Microbial Electrolysis Cells for Decentralised Wastewater Treatment: The Next Steps
Abstract
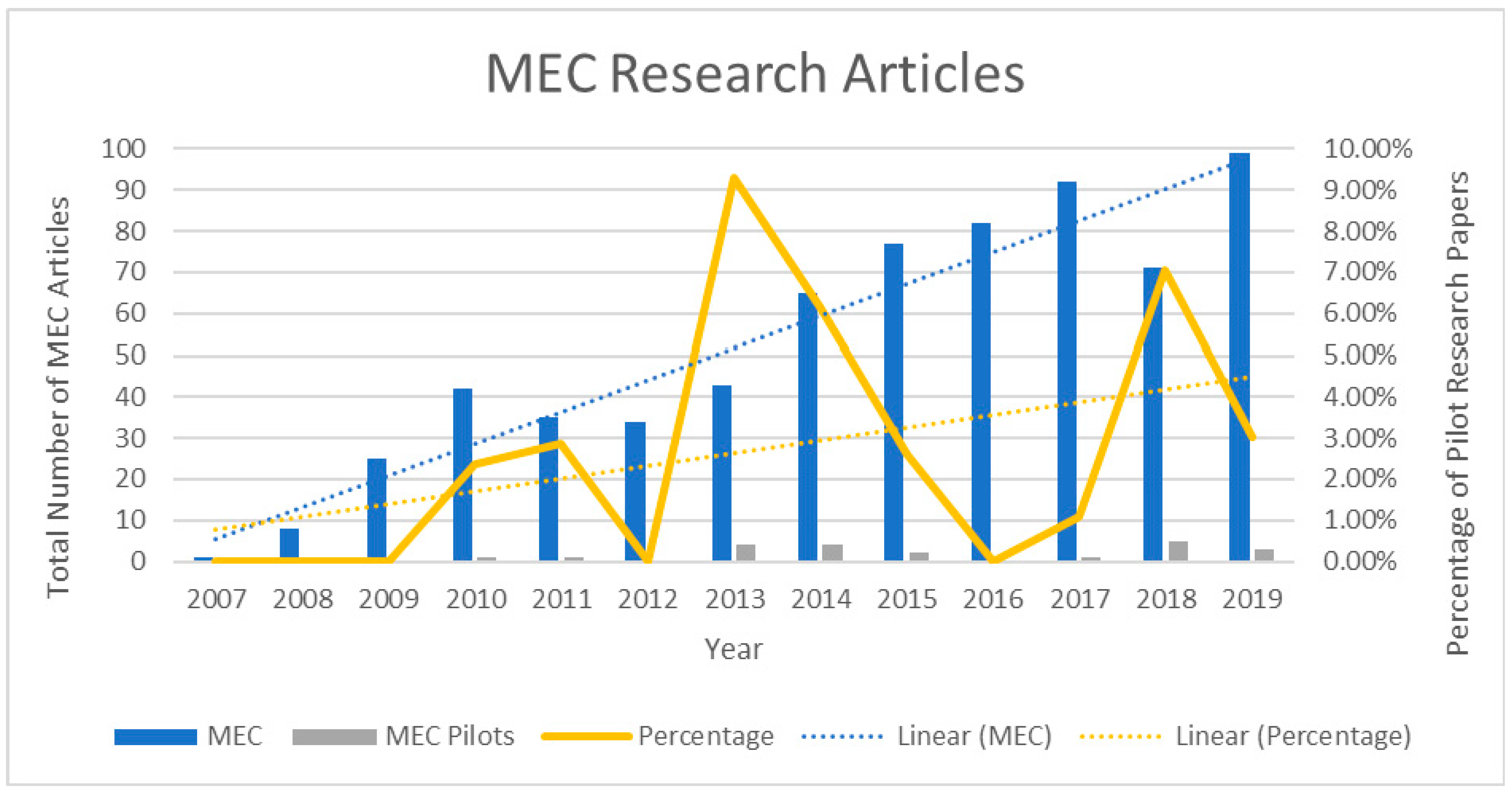
:1. Introduction
1.1. Current Wastewater Treatment Methods and Limitations
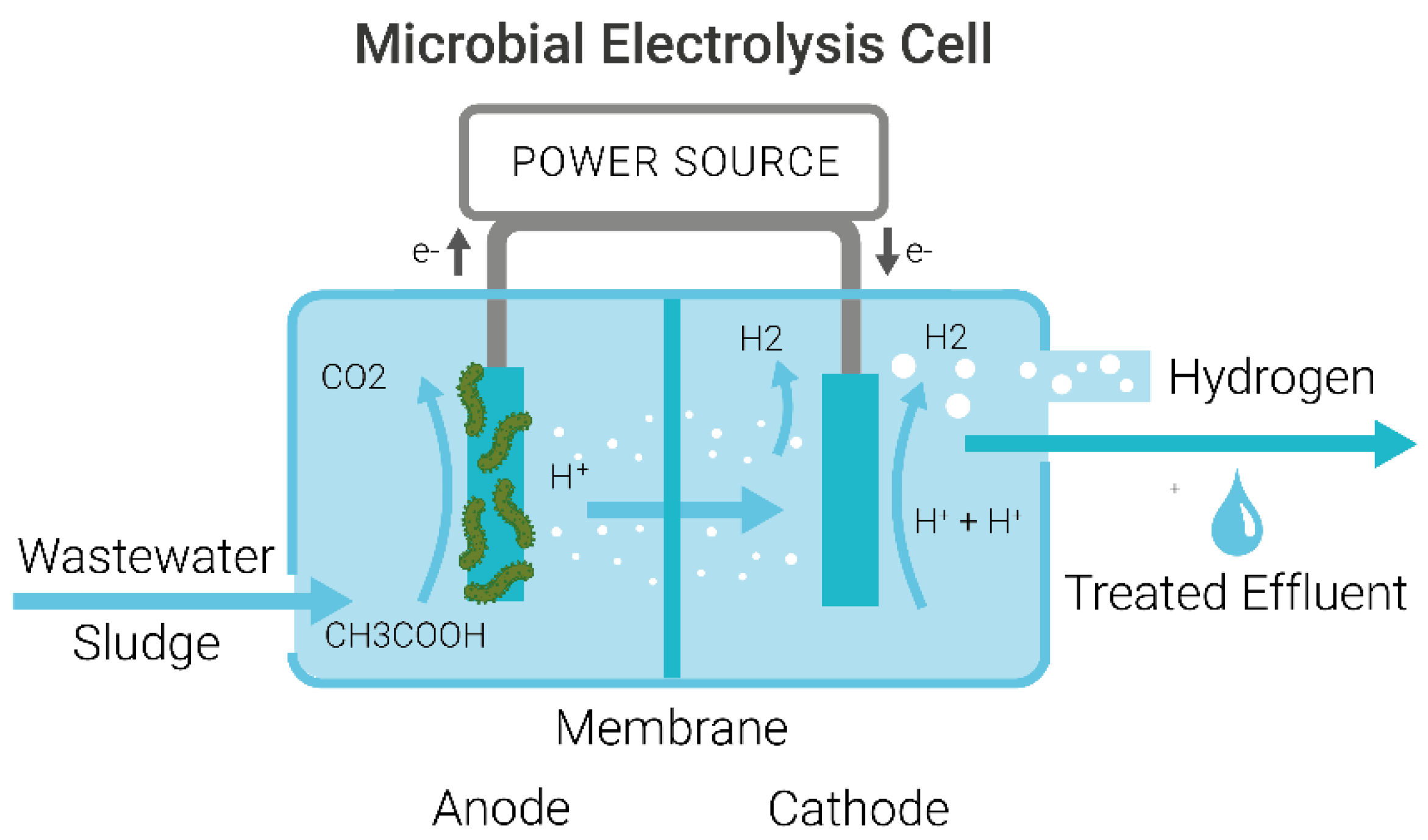
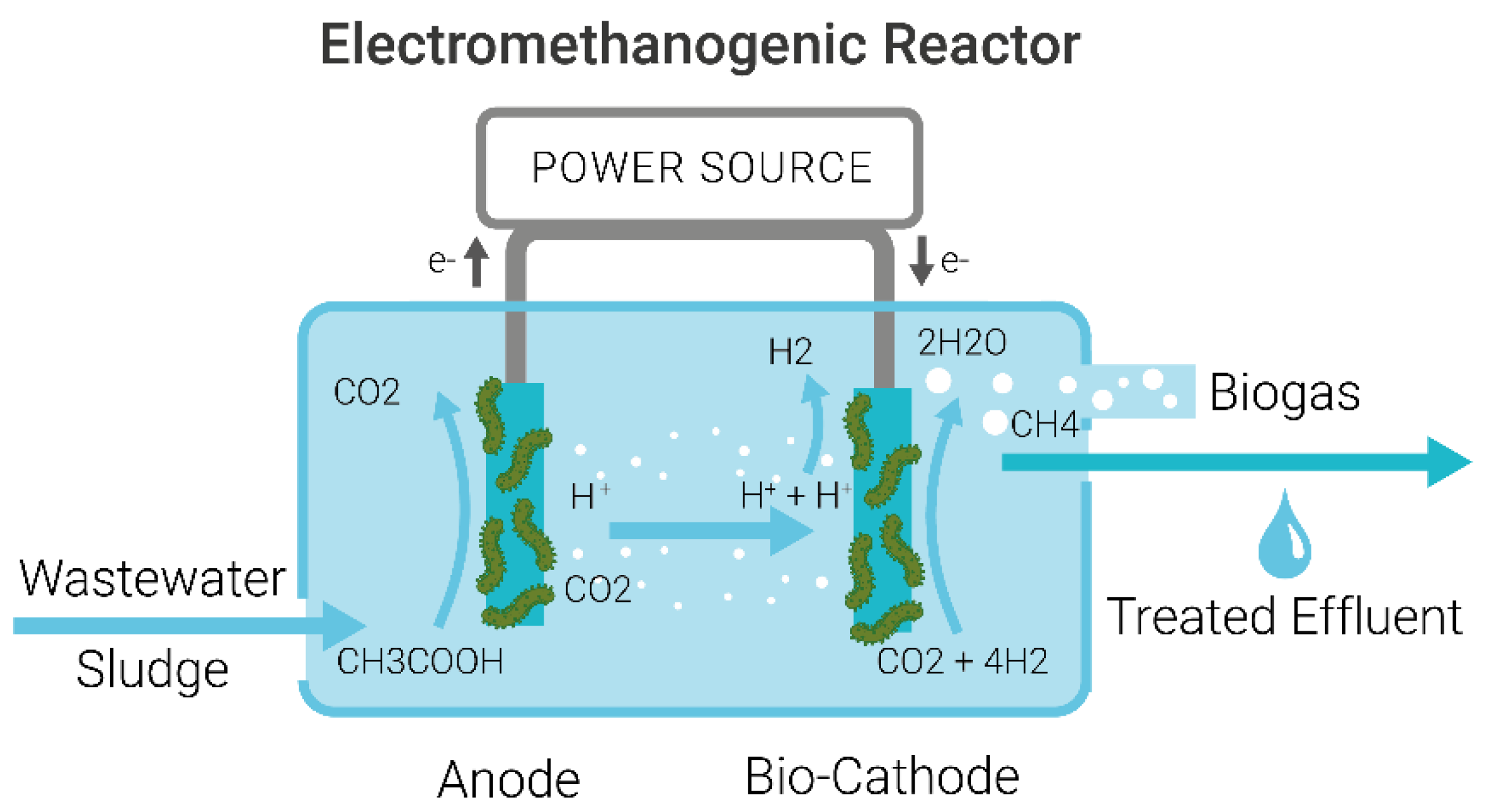
1.2. Mechanism of Microbial Electrolysis Cells for Wastewater Treatment
2. Parameters Affecting the Design of MEC Systems for Industrial Wastewater Treatment
2.1. Feedstock
2.2. Inoculation
2.3. Anode Material
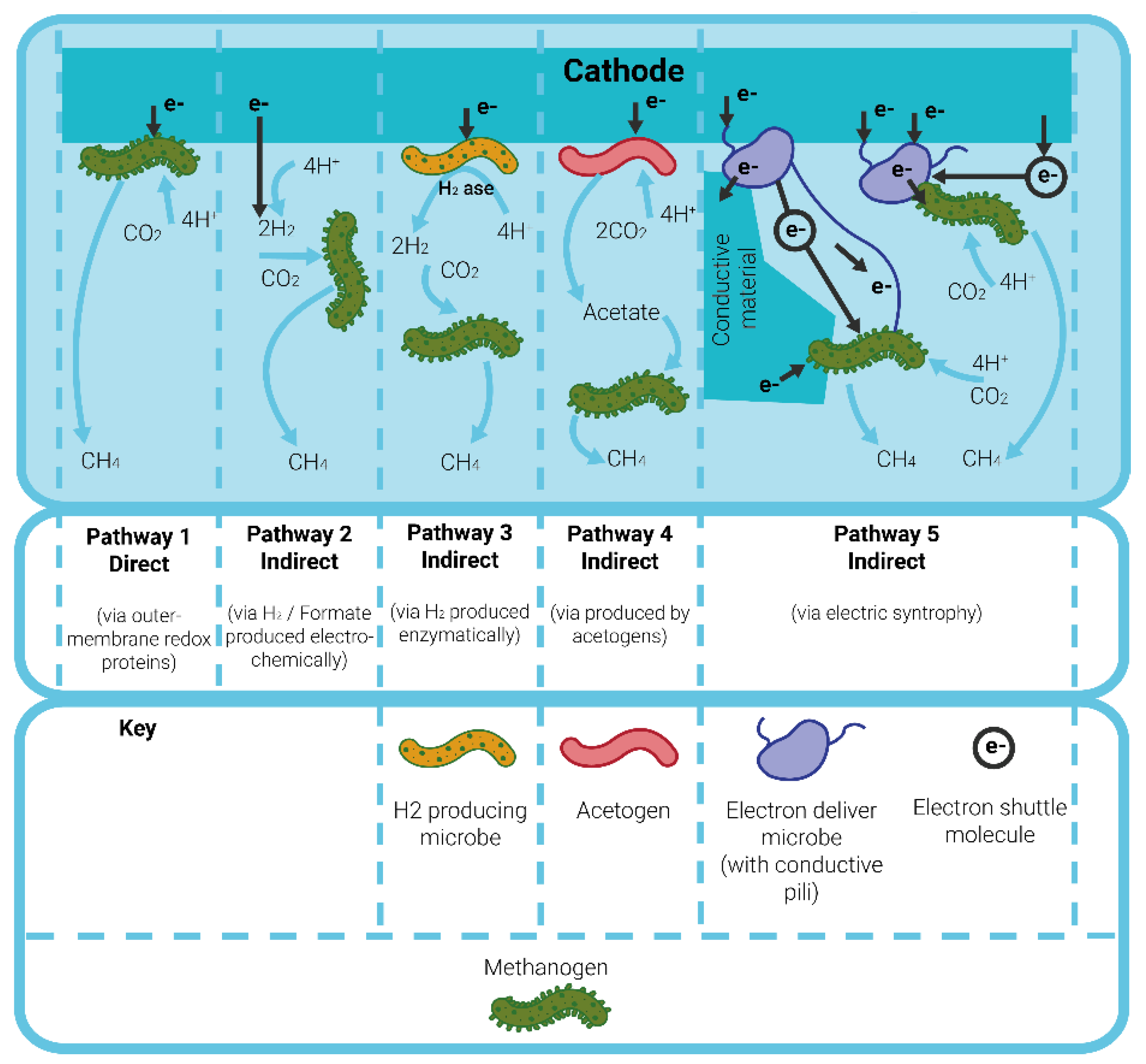
2.4. Cathode Material
2.5. Systems Architecture
3. Evaluation of the Barriers to Commercialisation of MECs for Wastewater Treatment
3.1. Economic and Cost Analysis
3.2. Scale-up Strategies
3.3. Analysis of Hydrogen or Methane Production and Its Implications for Industrial Implementation
4. Conclusions and Outlook
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BES | Bioelectrochemical System |
| MEC | Microbial Electrolysis Cell |
| MEC-AD | Microbial Electrolysis Cell Anaerobic Digester |
| MMEC | Methanogenic Microbial Electrolysis Cell |
| EMR | Electro-methanogenic Reactor |
| AD | Anaerobic Digestion |
| OLR | Organic Loading Rate |
| CF | Carbon Fibre |
| TRL | Technology Readiness Level |
| EAB | Electro-active Bacteria |
| NPV | Net Present Value |
| SRT | Solid Retention Time |
| CAPEX | Capital Expenditure |
| OPEX | Operational Expenditure |
References
- OECD. Water. In OECD Environmental Outlook to 2050; OECD: Paris, France, 2012; Chapter 5; pp. 207–274. [Google Scholar] [CrossRef]
- WWAP (United Nations World Water Assessment Programme). The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource; UNESCO: Paris, France, 2017.
- Zhang, Y.; Merrill, M.D.; Logan, B.E. The use and optimization of stainless steel mesh cathodes in microbial electrolysis cells. Int. J. Hydrogen Energy 2010, 35, 2020–12028. [Google Scholar] [CrossRef]
- Moreno, R.; San-Martín, M.I.; Escapa, A.; Morán, A. Domestic wastewater treatment in parallel with methane production in a microbial electrolysis cell. Renew. Energy 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Pandev, M.; Lucchese, P.; Mansilla, C.; Duigou, A.L.; Abrashev, B.; Vladikova, D. Hydrogen Economy: The future for a sustainable and green society. Bulg. Chem. Commun. 2017, 49, 84–92. [Google Scholar]
- Werner, C.M.; Katuri, K.P.; Hari, A.R.; Chen, W.; Lai, Z.; Logan, B.E.; Amy, G.L.; Saikaly, P.E. Graphene-Coated Hollow Fiber Membrane as the Cathode in Anaerobic Electrochemical Membrane Bioreactors—Effect of Configuration and Applied Voltage on Performance and Membrane Fouling. Environ. Sci. Technol. 2016, 50, 4439–4447. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Baserba, M.; Vinardell, S.; Molinos-Senante, M.; Rosso, D.; Poch, M. The Economics of Wastewater Treatment Decentralization: A Techno-economic Evaluation. Environ. Sci. Technol. 2018, 52, 8965–8976. [Google Scholar] [CrossRef] [PubMed]
