1. Introduction
Riverbank filtration is categorized as one of the natural and sustainable techniques that can be used for water purification. This filtration system is mainly based on the action of the soil of the banks of the water source as a natural filtration surface to the infiltrated water [
1]. In Egypt, the growing need for drinkable water has promoted the use of riverbank filtration as an economical method for water purification [
2]. The main aim of this research is to focus on protecting and improving the quality of water by spotlighting pollutants that affect water and how to eliminate them by using a compelling method to treat water that is considered a solution to water problems. The advancement of the technology of riverbank filtration is one of the most considerable and quickest solutions that should be embraced by the government and responsible parties [
3]. Riverbank filtration is considered a regulatory system used to eliminate heavy metals and microorganisms and is environmentally friendly because of the absence of chemicals in this technique [
4]. It is very important to investigate the degree of efficiency of the riverbank filtration process before applying, especially in developing counties that have a high-water demand [
5]. The utilization of RBF has expanded widely in Egypt, many governorates in Egypt have started to use this filtration system such as Giza, Beni Suef, Minia, Suhag, and Qena.
The quality of water is one of the essential factors that affect living organisms whether used for drinking, home, or factories. Water quality expresses the circumstances of the water’s inclusive biological, physical, and chemical characteristics. Riverbank filtration is more advantageous than traditional water resources development modes in that it improves water quality by percolation [
6]. By detecting the concentration of it is parameters and components it is possible to compare these results with standard components for potable water [
7]. Freshwater supports our plants, as well as animals and humans. It is definite that our activities and actions cause an effect on water quality, whether positive or negative [
8]. With time, the quality of water is threatened due to all kinds of sources of pollution such as nutrients and phosphates that come from fertilizers, industrialization, irrigation, and agricultural activities, which places humans at risk [
9]. As a result of increasing populations and industries, the size of a wide range of pollutions such as water pollution, air pollution, and soil pollution are huge compared to the past [
10]. For drinking water quality, riverbank filtration is guaranteed as an effective method for removing many water contaminants. Along the stream of one of the Indian rivers (Yamuna), the removal efficiency of dissolved organic matter using RBF exceeds the 50% removal [
11]. In some conditions, riverbank filtration is used as a pre-step in the water purification process, for better water quality, and energy cost reduction [
12].
Surface water contains specific concentrations of heavy metals such as Iron (Fe), Mercury (Hg), Copper (Cu), Nickel (Ni), Zinc (Zn), Cobalt (Co), and Lead (Pb) that may cause many diseases to human beings. Due to the toxicity of heavy metals, various techniques have been used to remove them from water such as adsorption, membrane filtration, precipitation, and Flocculation [
13]. Most of the dissolved iron in water forms stable compounds with fulvic acid, that can move with the water stream into the intake wells during riverbank filtration [
14]. The site conditions, type, and concentration of heavy metals are the most influencing factors on the efficiency of removal using riverbank filtration. In addition, it has been declared that the water characteristics such as pH value, dissolved oxygen, and dissolved organic matter concentrations have a major impact upon the adsorption process of heavy metals during riverbank filtration [
15]. Sediments on the riverbed or aquifer may contain a sufficient concentration of iron, manganese minerals, and hydroxides that may contaminate water by dissolving [
16]. Redox conditions are one of the dependent factors on the riverbank filtration RBF process especially on the removal efficiency of micropolluants [
17]. The media of redox is mainly identified by the process of microbial respiration, at which the natural organic matters NOM is the electron donor and oxygen, ni-trate, Mn(III/IV)- and Fe(III) hydroxides or sulfate are the electron acceptors [
18].
In Egypt, there are many types of contamination that affect water sources such as heavy metals. In our country, we have a lot of sources of freshwater in comparison with many other countries. They have to begin using efficient and cost-effective water purification methods, such as riverbank filtration [
19]. Riverbank filtration is a technique that provides an ordinary filtration process. During this process when groundwater passes through the soil along the bank of the Nile, a group of chemical, physical and biological processes takes place, which are essential components of the treatment process [
20]. The environmental parameters such as biodegradation and adsorption of organic contaminants are still typically unquantified [
21]. Some of the literature has investigated the degradation and sorption of various emerging organic contaminants (EOC) such as adsorbable organic halogens (AOX), naphthalenedisulfonic (NDSA), phenazon, clindamycin, carbamazepine, sulfa-methoxazole (SMOX), methyl tert-butylether (MTBE), and adsorbable organic iodines (AOI). They observed that mostly a decrease in EOC concentrations have happened after riverbank filtration.
