2017, 23, 271–298. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.S.; Koh, D.C.; Lee, D.H.; Lee, S.G.; Park, W.B.; Woo, N.C. Hydrogeochemical and isotopic evidence of groundwater salinization in a coastal aquifer: A case study in Jeju volcanic island, Korea. J. Hydrol. 2003, 270, 282–294. [Google Scholar] [CrossRef]
- Barzegar, R.; Moghaddam, A.A.; Tziritis, E.; Fakhri, M.S.; Soltani, S. Identification of hydrogeochemical processes and pollution sources of groundwater resources in the Marand plain, northwest of Iran. Environ. Earth Sci. 2017, 76, 297. [Google Scholar] [CrossRef]
- Iqbal, J.; Nazzal, Y.; Howari, F.; Xavier, C.; Yousef, A. Hydrochemical processes determining the groundwater quality for irrigation use in an arid environment: The case of Liwa Aquifer, Abu Dhabi, United Arab Emirates. Groundw. Sustain. Dev. 2018, 7, 212–219. [Google Scholar] [CrossRef]
- Wang, Z.; Torres, M.; Paudel, P.; Hu, L.; Yang, G.; Chu, X. Assessing the Karst Groundwater Quality and Hydrogeochemical Characteristics of a Prominent Dolomite Aquifer in Guizhou, China. Water 2020, 12, 2584. [Google Scholar] [CrossRef]
- Zancanaro, E.; Teatini, P.; Scudiero, E.; Morari, F. Identification of the Origins of Vadose-Zone Salinity on an Agricultural Site in the Venice Coastland by Ionic Molar Ratio Analysis. Water 2020, 12, 3363. [Google Scholar] [CrossRef]
- Domingo-Pinillos, J.C.; Senent-Aparicio, J.; García-Aróstegui, J.L.; Baudron, P. Long term hydrodynamic effects in a semi-arid Mediterranean multilayer aquifer: Campo de Cartagena in south-eastern Spain. Water 2018, 10, 1320. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liang, X.; Xiao, C.; Cao, Y.; Hu, S. Hydrochemical Evolution of Groundwater in a Typical Semi-Arid Groundwater Storage Basin Using a Zoning Model. Water 2019, 11, 1334. [Google Scholar] [CrossRef] [Green Version]
- Cao, F.; Jaunat, J.; Vergnaud-Ayraud, V.; Devau, N.; Labasque, T.; Guillou, A.; Ollivier, P. Heterogeneous behaviour of unconfined Chalk aquifers infer from combination of groundwater residence time, hydrochemistry and hydrodynamic tools. J. Hydrol. 2020, 581, 124433. [Google Scholar] [CrossRef]
- Murgulet, D.; Murgulet, V.; Spalt, N.; Douglas, A.; Hay, R.G. Impact of hydrological alterations on river-groundwater exchange and water quality in a semi-arid area: Nueces River, Texas. Sci. Total Environ. 2016, 572, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Nigro, A.; Petitta, M. Groundwater mixing in the discharge area of San Vittorino Plain (Central Italy): Geochemical characterization and implication for drinking uses. Environ. Earth Sci. 2017, 76, 393. [Google Scholar] [CrossRef]
- Maest, A.; Prucha, R.; Wobus, C. Hydrologic and Water Quality Modeling of the Pebble Mine Project Pit Lake and Downstream Environment after Mine Closure. Minerals 2020, 10, 727. [Google Scholar] [CrossRef]
- Güler, C.; Kurt, M.A.; Alpaslan, M.; Akbulut, C. Assessment of the impact of anthropogenic activities on the groundwater hydrology and chemistry in Tarsus coastal plain (Mersin, SE Turkey) using fuzzy clustering, multivariate statistics and GIS techniques. J. Hydrol. 2012, 414, 435–451. [Google Scholar] [CrossRef]
- Voutsis, N.; Kelepertzis, E.; Tziritis, E.; Kelepertsis, A. Assessing the hydrogeochemistry of groundwaters in ophiolite areas of Euboea Island, Greece, using multivariate statistical methods. J. Geochem. Explor. 2015, 159, 79–92. [Google Scholar] [CrossRef]