- United Nations. The World ’s Cities in 2018; World’s Cities 2018—Data Booklet; United Nations: New York, NY, USA, 2018; p. 34. [Google Scholar]
- Starkl, M.; Brunner, N.; Feil, M.; Hauser, A. Ensuring Sustainability of Non-Networked Sanitation Technologies: An Approach to Standardization. Environ. Sci. Technol. 2015, 49, 6411–6418. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.N.; Lee, S.H.; Kim, H.; Park, Y.H.; In, S.-I. Improved Microbial Electrolysis Cell Hydrogen Production by Hybridization with a TiO2 Nanotube Array Photoanode. Energies 2018, 11, 3184. [Google Scholar] [CrossRef] [Green Version]
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewatertreatment and energy recovery. From laboratory to pilot plantand beyond. Renew. Sustain. Energy Rev. 2015, 55, 942–956. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Ter Heijne, A.; Buisman, C.J.N.; Hamelers, H.V.M. Microbial electrolysis cells for production of methane from CO2: Long-term performance and perspectives. Int. J. Energy Res. 2011, 36, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass-Valoriz. 2017, 8, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Kitching, M.; Butler, R.; Marsili, E. Microbial bioelectrosynthesis of hydrogen: Current challenges and scale-up. Enzym. Microb. Technol. 2017, 96, 1–13. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Olsen, S.I.; Nigam, P.S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. R. Soc. Chem. Adv. 2011, 2, 1248–1263. [Google Scholar]
- Sleutels, T.H.; Ter Heijne, A.; Buisman, C.J.; Hamelers, H.V. Bioelectrochemical Systems: An Outlook for Practical Applications. ChemSusChem 2012, 6, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Leng, X.; Zhao, S.; Ji, J.; Zhou, T.; Khan, A.; Kakde, A.; Liu, P.; Li, X. A review on the applications of microbial electrolysis cells in anaerobic digestion. Bioresour. Technol. 2018, 255, 340–348. [Google Scholar] [CrossRef]
- Siegert, M.; Yates, M.D.; Spormann, A.M.; Logan, B.E. Methanobacterium Dominates Biocathodic Archaeal Communities in Methanogenic Microbial Electrolysis Cells. ACS Sustain. Chem. Eng. 2015, 3, 1668–1676. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysiscells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.D.; Colprim, J.; Puig, S. On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874. [Google Scholar] [CrossRef] [PubMed]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of Organic Compounds from Carbon Dioxide Is Catalyzed by a Diversity of Acetogenic Microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef] [Green Version]