Treatment is a process to improve water quality to match with the water quality standards that is suitable for human uses. The volume of treated water depends on the citizen’s consumption per day of water for drinking, cooking, and reactional purposes. Responsible ministry or the government should choose a suitable treatment system by taking into consideration the cost and design period.
Countries that have insufficient funds cannot build an effective treatment plant; therefore, it is hard to find safe water for human use. To balance out this issue, there are many economical solutions to treat water, such as using riverbank filtration and slow sand filter in the filtration stage [
22]. The treatment process is designed to make sure that water whether it is wastewater or potable water is safe for human uses in all it is forms. Treatment is considered the second stage in the water supply system. The quantity of water, geological conditions, and pollution on the surface bank are important factors that may hinder the success of the riverbank filtration process [
23]. Also, the seasonal variations may cause a decrease in RBF efficiency due to the change in microbial diversity during wet and dry seasons [
24]. Clogging in the riverbed is an important factor that affects the quantity and quality of water during the RBF process [
25].
Both human-made and natural impacts can affect the quality of water. The natural treatment uses natural geology in order to purify water without chemicals. Nowadays, many countries worldwide need to improve the quality of water without harm to the environment. There are many advantages for the natural treatment process such as; it is not a complicated process, there is no need for very skilled employees, it is not expensive like conventional treatment processes, it can be used widely, and it is easy to maintain. However, these processes are not suitable for all situations such as if there is a lot of and various chemical contaminations in the water. In this case, there must be human intervention [
26].
There are many examples of natural treatment that can remove contaminations efficiently, such as lagoons and RBF [
1]. Riverbank Filtration is a technique delivering a natural filtration process. Compared with the traditional treatment process, riverbank filtration is very economical. The total cost of the RBF unit is about 250,000 EGP, while the price of a conventional treatment plant is 10 million EGP [
27].
