Electrochemical Treatment of Effluent for the Removal of Contaminants of Emergent Concern and Culturable Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Effluent Sampling
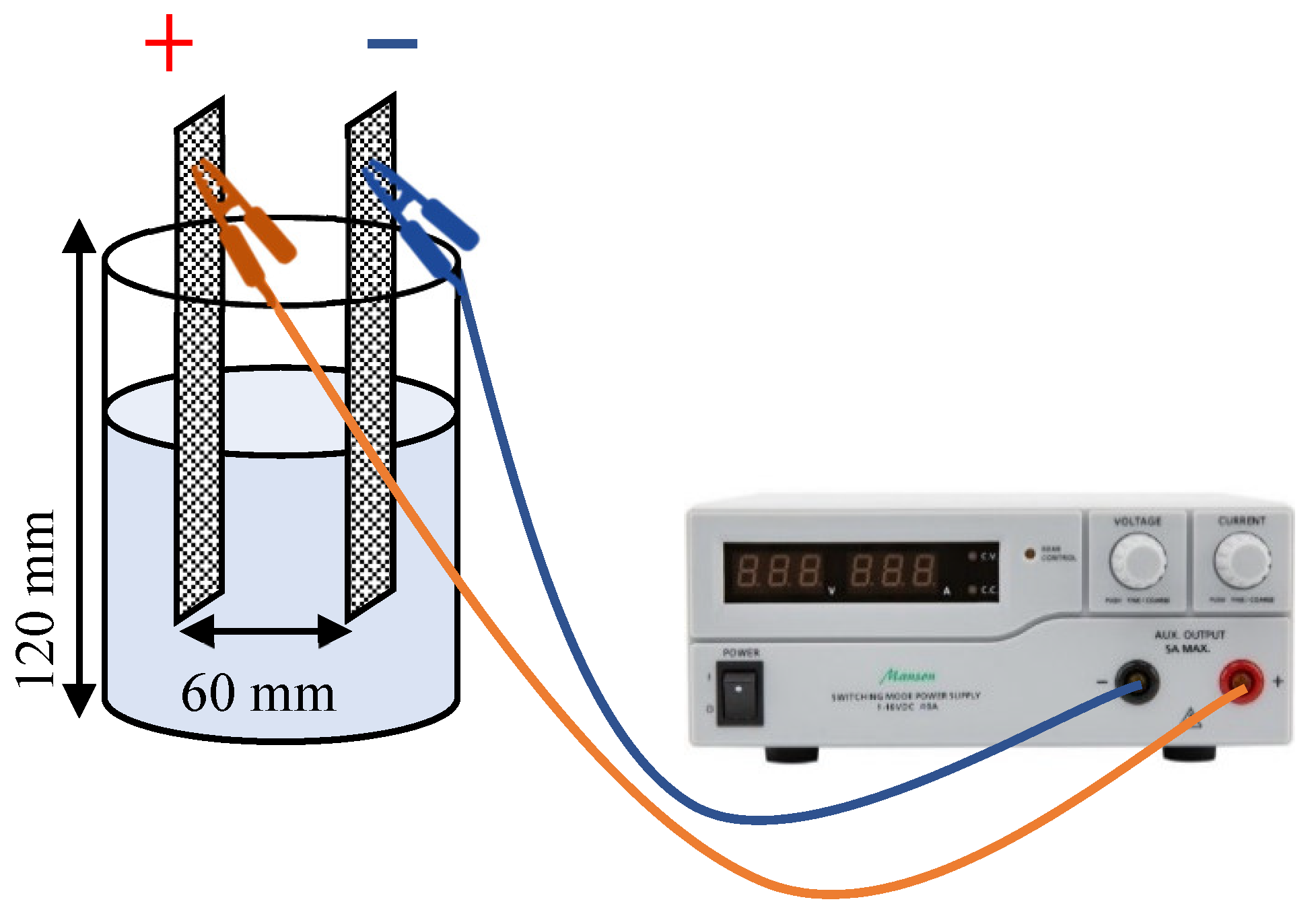
2.3. Electrochemical Reactor
2.4. Experimental Design
2.5. Analytical Methodologies
2.5.1. Effluent Characterization
2.5.2. CEC Extraction and Analysis
2.5.3. Culturable Microorganisms
2.6. Statistical Analysis
3. Results and Discussion
3.1. Definition of EC Treatment Duration
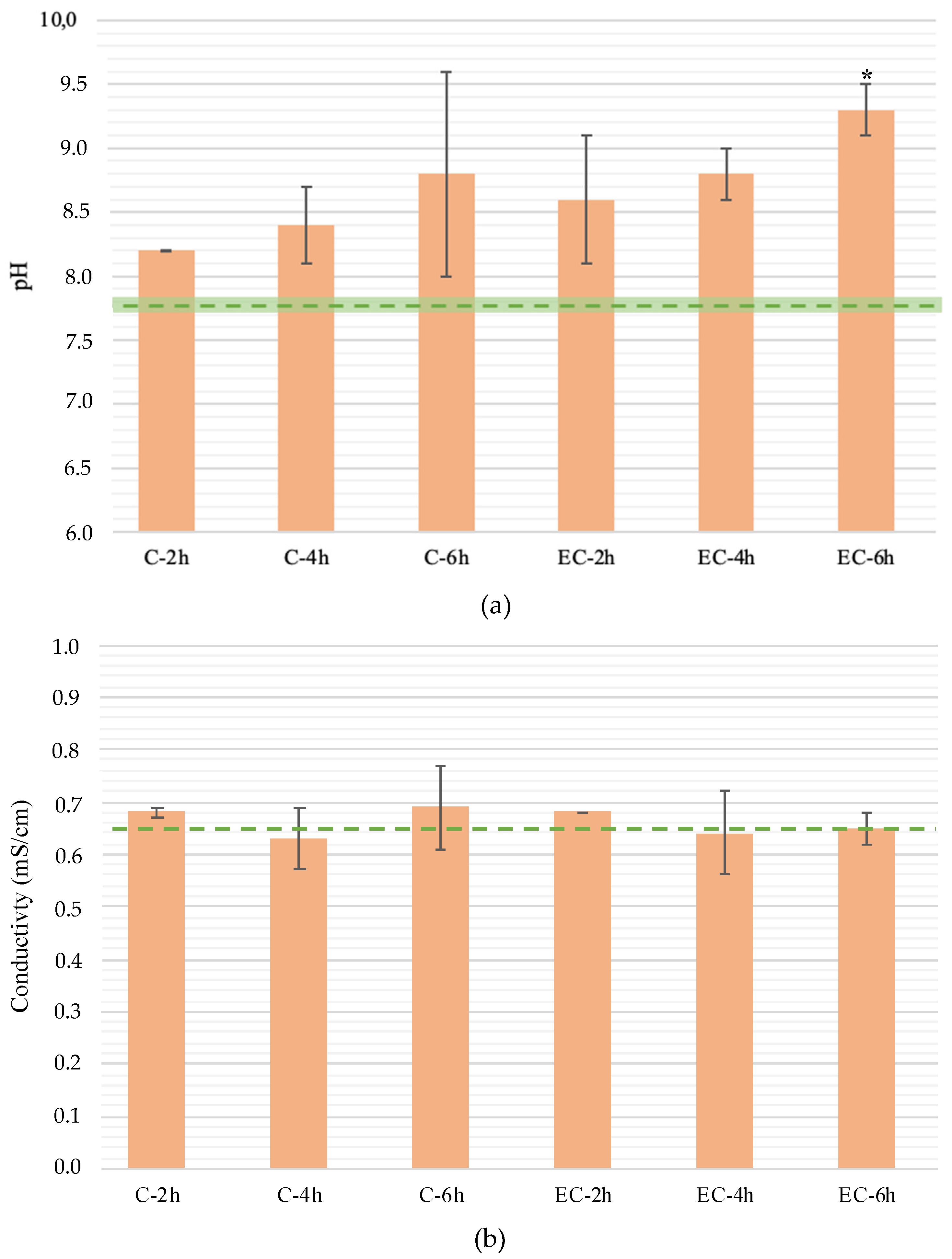
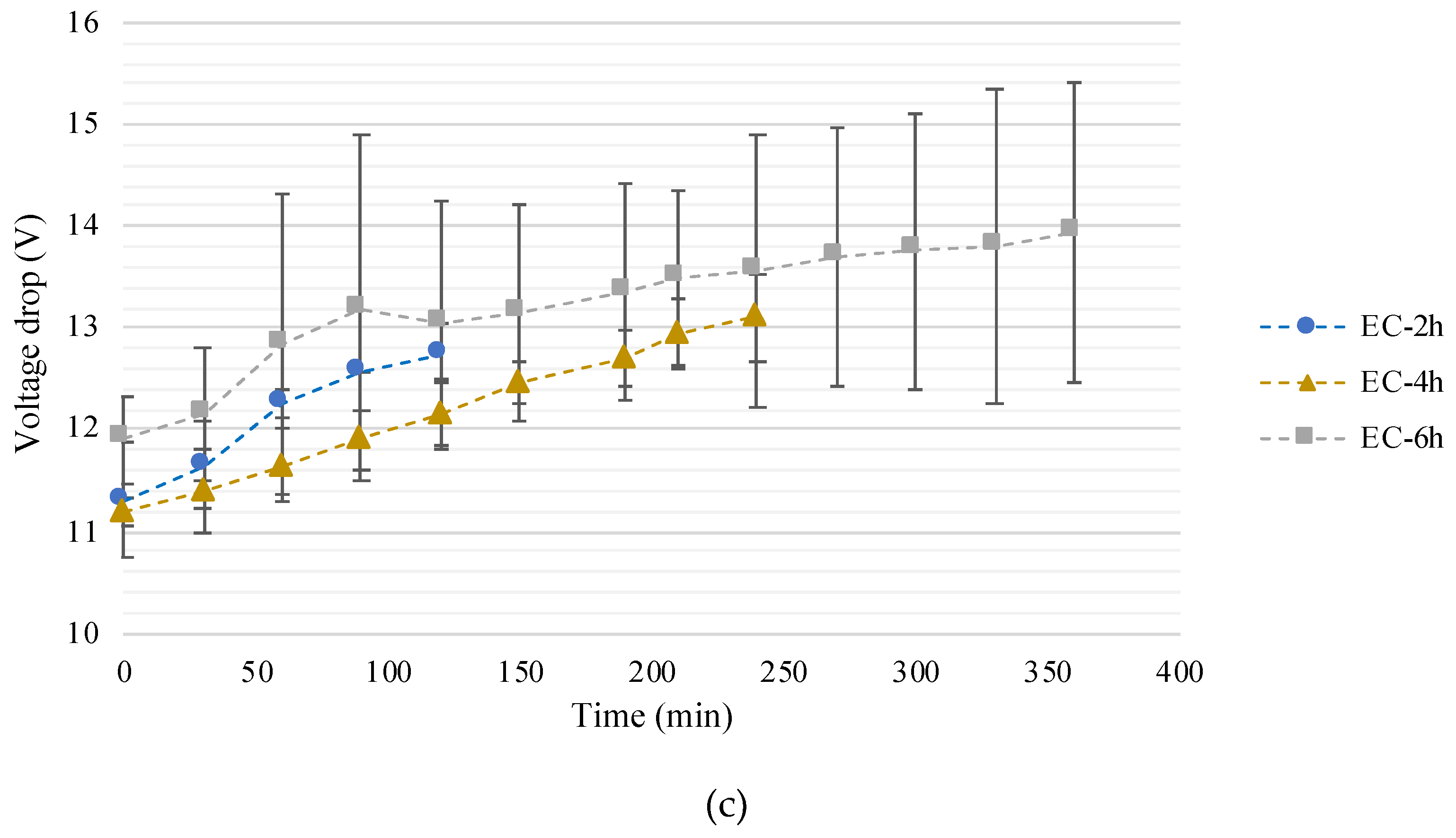
3.1.1. Treatment Time Influence on pH, Conductivity, and Voltage
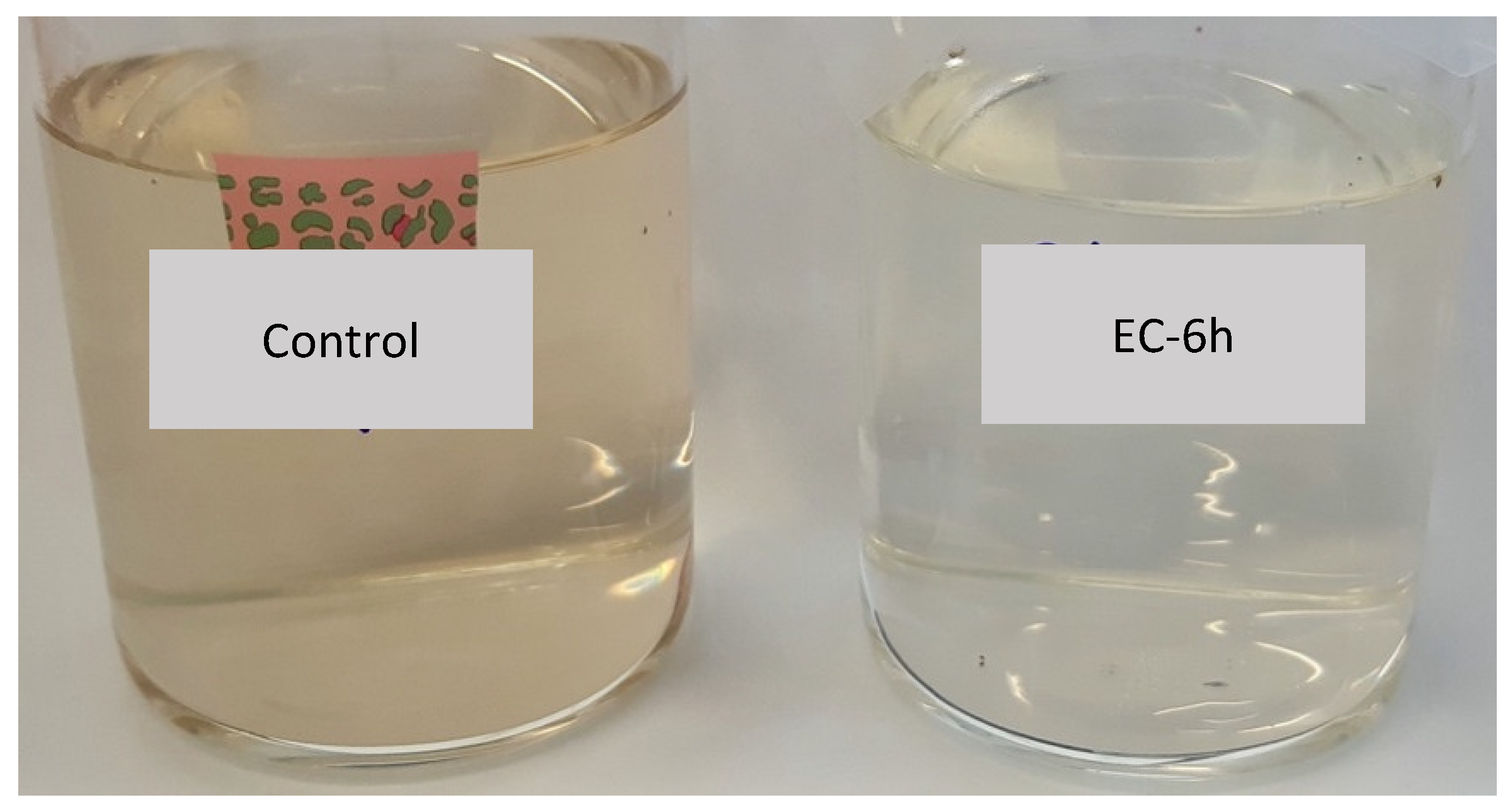
3.1.2. Treatment Time Influence on CEC and Microorganism Removal
3.2. Efficiency of EC Treatment in Different Effluent Samples
3.2.1. General Changes: pH, Conductivity, and Voltage
3.2.2. EC Efficiency on CECs and Microorganism Removal
3.2.3. EC Efficiency on a Complex Mixture of CEC and Microorganism Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Serna, R.; Posadas, E.; García-Encina, P.A.; Muñoz, R. Removal of Contaminants of Emerging Concern from Urban Wastewater in Novel Algal-Bacterial Photobioreactors. Sci. Total Environ. 2019, 662, 32–40. [Google Scholar] [CrossRef] [Green Version]
- The European Parliament and the Council of the European Union. Regulation (EU) 2020/741, Minimum Requirements for Water Reuse. Off. J. Eur. Union 2020, 2019, L 177/32. [Google Scholar]