- Nelabhotla, A.B.T.; Dinamarca, C. Bioelectrochemical CO2 Reduction to Methane: MES Integration in Biogas Production Processes. Appl. Sci. 2019, 9, 1056. [Google Scholar] [CrossRef] [Green Version]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Saheb-Alam, S.; Singh, A.; Hermansson, M.; Persson, F.; Schnürer, A.; Wilén, B.M.; Modin, O. Effect of Start-Up Strategies and Electrode Materials on Carbon Dioxide Reduction on Biocathodes. Am. Soc. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.P.; Russell, T.P.; Lovley, D.R. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 2012, 6, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Aiken, D.C.; Curtis, T.P.; Heidrich, E.S. Avenues to the financial viability of microbial electrolysis cells [MEC] for domestic wastewater treatment and hydrogen production. Int. J. Hydrogen Energy 2019, 44, 2426–2434. [Google Scholar] [CrossRef]
- IPCC. Wastewater Treatment and Discharge. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 2006; Volume 5, pp. 6.1–6.28. [Google Scholar]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef]
- Sangeetha, T.; Guo, Z.; Liu, W.; Cui, M.; Yang, C.; Wang, L.; Wang, A. Cathode material as an influencing factor on beer wastewater treatment and methane production in a novel integrated upflow microbial electrolysis cell (Upflow-MEC). Int. J. Hydrogen Energy 2016, 41, 2189–2196. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.-Y.; Kim, H.W.; Lim, K.-H.; Shin, H.-S. Effects of organic loading rates on the continuous electricity generation from fermented wastewater using a single-chamber microbial fuel cell. Bioresour. Technol. 2010, 101, S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Rago, L.; Baeza, J.A.; Guisasola, A. Bioelectrochemical hydrogen production with cheese whey as sole substrate. J. Chem. Technol. Biotechnol. 2016, 92, 173–179. [Google Scholar] [CrossRef]
- Mohammed, A.J.; Ismail, Z.Z. Slaughterhouse wastewater biotreatment associated with bioelectricity generation and nitrogen recovery in hybrid system of microbial fuel cell with aerobic and anoxic bioreactors. Ecol. Eng. 2018, 125, 119–130. [Google Scholar] [CrossRef]
- Christwardana, M.; Prabowo, A.K.; Tiarasukma, A.P.; Ariyanti, D. Microbial Fuel Cells for Simultaneous Electricity Generation and Organic Degradation from Slaughterhouse Wastewater. Int. J. Renew. Energy Dev. 2016, 5, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Noori, T.; Ghangrekar, M.M.; Mitra, A.; Mukherjee, C. Enhanced Power Generation in Microbial Fuel Cell Using MnO2-Catalyzed Cathode Treating Fish Market Wastewater; Springer: New Delhi, India, 2016; pp. 285–294. [Google Scholar]
- Taiganides, P.E. Pig Waste Management & Recycling—The Singapore Experience; International Development Research Centre: Ottawa, ON, Canada, 1992. [Google Scholar]
- Wagner, R.C.; Regan, J.M.; Oh, S.-E.; Zuo, Y.; Logan, B.E. Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res. 2009, 43, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- San-Martín, M.I.; Sotres, A.; Alonso, R.M.; Díaz-Marcos, J.; Morán, A.; Escapa-González, A. Assessing anodic microbial populations and membrane ageing in a pilot microbial electrolysis cell. Int. J. Hydrogen Energy 2019, 44, 17304–17315. [Google Scholar] [CrossRef]
- Viana, M.B.; Freitas, A.V.; Leitão, R.C.; Pinto, G.A.; Santaella, S.T. Anaerobic digestion of crude glycerol: A review. Environ. Technol. Rev. 2012, 1, 81–92. [Google Scholar] [CrossRef]