The main objective of this research is to study the removal behavior of different heavy metals from water coming from the river Nile. This objective will lead to identifying the possibility of the riverbank filtration process being a reliable and successful technique that can be used separately or as a pretreatment stage to remove heavy metals from water.
2. Materials and Methods
2.1. Work Plan Model
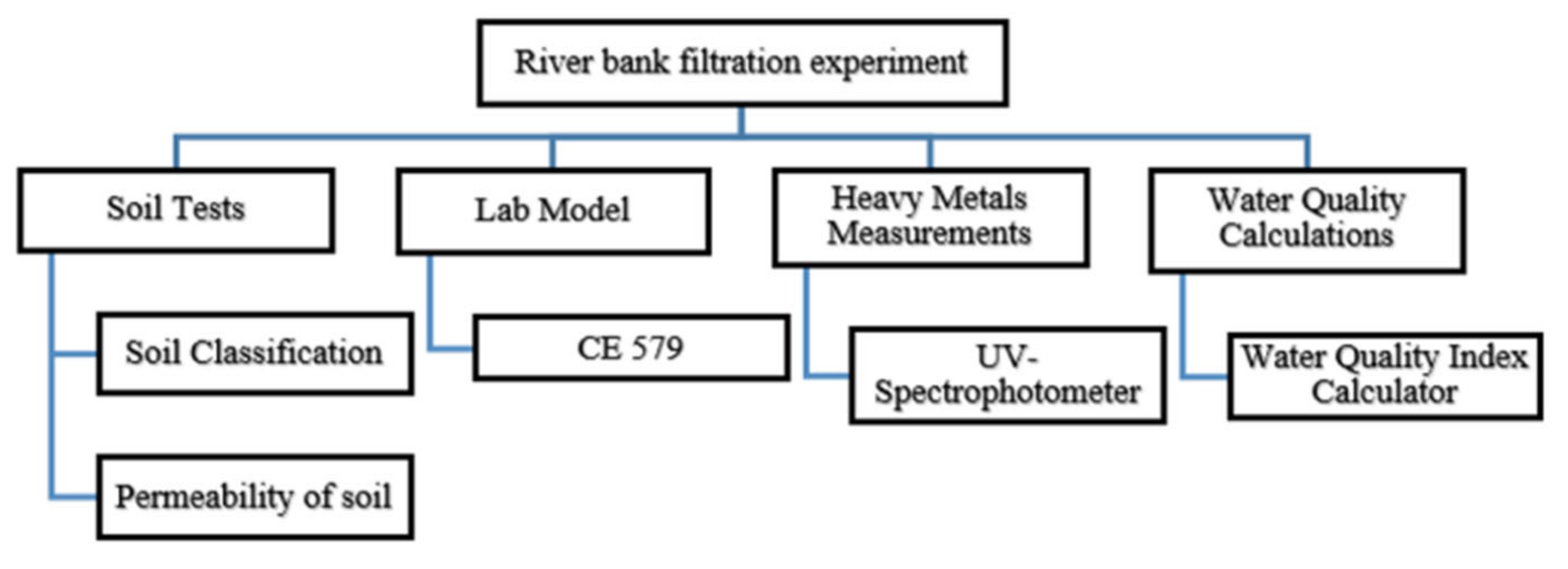
The algorithm of this study is shown in
Figure 1. The working map is ramified into four components. It includes the soil tests, measurement of heavy metals concentration, and the calculation of water quality in addition to the riverbank filtration lab model.
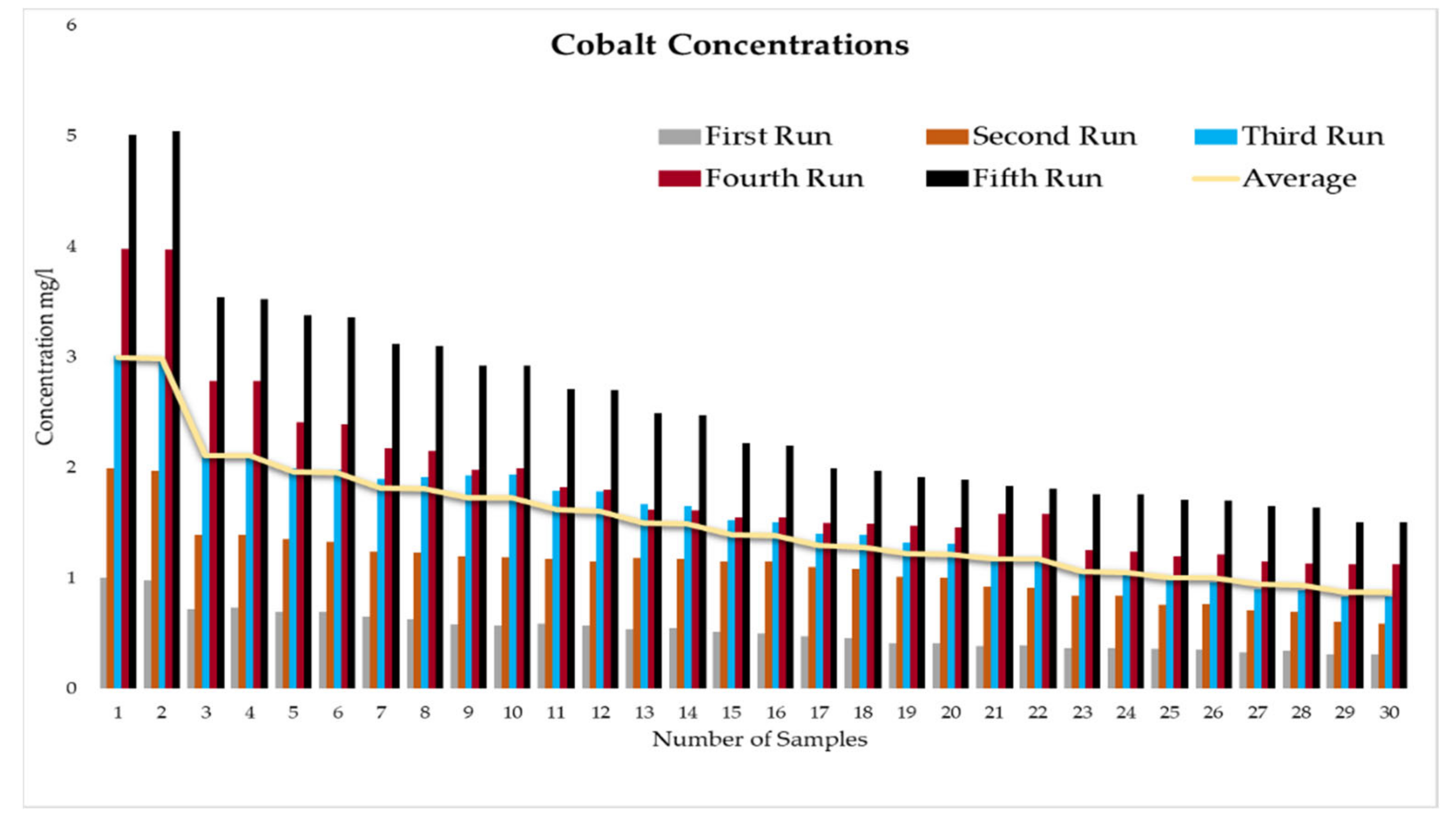
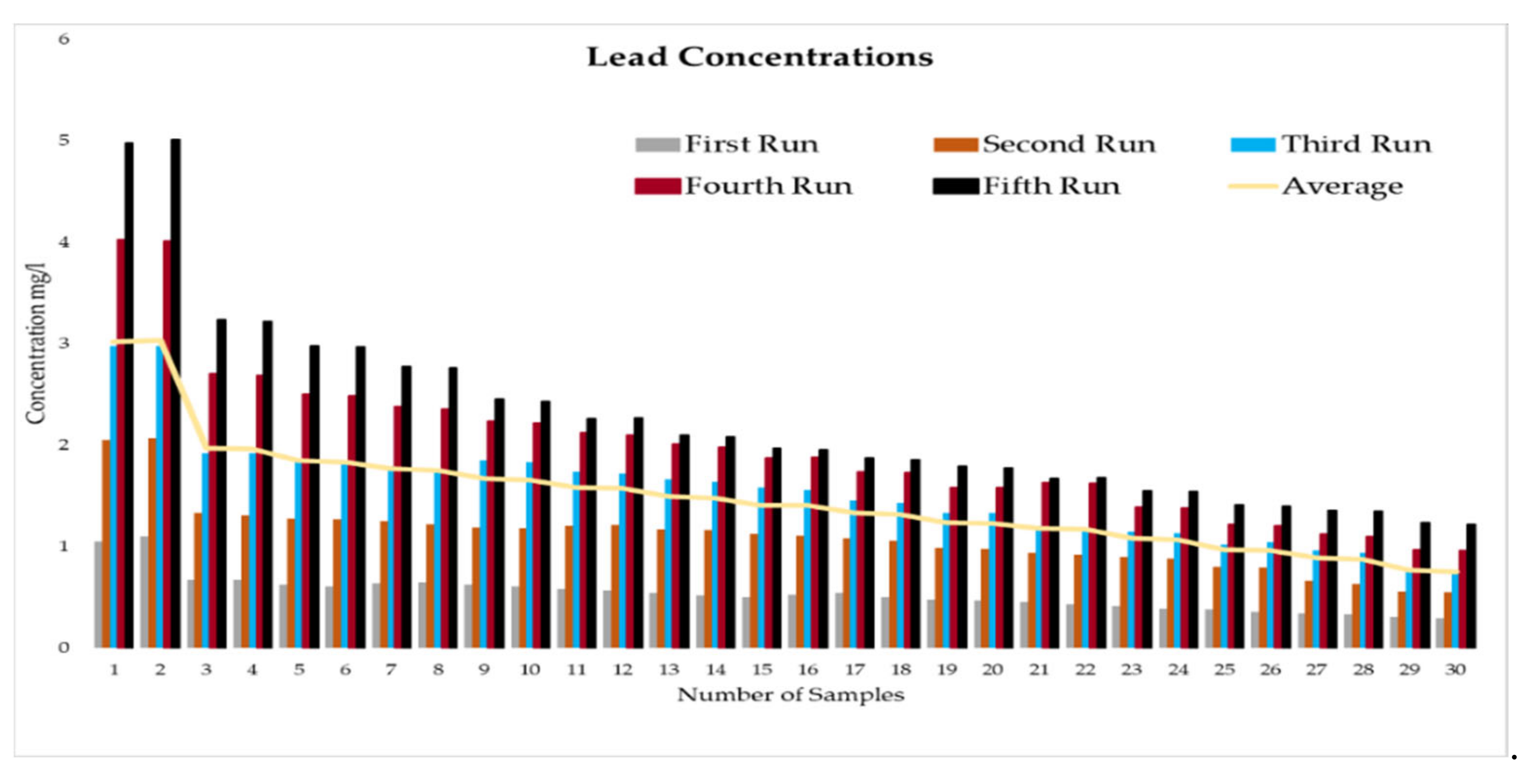
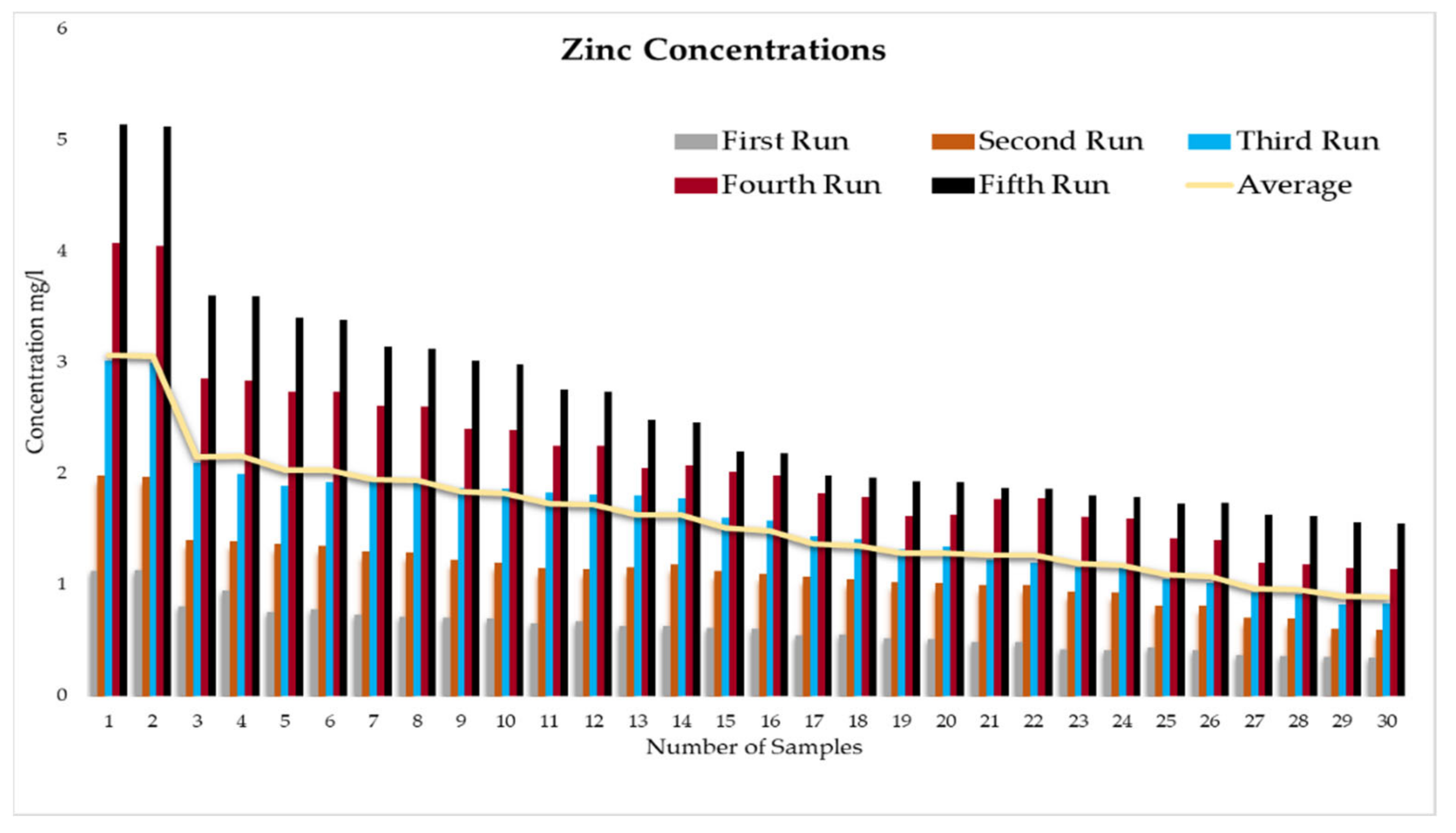
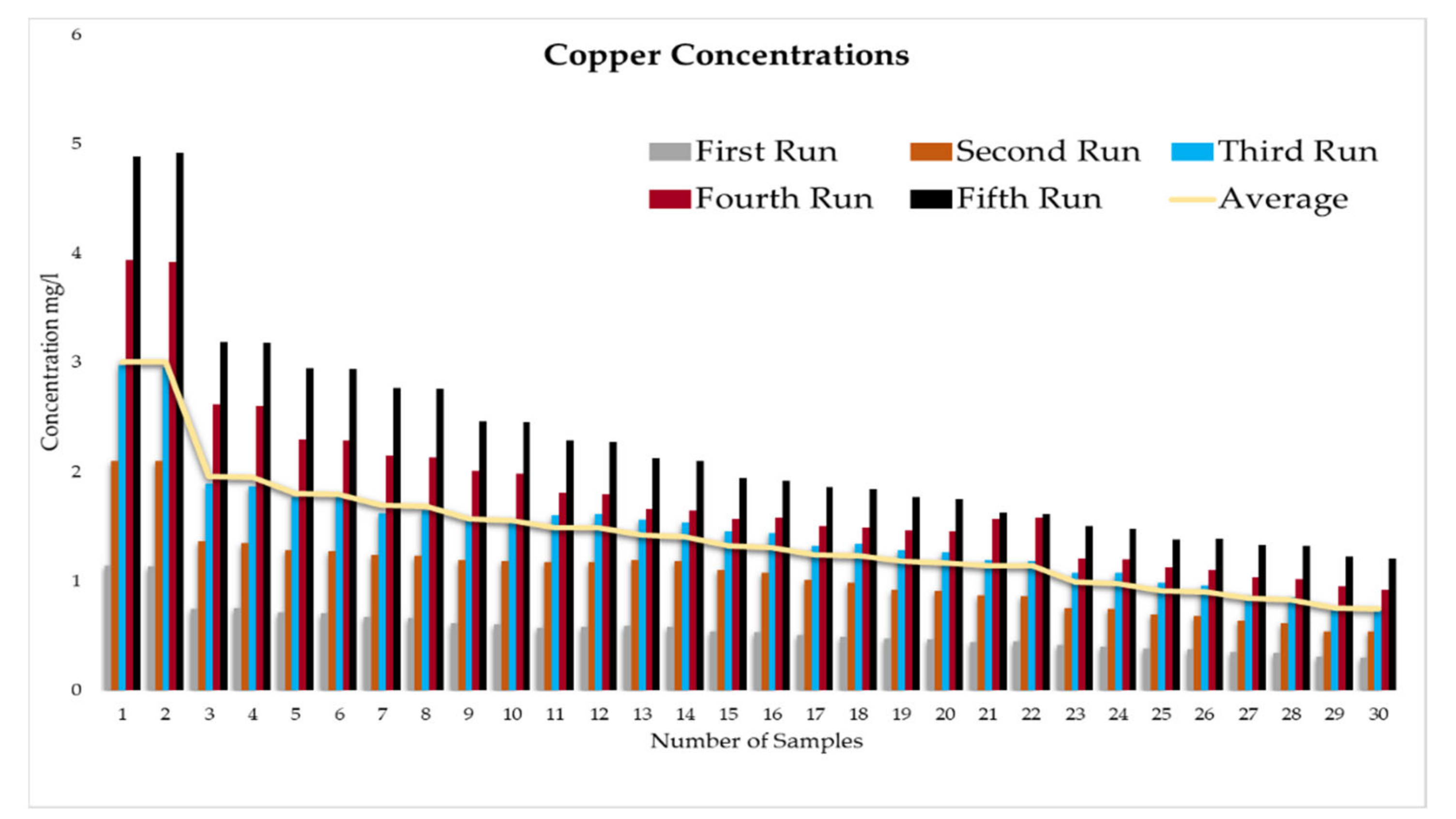
This experiment was divided into five runs. In each run, the concentration of the heavy metals is gradually increased starting from a concentration of 1 mg/L of metal and ending with 5 mg/L. During the experiment two samples were taken daily before the operation of the RBF Unit; and within a period of time, two samples were taken every half an hour. More than two hundred water samples were taken during a working period of 4 months from the inlet and outlet of the lab filtration model (CE 579 Unit). The samples were conveyed to water quality laboratories in the faculty of engineering in Ain shams University (Egypt). In the lab, all the measuring experiments were done to determine the concentration of heavy metal in the water before and after filtration to identify the effect of riverbank filtration on the quality of water from the River Nile.