- Nawaz, T.; Sengupta, S. Chapter 4—Contaminants of Emerging Concern: Occurrence, Fate, and Remediation. In Advances in Water Purification Techniques: Meeting the Needs of Developed and Developing Countries; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–114. [Google Scholar]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of Emerging Concern: A Review of New Approach in AOP Technologies. Environ. Monit. Assess. 2017, 189. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Amorim, C.C.; Leão, M.M.D. Occurrence, Control and Fate of Contaminants of Emerging Concern in Environmental Compartments in Brazil. J. Hazard. Mater. 2019, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of Secondary Wastewater Treatment Methods for the Removal of Contaminants of Emerging Concern Implicated in Crop Uptake and Antibiotic Resistance Spread: A Review. Sci. Total Environ. 2019, 1052–1081. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.C. Electrokinetic Improvement of Soft Clay Using Electrical Vertical Drains. In Ground Improvement Case Histories: Chemical, Electrokinetic, Thermal and Bioengineering Methods; Butterworth Heinemann: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the Main Disinfection Processes for Wastewater and Drinking Water Treatment Plants. Sustainability 2018, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Kauser, I.; Ciesielski, M.; Poretsky, R.S. Ultraviolet Disinfection Impacts the Microbial Community Composition and Function of Treated Wastewater Effluent and the Receiving Urban River. PeerJ 2019, 2019. [Google Scholar] [CrossRef]