- Chookaew, T.; Prasertsan, P.; Ren, Z.J. Two-stage conversion of crude glycerol to energy using dark fermentation linked with microbial fuel cell or microbial electrolysis cell. New Biotechnol. 2014, 31, 179–184. [Google Scholar] [CrossRef]
- Selembo, P.A.; Perez, J.M.; Lloyd, W.A.; Logan, B.E. High hydrogen production from glycerol or glucose by electrohydrogenesis using microbial electrolysis cells. Int. J. Hydrogen Energy 2009, 34, 5373–5381. [Google Scholar] [CrossRef]
- Escapa-González, A.; Manuel, M.-F.; Morán, A.; Gómez, X.; Guiot, S.R.; Tartakovsky, B. Hydrogen Production from Glycerol in a Membraneless Microbial Electrolysis Cell. Energy Fuels 2009, 23, 4612–4618. [Google Scholar] [CrossRef] [Green Version]
- Moreno, R.; Escapa-González, A.; Cara, J.; Carracedo, B.; Gómez, X. A two-stage process for hydrogen production from cheese whey: Integration of dark fermentation and biocatalyzed electrolysis. Int. J. Hydrogen Energy 2015, 40, 168–175. [Google Scholar] [CrossRef]
- Montpart, N.; Rago, L.; Baeza, J.A.; Guisasola, A. Hydrogen production in single chamber microbial electrolysis cells with different complex substrates. Water Res. 2015, 68, 601–615. [Google Scholar] [CrossRef]
- Arvin, A.; Hosseini, M.; Amin, M.-M.; Najafpour-Darzi, G.; Ghasemi, Y. Efficient methane production from petrochemical wastewater in a single membrane-less microbial electrolysis cell: The effect of the operational parameters in batch and continuous mode on bioenergy recovery. J. Environ. Heal. Sci. Eng. 2019, 17, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. A new upgraded biogas production process: Coupling microbial electrolysis cell and anaerobic digestion in single-chamber, barrel-shape stainless steel reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Gikas, P. Towards energy positive wastewater treatment plants. J. Environ. Manag. 2017, 203, 621–629. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Effect of Combined Inoculation on Biogas Production from Hardly Degradable Material. Energies 2019, 12, 217. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.S.; Fontmorin, J.-M.; Izadi, P.; Daud, W.R.W.; Scott, K.; Yu, E.H. Impact of applied cell voltage on the performance of a microbial electrolysis cell fully catalysed by microorganisms. Int. J. Hydrogen Energy 2020, 45, 2557–2568. [Google Scholar] [CrossRef]
- Guo, K.; Freguia, S.; Dennis, P.G.; Chen, X.; Donose, B.C.; Keller, J.; Gooding, J.J.; Rabaey, K. Effects of Surface Charge and Hydrophobicity on Anodic Biofilm Formation, Community Composition, and Current Generation in Bioelectrochemical Systems. Environ. Sci. Technol. 2013, 47, 7563–7570. [Google Scholar] [CrossRef]