- Barzegar, R.; Moghaddam, A.A.; Tziritis, E. Assessing the hydrogeochemistry and water quality of the Aji-Chay River, northwest of Iran. Environ. Earth Sci. 2016, 75, 1486. [Google Scholar] [CrossRef]
- Celestino, A.E.M.; Ramos-Leal, J.; Cruz, D.A.M.; Tuxpan, J.; Bashulto, J.D.L.; Ramírez, J.M. Identification of the hydrogeochemical processes and assessment of groundwater quality, using multivariate statistical approaches and water quality index in a wastewater irrigated region. Water 2019, 11, 1702. [Google Scholar] [CrossRef] [Green Version]
- Walter, J.; Chesnaux, R.; Gaboury, D.; Cloutier, V. Subsampling of Regional-Scale Database for improving Multivariate Analysis Interpretation of Groundwater Chemical Evolution and Ion Sources. Geosciences 2019, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.; Xiao, C.; Li, M.; Liang, X. Hydrogeochemical Characteristics and Groundwater Quality Evaluation Based on Multivariate Statistical Analysis. Water 2020, 12, 2792. [Google Scholar] [CrossRef]
- Tziritis, E. Stable isotope study of a karstic aquifer in Central Greece. Composition, variations and controlling factors. In Advances in the Research of Aquatic Environment; Springer: Berlin/Heidelberg, Germany, 2011; pp. 193–200. [Google Scholar] [CrossRef]
- Joshi, S.K.; Rai, S.P.; Sinha, R.; Gupta, S.; Densmore, A.L.; Rawat, Y.S.; Shekhar, S. Tracing groundwater recharge sources in the northwestern Indian alluvial aquifer using water isotopes (δ18O, δ2H and 3H). J. Hydrol. 2018, 559, 835–847. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Wang, L.; Shi, L.; Song, X.; Yeh, T.C.J.; Zhen, P. Coupling hydrochemistry and stable isotopes to identify the major factors affecting groundwater geochemical evolution in the Heilongdong Spring Basin, North China. J. Geochem. Explor. 2019, 205, 106352. [Google Scholar] [CrossRef]
- Zamora, H.A.; Eastoe, C.J.; Wilder, B.T.; McIntosh, J.C.; Meixner, T.; Flessa, K.W. Groundwater Isotopes in the Sonoyta River Watershed, USA-Mexico: Implications for Recharge Sources and Management of the Quitobaquito Springs. Water 2020, 12, 3307. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Mu, W.; Zhu, G. Using Isotopes (H, O, and Sr) and Major Ions to Identify Hydrogeochemical Characteristics of Groundwater in the Hongjiannao Lake Basin, Northwest China. Water 2020, 12, 1467. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Arampatzis, G.; Tziritis, E.; Pisinaras, V.; Herrmann, F.; Kunkel, R.; Wendland, F. Assessment of climate change impact in the hydrological regime of River Pinios Basin, central Greece. Desalination Water Treat. 2016, 57, 2256–2267. [Google Scholar] [CrossRef]
- Alexandridis, T.; Panagopoulos, A.; Galanis, G.; Alexiou, I.; Cherif, I.; Chemin, Y.; Stavrinos, E.; Bilas, G.; Zalidis, G. Combining remotely sensed surface energy fluxes and GIS analysis of groundwater parameters for irrigation assessment. Irrig. Sci. 2014, 32, 127–140. [Google Scholar] [CrossRef]
- Vrouhakis, I.; Panagopoulos, A.; Stamatis, G. Current quality and quantity status of Tirnavos sub-basin water system—Central Greece. In Proceedings of the 11th International Hydrogeological Congress of the Greece, Athens, Greece, 4–6 October 2017. [Google Scholar]