- Mamane, H. Impact of Particles on UV Disinfection of Water and Wastewater Effluents: A Review. Hadas Mamane School of Mechanical Engineering, Faculty of Engineering, Tel-Aviv. Rev. Chem. Eng. 2008, 24, 67–157. [Google Scholar] [CrossRef]
- Tröster, I.; Fryda, M.; Herrmann, D.; Schäfer, L.; Hänni, W.; Perret, A.; Blaschke, M.; Kraft, A.; Stadelmann, M. Electrochemical Advanced Oxidation Process for Water Treatment Using DiaChem® Electrodes. Diam. Relat. Mater. 2002, 11, 640–645. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Couto, N.; Guedes, P.; Pinto, J.; Mateus, E.P.; Ribeiro, A.B. Electrodialytic 2-Compartment Cells for Emerging Organic Contaminants Removal from Effluent. J. Hazard. Mater. 2018, 358, 467–474. [Google Scholar] [CrossRef]
- Magro, C.; Mateus, E.P.; Paz-Garcia, J.M.; Ribeiro, A.B. Emerging Organic Contaminants in Wastewater: Understanding Electrochemical Reactors for Triclosan and Its by-Products Degradation. Chemosphere 2020, 247. [Google Scholar] [CrossRef] [Green Version]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Morais, R.L.; Garcia, L.F.; Moreno, E.K.G.; Thomaz, D.V.; de Brito Rodrigues, L.; Brito, L.B.; Lobón, G.S.; de Oliveira, G.A.R.; Rodrigues, M.F.; Vaz, B.G.; et al. Electrochemical Remediation of Industrial Pharmaceutical Wastewater Containing Hormones in a Pilot Scale Treatment System. Eclet. Quim. 2019, 44, 40–52. [Google Scholar] [CrossRef]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A Comprehensive Review on Contaminants Removal from Pharmaceutical Wastewater by Electrocoagulation Process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef] [PubMed]