- Escapa-González, A.; San-Martín, M.; Mateos, R.; Morán, A. Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical hydrogen production from urban wastewater on a pilot scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Luo, L.; Liu, H. Startup performance of microbial electrolysis cell assisted anaerobic digester (MEC-AD) with pre-acclimated activated carbon. Bioresour. Technol. Rep. 2019, 5, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Yu, E.H.; Daud, W.R.W.; Kim, B.H.; Scott, K. Bioanode as a limiting factor to biocathode performance in microbial electrolysis cells. Bioresour. Technol. 2017, 238, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.G.; Peixoto, L.; Soares, O.S.G.P.; Pereira, M.F.R.; Ter Heijne, A.; Kuntke, P.; Alves, M.M.; Pereira, M.A. Influence of carbon anode properties on performance and microbiome of Microbial Electrolysis Cells operated on urine. Electrochim. Acta 2018, 267, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.P.; Pandit, S. Important Factors InfluencingMicrobial Fuel Cell Performance; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, S.; Cheng, C.; Thomas, A. Carbon-Based Microbial-Fuel-Cell Electrodes: From Conductive Supports to Active Catalysts. Adv. Mater. 2017, 29, 1602547. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Roca-Puigros, M.; Geppert, F.; Caizán-Juanarena, L.; Na Ayudthaya, S.P.; Buisman, C.; Ter Heijne, A. Granular Carbon-Based Electrodes as Cathodes in Methane-Producing Bioelectrochemical Systems. Front. Bioeng. Biotechnol. 2018, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Prévoteau, A.; Patil, S.A.; Rabaey, K. Engineering electrodes for microbial electrocatalysis. Curr. Opin. Biotechnol. 2015, 33, 149–1565. [Google Scholar] [CrossRef]
- Carlotta-Jones, D.I.; Purdy, K.; Kirwan, K.; Stratford, J.; Coles, S.R. Improved hydrogen gas production in microbial electrolysis cells using inexpensive recycled carbon fibre fabrics. Bioresour. Technol. 2020, 304, 122983. [Google Scholar] [CrossRef]
- Pötschke, L.; Huber, P.; Schriever, S.; Rizzotto, V.; Gries, T.; Blank, L.M.; Rosenbaum, M.A. Rational Selection of Carbon Fiber Properties for High-Performance Textile Electrodes in Bioelectrochemical Systems. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Cotterill, S.; Dolfing, J.; Jones, C.; Curtis, T.P.; Heidrich, E.S. Low Temperature Domestic Wastewater Treatment in a Microbial Electrolysis Cell with 1 m2Anodes: Towards System Scale-Up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Cotterill, S.; Dolfing, J.; Curtis, T.P.; Heidrich, E.S. Community Assembly in Wastewater-Fed Pilot-Scale Microbial Electrolysis Cells. Front. Energy Res. 2018, 6, 98. [Google Scholar] [CrossRef]
- Santoro, C.M.; Babanova, S.; Artyushkova, K.; Cornejo, J.A.; Ista, L.K.; Bretschger, O.; Marsili, E.; Atanassov, P.; Schuler, A.J. Influence of anode surface chemistry on microbial fuel cell operation. Bioelectrochemistry 2015, 106, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, T.; Yokoyama, H. Molybdenum anode: A novel electrode for enhanced power generation in microbial fuel cells, identified via extensive screening of metal electrodes. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocaznoi, D.; Calmet, A.; Etcheverry, L.; Erable, B.; Bergel, A. Stainless steel is a promising electrode material for anodes of microbial fuel cells. Energy Environ. Sci. 2012, 5, 9645–9652. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Donose, B.C.; Soeriyadi, A.H.; Prévoteau, A.; Patil, S.A.; Freguia, S.; Gooding, J.J.; Rabaey, K. Flame Oxidation of Stainless Steel Felt Enhances Anodic Biofilm Formation and Current Output in Bioelectrochemical Systems. Environ. Sci. Technol. 2014, 48, 7151–7156. [Google Scholar] [CrossRef]
- Ahn, Y.; Im, S.; Chung, J.W. Optimizing the operating temperature for microbial electrolysis cell treating sewage sludge. Hydrog. Energy 2017, 42, 27784–27791. [Google Scholar] [CrossRef]
- Wang, L.; He, Z.; Guo, Z.; Sangeetha, T.; Yang, C.; Gao, L.; Wang, A.; Liu, W. Microbial community development on different cathode metals in a bioelectrolysis enhanced methane production system. J. Power Sources 2019, 444, 227306. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Siegert, M.; Yates, M.D.; Call, D.F.; Zhu, X.; Spormann, A.; Logan, B.E. Comparison of Nonprecious Metal Cathode Materials for Methane Production by Electromethanogenesis. ACS Sustain. Chem. Eng. 2014, 2, 910–917. [Google Scholar] [CrossRef] [Green Version]
- Kellenberger, A.; Vaszilcsin, N.; Brandl, W.; Duteanu, N. Kinetics of hydrogen evolution reaction on skeleton nickel and nickel–titanium electrodes obtained by thermal arc spraying technique. Int. J. Hydrogen Energy 2007, 32, 3258–3265. [Google Scholar] [CrossRef]
- Ma, X.; Li, Z.; Zhou, A.; Yue, X. Energy recovery from tubular microbial electrolysis cell with stainless steel mesh as cathode. R. Soc. Open Sci. 2017, 4, 170967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokabian, B.; Gude, V.G. Role of membranes in bioelectrochemical systems. Membr. Water Treat. 2015, 6, 53–75. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically Assisted Microbial Production of Hydrogen from Acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.; Euverink, G.J.; Metz, S.J.; Buisman, C.J. Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int. J. Hydrogen Energy 2006, 31, 1632–1640. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Sleutels, T.H.J.A.; Hamelers, H.V.; Buisman, C.J.N. Effect of the type of ion exchange membrane on performance, ion transport, and pH in biocatalyzed electrolysis of wastewater. Water Sci. Technol. 2008, 57, 1757–1762. [Google Scholar] [CrossRef]
- Escapa, A.; Gómez, X.; Tartakovsky, B.; Morán, A. Estimating microbial electrolysis cell (MEC) investment costs in wastewater treatment plants: Case study. Int. J. Hydrogen Energy 2012, 37, 18641–18653. [Google Scholar] [CrossRef]