- Plastiras, V. Geological Map of Greece, Larissa Sheet; Institute of Geology and Mineral Exploitation: Athens, Greece, 1982. [Google Scholar]
- Miggiros, G. Geological Map of Greece, Gonnoi Sheet; Institute of Geology and Mineral Exploitation: Athens, Greece, 1980. [Google Scholar]
- Panagopoulos, A. A methodology for groundwater resources management of a typical alluvial aquifer system in Greece. Ph.D. Thesis, School of Earth Sciences, Faculty of Science, University of Birmingham, Birmingham, UK, 1995. [Google Scholar]
- Vrouhakis, I.; Tziritis, E.; Panagopoulos, A.; Kulls, C.; Stamatis, G. The use of environmental stable isotopes at the Tirnavos alluvial basin (Central Greece). In Proceedings of the 15th International Congress of the Geological Society of Greece, Athens, Greece, 22–24 May 2019. [Google Scholar]
- Demitrack, A. The Late Quaternary Geologic History of the Larissa Plain, Thessaly, Greece: Tectonic, Climatic, and Human Impact on the Landscape. Ph.D. Thesis, Stanford University, Stanford, UK, 1986. (Unpublished). [Google Scholar]
- Aggarwal, P.K.; Araguas, L.; Garner, W.A.; Groeninig, M.; Kulkarni, K. Introduction to Water Sampling Analysis for Isotope Hydrology. Water Resources Programme-IAEA. Available online: www-naweb.iaea.org/napc/ih/documents/other/Sampling%20booklet%20web.pdf (accessed on 1 December 2009).
- Ministry of the Environment Energy and Climate Change. Development of river Basin Management Plans for the Water District of Thessalia, Epirus, Western Sterea Ellada, in Accordance with the Directive 2000/60/EC, the Law 3199/2003 and the P.D. 51/2007; Special Secretariat for Water: Athens, Greece, 2014.
- Burdon, D.; Mazloum, S. Some Chemical Types of Ground-Water from Syria; David, J.B., Soubhi, M., Eds.; UNESCO: Paris, France, 1958. [Google Scholar]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Lloyd, J.; Heathcote, J. Natural Inorganic Hydrochemistry in Relation to Ground Water; Oxford Science Publications: Oxford, UK, 1985. [Google Scholar]
- Ravikumar, P.; Somashekar, R.K.; Prakash, K.L. A comparative study on usage of Durov and Piper diagrams to interpret hydrochemical processes in groundwater from SRLIS river basin, Karnataka, India. Elixir Int. J. 2015, 80, 31073–31077. [Google Scholar]
- Shyu, G.S.; Cheng, B.Y.; Chiang, C.T.; Yao, P.H.; Chang, T.K. Applying factor analysis combined with kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. Int. J. Environ. Res. Public Health 2011, 8, 1084–1109. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Lin, K.H.; Kuo, Y.M. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]
- Panda, U.C.; Sundaray, S.K.; Rath, P.; Nayak, B.B.; Bhatta, D. Application of factor and cluster analysis for characterization of river and estuarine water systems–a case study: Mahanadi River (India). J. Hydrol. 2006, 331, 434–445. [Google Scholar] [CrossRef]
- Tziritis, E.P. Environmental monitoring of Micro Prespa Lake basin (Western Macedonia, Greece): Hydrogeochemical characteristics of water resources and quality trends. Environ. Monit. Assess. 2014, 186, 4553–4568. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Reimann, C.; Filzmoser, P.; Garrett, R.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics with R.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. PHREEQC2 User’s Manual and Program; Water-Resources Investigations Report; US Geological Survey: Denver, CO, USA, 2004.