- Bahlmann, A.; Brack, W.; Schneider, R.J.; Krauss, M. Carbamazepine and Its Metabolites in Wastewater: Analytical Pitfalls and Occurrence in Germany and Portugal. Water Res. 2014, 57, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.; Liu, X.; Zhang, Q.; Liu, R.; Wang, Z.; Shi, X.; Quan, J.; Shen, X.; Zhang, F. Removal of Sulfamethoxazole and Trimethoprim from Reclaimed Water and the Biodegradation Mechanism. Front. Environ. Sci. Eng. 2018, 12. [Google Scholar] [CrossRef]
- Vieno, N.; Sillanpää, M. Fate of Diclofenac in Municipal Wastewater Treatment Plant—A Review. Environ. Int. 2014, 28–39. [Google Scholar] [CrossRef]
- von Sperling, M.; Verbyla, M.E.; Oliveira, S.M.A.C. Removal Efficiencies. In Assessment of Treatment Plant Performance and Water Quality Data: A Guide for Students, Researchers and Practitioners; IWA Publishing: London, UK, 2020; pp. 181–206. [Google Scholar]
- Tung, C.H.; Shen, S.Y.; Chang, J.H.; Hsu, Y.M.; Lai, Y.C. Treatment of Real Printing Wastewater with an Electrocatalytic Process. Sep. Purif. Technol. 2013, 117, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Basu, S.; Page, J.; Wei, I.W. UV Disinfection of Treated Wastewater Effluent: Influence of Color, Reactivation and Regrowth of Coliform Bacteria. 2007. Available online: https://repository.library.northeastern.edu/files/neu:329986; http://hdl.handle.net/2047/D20237267 (accessed on 15 December 2020).