- Rajendran, K.; Murthy, G.S. Techno-economic and life cycle assessments of anaerobic digestion—A review. Biocatal. Agric. Biotechnol. 2019, 20, 101207. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.; Molenkamp, R.J.; Buisman, C.J.N. Performance of single chamber biocatalyzed electrolysis with different types of ion exchange membranes. Water Res. 2007, 41, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Premier, G.; Michie, I.; Boghani, H.C.; Fradler, K.; Kim, J. Reactor design and scale-up. In Microbial Electrochemical and Fuel Cells; Scott, K., Yu, E.H., Eds.; Wood House Publishing: Chippenham, UK, 2016; pp. 215–239. [Google Scholar]
- Ki, D.; Popat, S.C.; Torres, C.I. Reduced overpotentials in microbial electrolysis cells through improved design, operation, and electrochemical characterization. Chem. Eng. J. 2016, 287, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Rivera, I.; Bakonyi, P.; Buitrón, G. H2 production in membraneless bioelectrochemical cells with optimized architecture: The effect of cathode surface area and electrode distance. Chemosphere 2017, 171, 379–385. [Google Scholar] [CrossRef]
- Lu, L.; Ren, Z.J. Microbial electrolysis cells for waste biorefinery: A state of the art review. Bioresour. Technol. 2016, 215, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Logan, B.E. High hydrogen production rate of microbial electrolysis cell (MEC) with reduced electrode spacing. Bioresour. Technol. 2010, 102, 3571–3574. [Google Scholar] [CrossRef]
- Gil-Carrera, L.; Mehta, P.; Escapa-González, A.; Morán, A.; García, V.; Guiot, S.R.; Tartakovsky, B. Optimizing the electrode size and arrangement in a microbial electrolysis cell. Bioresour. Technol. 2011, 102, 9593–9598. [Google Scholar] [CrossRef]
- Muoio, R.; Palli, L.; Ducci, I.; Coppini, E.; Bettazzi, E.; Daddi, D.; Fibbi, D.; Gori, R. Optimization of a large industrial wastewater treatment plant using a modeling approach: A case study. J. Environ. Manag. 2019, 249, 109436. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2012, 97, 6979–6989. [Google Scholar] [CrossRef] [PubMed]
- Fornero, J.J.; Rosenbaum, M.; Angenent, L.T. Electric Power Generation from Municipal, Food, and Animal Wastewaters Using Microbial Fuel Cells. Electroanalysis 2010, 22, 832–843. [Google Scholar] [CrossRef]
- Guo, H.; Kim, Y. Stacked multi-electrode design of microbial electrolysis cells for rapid and low-sludge treatment of municipal wastewater. Biotechnol. Biofuels 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, C.; Zhu, G. Performance, process kinetics and functional microbial community of biocatalyzed electrolysis-assisted anaerobic baffled reactor treating carbohydrate-containing wastewater. RSC Adv. 2018, 8, 41150–41162. [Google Scholar] [CrossRef] [Green Version]
- Gil-Carrera, L.; Escapa, A.; Moreno, R.; Morán, A. Reduced energy consumption during low strength domestic wastewater treatment in a semi-pilot tubular microbial electrolysis cell. J. Environ. Manag. 2013, 122, 1–7. [Google Scholar] [CrossRef]