- Ministry of Environment; Energy and Climate Change; Special Secretariat for Water. River Basin Management Plan of Thessaly Water District (GR08). In Analysis of Anthropogenic Pressures and Their Impact on Surface Water Systems and Aquifer Systems; Special Secretariat for Water: Athens, Greece, 2014. [Google Scholar]
- Sharma, L.; Greskowiak, J.; Ray, C.; Eckert, P.; Prommer, H. Elucidating temperature effects on seasonal variations of biogeochemical turnover rates during riverbank filtration. J. Hydrol. 2012, 428, 104–115. [Google Scholar] [CrossRef]
- Menberg, K.; Blum, P.; Kurylyk, B.L.; Bayer, P. Observed groundwater temperature response to recent climate change. Hydrol. Earth Syst. Sci. 2014. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, M. Analysing the long term reduction in groundwater temperature due to pun pumping. Hydrol. Sci. J. 1995, 40, 407–421. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Kassapi, K.A.; Arampatzis, G.; Perleros, B.; Drakopoulou, S.; Tziritis, E.; Chrysafi, A.; Vrouhakis, I. Assessment of chemical and quantitative status of groundwater systems in Pinios hydrological basin-Greece. In Proceedings of the XI Int. Conference Protection and Restoration of the Environment, Thessaloniki, Greece, 3–6 July 2012. [Google Scholar]
- Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31998L0083 (accessed on 19 March 2019).
- World Health Organization (WHO). Guidelines for Drinking Water Quality, 2nd ed.; Health Criteria and Other Supporting Information; World Health Organization (WHO): Vienna, Austria, 1996; Volume 2. [Google Scholar]
- Al-Bassam, A.M.; Khalil, A.R. DurovPwin: A new version to plot the expanded Durov diagram for hydro-chemical data analysis. Comput. Geosci. 2012, 42, 1–6. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Lloyd, J.; Fitzsimons, V. Groundwater evolution of the Tirnavos alluvial basin, central Greece, as indicated by hydrochemistry. In Proceedings of the 3rd Hydrogeological Conference of the Hellenic Chapter of IAH, Heraklion, Greece, 3–5 November 1995. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Boghici, R.; Van Broekhoven, N.G. Hydrogeology of the Rustler Aquifer, Trans-Pecos, Texas; in Aquifers of West Texas. Tex. Water Dev. Board Rep. 2001, 356, 207–225. [Google Scholar]
- Jalali, M. Hydrochemical characteristics and sodification of groundwater in the Shirin Sou, Hamedan, Western Iran. Nat. Resour. Res. 2012, 21, 61–73. [Google Scholar] [CrossRef]
- Jalali, M. Major ion chemistry of groundwaters in the Bahar area, Hamadan, western Iran. Environ. Geol. 2005, 47, 763–772. [Google Scholar] [CrossRef]
- Esmaeili-Vardanjani, M.; Rasa, I.; Amiri, V.; Yazdi, M.; Pazand, K. Evaluation of groundwater quality and assessment of scaling potential and corrosiveness of water samples in Kadkan aquifer, Khorasan-e-Razavi Province, Iran. Environ. Monit. Assess. 2015, 187, 53. [Google Scholar] [CrossRef] [PubMed]
- Kalantary, N.; Rahimi, M.; Charchi, A. Use of composite diagram, factor analyses and saturation index for quantification of Zaviercherry and Kheran plain groundwaters. J. Eng. Geol. 2007, 2, 339–356. [Google Scholar]