- Huang, J.J.; Xi, J.; Hu, H.Y.; Li, Y.; Lu, S.Q.; Tang, F.; Pang, Y.C. UV Light Tolerance and Reactivation Potential of Tetracycline-Resistant Bacteria from Secondary Effluents of a Wastewater Treatment Plant. J. Environ. Sci. 2016, 41, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of Residual Pharmaceuticals from Aqueous Systems by Advanced Oxidation Processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Guedes, P.; Couto, N.; Almeida, J.; Rodrigues, A.M.; Mateus, E.P.; Ribeiro, A.B. Electrodialytic Treatment of Sewage Sludge: Influence on Microbiological Community. Int. J. Environ. Sci. Technol. 2018, 15, 1103–1112. [Google Scholar] [CrossRef]
- Oh, S.; Shin, W.S.; Kim, H.T. Effects of PH, Dissolved Organic Matter, and Salinity on Ibuprofen Sorption on Sediment. Environ. Sci. Pollut. Res. 2016, 23, 22882–22889. [Google Scholar] [CrossRef] [Green Version]
- Dalmázio, I.; Santos, L.S.; Lopes, R.P.; Eberlin, M.N.; Augusti, R. Advanced Oxidation of Caffeine in Water: On-Line and Real-Time Monitoring by Electrospray Ionization Mass Spectrometry. Environ. Sci. Technol. 2005, 39, 5982–5988. [Google Scholar] [CrossRef]
- Ye, Z.; Steter, J.R.; Centellas, F.; Lluís Cabot, P.; Sirés, I. Photoelectro-Fenton as Post-Treatment for Electrocoagulated 2 Benzophenone-3-Loaded Synthetic and Urban Wastewater. J. Clean. Prod. 2019, 208, 1393–1402. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Wu, L. Sorption and Degradation of Pharmaceuticals and Personal Care Products (PPCPs) in Soils. Environ. Sci. Pollut. Res. 2013, 20, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Gao, S.; Zhu, J.; Wang, L.; Xiong, Y.; Xia, X.; Yang, L. The Enhanced Photocatalytic Performance toward Carbamazepine by Nitrogen-Doped Carbon Dots Decorated on BiOBr/CeO2: Mechanism Insight and Degradation Pathways. Chem. Eng. J. 2020, 391. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Liu, Y.; Zhang, L.; Feng, L. Modelling Study on the Effects of Chloride on the Degradation of Bezafibrate and Carbamazepine in Sulfate Radical-Based Advanced Oxidation Processes: Conversion of Reactive Radicals. Chem. Eng. J. 2019, 358, 1332–1341. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.Y.; Yoon, J. The Role of Reactive Oxygen Species in the Electrochemical Inactivation of Microorganisms. Environ. Sci. Technol. 2006, 40, 6117–6122. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, Z.; Duan, J.; Li, N.; Li, B.; Song, T.; Sardar, M.F.; Lv, X.; Zhu, C. Electrochemical Disinfection of Secondary Effluent from a Wastewater Treatment Plant: Removal Efficiency of ARGs and Variation of Antibiotic Resistance in Surviving Bacteria. Chem. Eng. J. 2020, 392. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Y.; Tao, H.; Zhou, S.; Zeng, Y. Bio-Electro-Fenton System for Enhanced Estrogens Degradation. Bioresour. Technol. 2013, 138, 136–140. [Google Scholar] [CrossRef]
- García-Espinoza, J.D.; Mijaylova-Nacheva, P.; Avilés-Flores, M. Electrochemical Carbamazepine Degradation: Effect of the Generated Active Chlorine, Transformation Pathways and Toxicity. Chemosphere 2018, 192, 142–151. [Google Scholar] [CrossRef]