- Katuri, K.P.; Ali, M.; Saikaly, P.E. The role of microbial electrolysis cell in urban wastewater treatment: Integration options, challenges, and prospects. Curr. Opin. Biotechnol. 2019, 57, 101–110. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kiely, P.D.; Logan, B.E. A monetary comparison of energy recovered from microbial fuel cells and microbial electrolysis cells fed winery or domestic wastewaters. Int. J. Hydrogen Energy 2010, 35, 8855–8861. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.-J.; Kim, K.-Y.; Ajayi, F.F.; Chang, I.-S.; Kim, I.S. A Solar-Powered Microbial Electrolysis Cell with a Platinum Catalyst-Free Cathode To Produce Hydrogen. Environ. Sci. Technol. 2009, 43, 9525–9530. [Google Scholar] [CrossRef]
- Wan, L.-L.; Li, X.-J.; Zang, G.-L.; Wang, X.; Zhang, Y.-Y.; Zhou, Q. A solar assisted microbial electrolysis cell for hydrogen production driven by a microbial fuel cell. RSC Adv. 2015, 5, 82276–82281. [Google Scholar] [CrossRef]
- Andrews, J.; Shabani, B. Where does Hydrogen Fit in a Sustainable Energy Economy? Procedia Eng. 2012, 49, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Bastidas-Oyanedel, J.R.; Schmidt, J.E. Increasing Profits in Food Waste Biorefinery—A Techno-Economic Analysis. Energies 2018, 11, 1551. [Google Scholar] [CrossRef] [Green Version]
| Industry | COD Range (g/L) | Required HRT to Obtain OLR of 1400 mg COD/L/day (hrs) | BES Research Papers |
|---|---|---|---|
| Urban wastewater (WW) | 0.3–0.5 [28] | 5–9 | [29] |
| Alcohol Refining | 5–22 [28] | 86–377 | [30] |
| Beer & Malt | 2–7 [28] | 34–120 | [31] |
| Coffee | 3–15 [28] | 51–257 | [32] |
| Dairy Processing | 1.5–5.2 [28] | 26–89 | [33] |
| Meat & Poultry | 2–7 [28] | 34–120 | [34,35] |
| Fish Processing | 2.5 [28] | 43 | [36] |
| Swine waste | 18.3 [37] | 314 | [38,39] |
| Crude glycerol | 925–1600 [40] | 15,857–27,429 | [41,42,43] |
| Cheese Whey | 50–102.1 [44] | 857–1750 | [44] |
| Parameters | Scenario 2 | Scenario 3 |
|---|---|---|
| COD Removal | 44% | 44% |
| Energy Consumption | 1 kWh/kg-COD | 0.9 kWh/kg-COD |
| Cathodic Coulombic Efficiency (CCE) | 50% | 50% |
| Hydrogen Production | 0.6 m3/m3/day | 0.8 m3 /m3/day |
| Organic Loading Rate | 8.8 kg/m3/day | 17.5 kg/m3/day |
| MEC Volume | 2200 m3 | 1100 m3 |
| MEC Durability | >7 years | >7 years |
| MEC Purchase Price | 396 *1 £/m3 | 1149 *1 £/m3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fudge, T.; Bulmer, I.; Bowman, K.; Pathmakanthan, S.; Gambier, W.; Dehouche, Z.; Al-Salem, S.M.; Constantinou, A. Microbial Electrolysis Cells for Decentralised Wastewater Treatment: The Next Steps. Water 2021, 13, 445. https://doi.org/10.3390/w13040445
Fudge T, Bulmer I, Bowman K, Pathmakanthan S, Gambier W, Dehouche Z, Al-Salem SM, Constantinou A. Microbial Electrolysis Cells for Decentralised Wastewater Treatment: The Next Steps. Water. 2021; 13(4):445. https://doi.org/10.3390/w13040445
Chicago/Turabian StyleFudge, Thomas, Isabella Bulmer, Kyle Bowman, Shangami Pathmakanthan, William Gambier, Zahir Dehouche, Sultan Majed Al-Salem, and Achilleas Constantinou. 2021. "Microbial Electrolysis Cells for Decentralised Wastewater Treatment: The Next Steps" Water 13, no. 4: 445. https://doi.org/10.3390/w13040445