- Dotsika, E.; Lykoudis, S.; Poutoukis, D. Spatial distribution of the isotopic composition of precipitation and spring water in Greece. Glob. Planet. Chang. 2010, 71, 141–149. [Google Scholar] [CrossRef]
- Sharp, Z. Stable Isotope Geochemistry; Pearson Education; Prentice Hall: New York, NY, USA, 2007; p. 344. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Argiriou, A.A.; Lykoudis, S. Isotopic composition of precipitation in Greece. J. Hydrol. 2006, 327, 486–495. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydro-Geology; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Matiatos, I. Hydrogeological and Isotopic Investigations at Regions of the Argolis Peninsula. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2010. [Google Scholar]
- Stamatis, G.; Miggiros, G. The relationship between fractured tectonic and groundwater reservoir of massive formations of Ossa Mountain (E. Thessaly, Greece). Bull. Geol. Soc. Greece 2004, 36, 2077–2086. [Google Scholar] [CrossRef]
- Payne, B.; Dimitroula, C.; Leondiadis, I.; Kallergis, G. Environmental Isotope Data in the Western Thessaly Valley, Greece: Use of Mathematical Model for Quantitative Evaluations with Tritium; Bulletin of the Geological Society of Greece: Athens, Greece, 1976. [Google Scholar]

| Variable | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Communality |
|---|---|---|---|---|---|---|
| pH | 0.149 | −0.867 | 0.333 | 0.131 | 0.046 | 0.903 |
| EC | 0.921 | 0.29 | 0.165 | −0.130 | −0.006 | 0.976 |
| K | 0.084 | 0.693 | 0.009 | 0.055 | 0.256 | 0.556 |
| Na | 0.918 | −0.252 | 0.094 | −0.157 | 0.062 | 0.944 |
| Ca | 0.017 | 0.883 | 0.232 | 0.133 | 0.085 | 0.859 |
| Mg | 0.673 | 0.421 | 0.147 | −0.232 | −0.312 | 0.803 |
| Cl | 0.941 | 0.17 | 0.019 | 0.02 | −0.015 | 0.915 |
| HCO3 | 0.075 | 0.508 | 0.547 | −0.52 | −0.09 | 0.841 |
| SO4 | 0.965 | −0.073 | 0.017 | 0.001 | 0.074 | 0.942 |
| B | 0.892 | −0.251 | −0.034 | −0.114 | 0.068 | 0.877 |
| Cu | −0.189 | 0.181 | 0.082 | 0.091 | 0.772 | 0.680 |
| Fe | 0.375 | −0.115 | 0.068 | −0.541 | 0.551 | 0.754 |
| Mn | 0.191 | −0.225 | −0.18 | −0.804 | 0.045 | 0.767 |
| NO3 | 0.017 | 0.815 | −0.052 | 0.189 | −0.06 | 0.707 |
| NH4 | −0.013 | −0.036 | 0.083 | −0.897 | −0.104 | 0.823 |
| SICalcite | −0.042 | 0.036 | 0.945 | 0.128 | 0.175 | 0.942 |
| SIDolomite | 0.291 | −0.142 | 0.927 | −0.081 | −0.055 | 0.973 |
| SIGypsum | 0.595 | 0.492 | 0.143 | 0.171 | −0.238 | 0.702 |
| SIHalite | 0.893 | 0.016 | 0.066 | −0.098 | −0.224 | 0.862 |
| Variance | 6.241 | 3.719 | 2.352 | 2.264 | 1.251 | 15.827 |
| % Var | 32.8 | 19.6 | 12.4 | 11.9 | 6.6 | 83.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrouhakis, I.; Tziritis, E.; Panagopoulos, A.; Stamatis, G. Hydrogeochemical and Hydrodynamic Assessment of Tirnavos Basin, Central Greece. Water 2021, 13, 759. https://doi.org/10.3390/w13060759
Vrouhakis I, Tziritis E, Panagopoulos A, Stamatis G. Hydrogeochemical and Hydrodynamic Assessment of Tirnavos Basin, Central Greece. Water. 2021; 13(6):759. https://doi.org/10.3390/w13060759
Chicago/Turabian StyleVrouhakis, Ioannis, Evangelos Tziritis, Andreas Panagopoulos, and Georgios Stamatis. 2021. "Hydrogeochemical and Hydrodynamic Assessment of Tirnavos Basin, Central Greece" Water 13, no. 6: 759. https://doi.org/10.3390/w13060759