- Canosa, P.; Morales, S.; Rodríguez, I.; Rubí, E.; Cela, R.; Gómez, M. Aquatic Degradation of Triclosan and Formation of Toxic Chlorophenols in Presence of Low Concentrations of Free Chlorine. Anal. Bioanal. Chem. 2005, 383, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Skoumal, M.; Rodríguez, R.M.; Cabot, P.L.; Centellas, F.; Garrido, J.A.; Arias, C.; Brillas, E. Electro-Fenton, UVA Photoelectro-Fenton and Solar Photoelectro-Fenton Degradation of the Drug Ibuprofen in Acid Aqueous Medium Using Platinum and Boron-Doped Diamond Anodes. Electrochim. Acta 2009, 54, 2077–2085. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Chen, J.; Niu, J. Electrochemical Degradation of Sunscreen Agent Benzophenone-3 and Its Metabolite by Ti/SnO2-Sb/Ce-PbO2 Anode: Kinetics, Mechanism, Toxicity and Energy Consumption. Sci. Total Environ. 2019, 688, 75–82. [Google Scholar] [CrossRef]
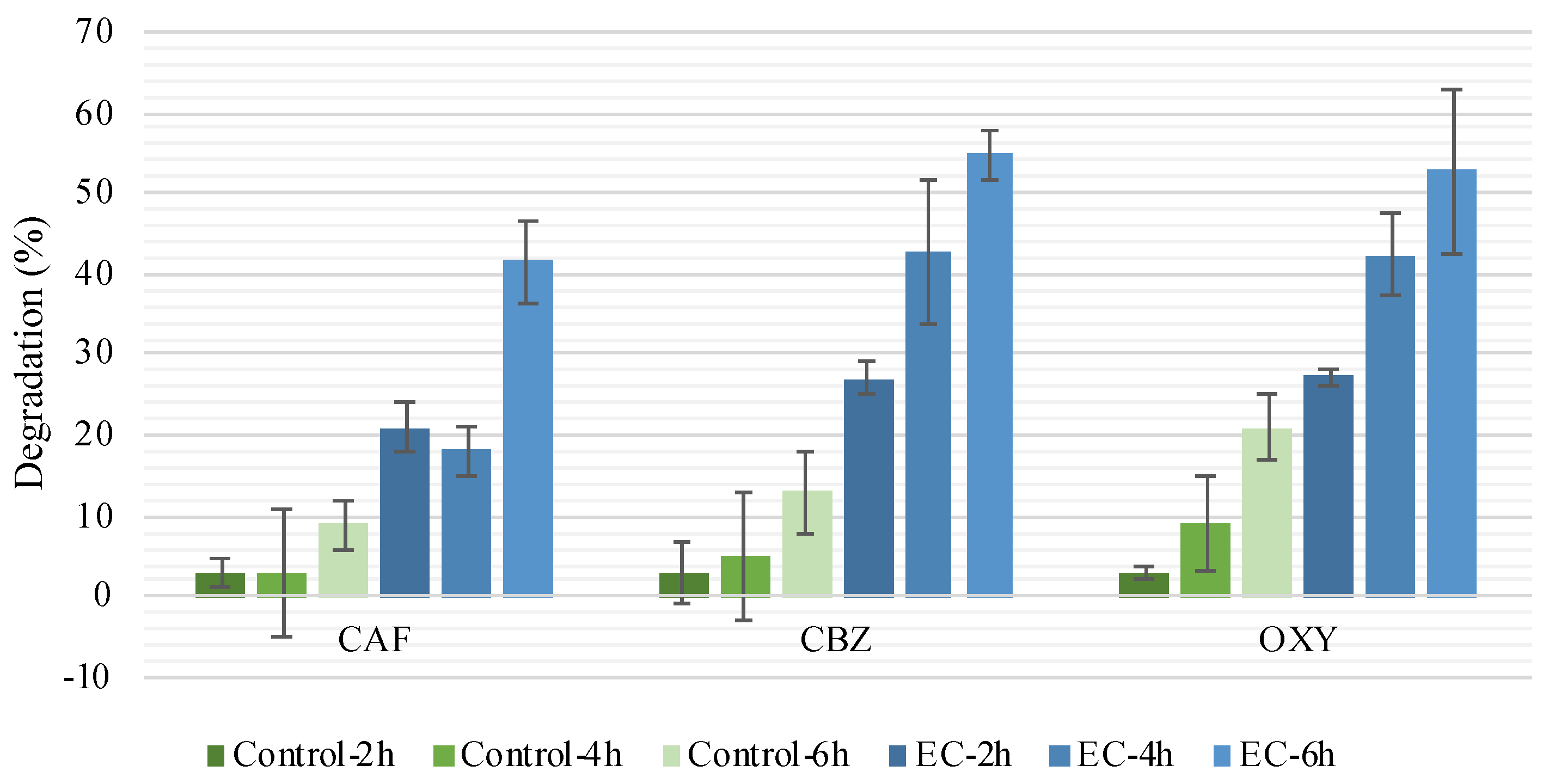
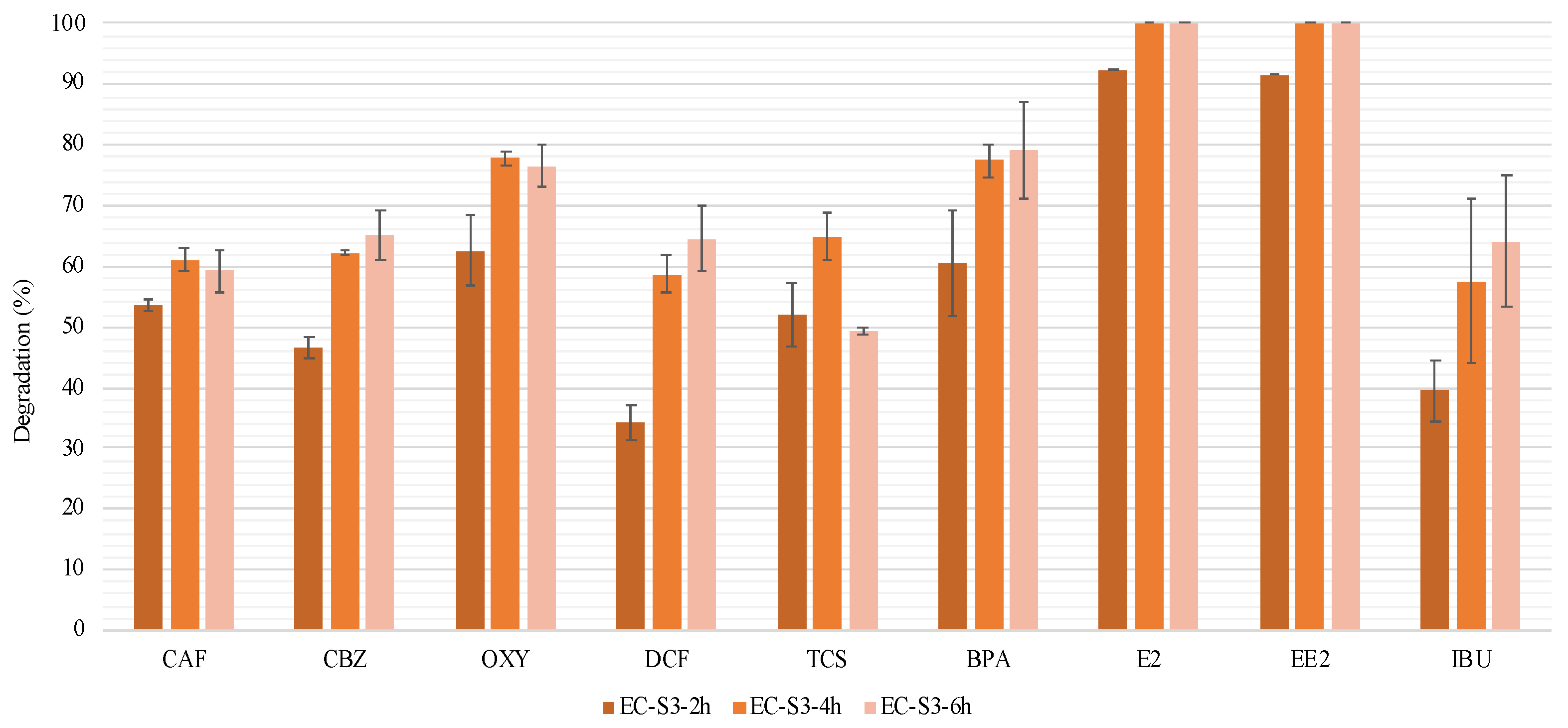
| Set | Experiment Code | EC a Duration (h) | Treatment Duration (h) | Number of CECs (spiked at 200 ppb) | Effluent Code | Sampling Date |
|---|---|---|---|---|---|---|
| 1 | EC-2h | 2 | 2 | 3 | - b | Sep. 2019 |
| EC-4h | 4 | 4 | ||||
| EC-6h | 6 | 6 | ||||
| C-2h | - | 2 | ||||
| C-4h | - | 4 | ||||
| C-6h | - | 6 | ||||
| 2 * | EC-S1-6h | 6 | 6 | 3 | S1 | Dec. 2019 |
| EC-S2-6h | 6 | 6 | 3 | S2 | Dec. 2019 | |
| EC-S3-2h | 2 | 2 | 9 | S3 | Oct. 2020 | |
| EC-S3-4h | 4 | 4 | 9 | S3 | Oct. 2020 | |
| EC-S3-6h | 6 | 6 | 9 | S3 | Oct. 2020 |
| Experiment Code | pH | Conductivity (mS/cm) | Voltage Drop (V) | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | |
| EC-S1-6h | 7.97 ± 0.00 | 9.06 ± 0.32 | 0.64 ± 0.00 | 0.59 ± 0.02 | 11.6 ± 0.4 | 13.5 ± 0.6 |
| EC-S2-6h | 8.13 ± 0.10 | 9.53 ± 0.33 | 0.48 ± 0.05 | 0.45 ± 0.00 | 13.4 ± 0.1 | 15.5 ± 0.8 |
| EC-S3-2h | 7.77 ± 0.00 | 9.54 ± 0.22 | 0.98 ± 0.00 | 0.91 ± 0.03 | 30.8 ± 2.8 | 31.8 ± 1.8 |
| EC-S3-4h | 9.16 ± 0.17 | 0.77 ± 0.02 | 26.7 ± 0.0 | 31.2 ± 1.0 | ||
| EC-S3-6h | 9.54 ± 0.20 | 0.77 ± 0.03 | 25.1 ± 4.9 | 35.2 ± 9.1 | ||
| Experiment Code | CAF | CBZ | OXY |
|---|---|---|---|
| EC-S1-6h | 19.0 ± 36.0 | 40.0 ± 21.0 | 95.0 ± 5.0 |
| EC-S2-6h | 38.5 ± 10.5 | 61.0 ± 8.0 | 99.0 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dionísio, J.; Gonçalves, C.; Guedes, P.; Ribeiro, A.B.; Couto, N. Electrochemical Treatment of Effluent for the Removal of Contaminants of Emergent Concern and Culturable Microorganisms. Water 2021, 13, 520. https://doi.org/10.3390/w13040520
Dionísio J, Gonçalves C, Guedes P, Ribeiro AB, Couto N. Electrochemical Treatment of Effluent for the Removal of Contaminants of Emergent Concern and Culturable Microorganisms. Water. 2021; 13(4):520. https://doi.org/10.3390/w13040520
Chicago/Turabian StyleDionísio, Joana, Cristiana Gonçalves, Paula Guedes, Alexandra B. Ribeiro, and Nazaré Couto. 2021. "Electrochemical Treatment of Effluent for the Removal of Contaminants of Emergent Concern and Culturable Microorganisms" Water 13, no. 4: 520. https://doi.org/10.3390/w13040520