2.2. Water Characteristics
Firstly, water samples were taken from the River Nile to be examined to determine and identify its degree of quality.
Table 1 represents the results of the water quality analysis of River Nile samples. The results show that most of the measured parameters’ average levels are below the standard limits mentioned of the Egyptian Law no. 48/1982, except the Turbidity [
28]. The redox conditions or the oxidation-reduction potentials ORP of water during the experiments was ranged between −65 mv to −180 mv.
Secondly, the concentration of the heavy metals under this study (Fe, Co, Pb, Zn, and Cu) was measured in the water samples taken to prepare the water for the experiments according to the fixed concentrations of heavy metals that were previously set for the tests (1 to 5 mg/L).
2.3. Soil Tests
The site used to take samples of soil for the study was located at 31°14′13.92″ E, 29°57′46.08″ N. These samples were taken for some experimental tests such as soil classification and the permeability of the soil. The soil was sieved and classified according to “ASTM D 2487-06 standard procedure”. Also, the concentration of some heavy metals that may be included as soil sediments were examined to make sure that it will not influence the research results. The soil investigation results are shown in
Table 2.
2.4. RBF Lab Model
Depth filtration by using soil filters is the main procedure in water purification. The device shown in
Figure 2 (CE 579) empowers this procedure to be established. The tower filter pipe is made from a transparent PMMA Plexiglas tube with dimensions 1660 mm high, and 150 mm and 200 mm internal and external diameter, respectively. The soil filter is integrated into the piping system with flange connections at the upper and lower end. The flanges on the tube side are also made of PMMA and are bonded to the tube. The counter flanges are made of PVC and are secured with knurled screws. The flange connections are sealed using rubber sealing rings.
The RBF model contains a filter and washing pumps with a maximum delivery rate of 5.0 and 3.0 m³/h respectively, under an average pressure of 1.5 bars. The empty bed contact time (EBCT) was based on the bed volume and the filtration rate was 2.16 min. Raw water that is contaminated with impurities and solids is dragged from the upside and moves to a soil filter bed. The solids and impurities are retained due to the raw water passing through the soil filter bed. The water passes due to gravity through the soil filter bed then appears at the bottom of the media. The treated raw water passes through to the outflow tank.
Supplementary solids and impurities are retained in the filter medium (filter bed), so that the resistance is increased. This process is done due to the difference in hydrostatic pressure between the inlet (soil filter) and the outlet. There is a software program used to control the operation and measure the data [
29].
The system model has four sampling valves. The sampling valves can optionally be integrated into the system. At the end of each valve, there is a lance with small holes through which samples can be taken.
The backwashing process for filter media is done periodically at the end of each run with the specified heavy metal concentration. During the transition stage of changing the type of heavy metal added to water, the soil inside the model is changed with a new soil having the same start-up characteristics to avoid any disturbance that can happen on the measured influent and effluent concentrations. Also, the influent tank is washed with diluted hydraulic acid and distilled water several times before feeding the model with influent water of any working runs.
2.5. Ultraviolet (UV) Spectrophotometer
There are many methods to determine the concentration of metals in raw water either with lab instruments such as UV-spectrophotometer or with titration methods. In this study, the UV-spectrophotometer is used to detect the concentration of heavy metals. The process of quantitative determination of heavy metals in the UV-Spectrophotometer occurs when the unknown metals absorb UV light generated from the instrument. The concentration of heavy metals is directly proportional to the intensity of light [
30].
2.6. Water Quality Index (WQI) Calculator
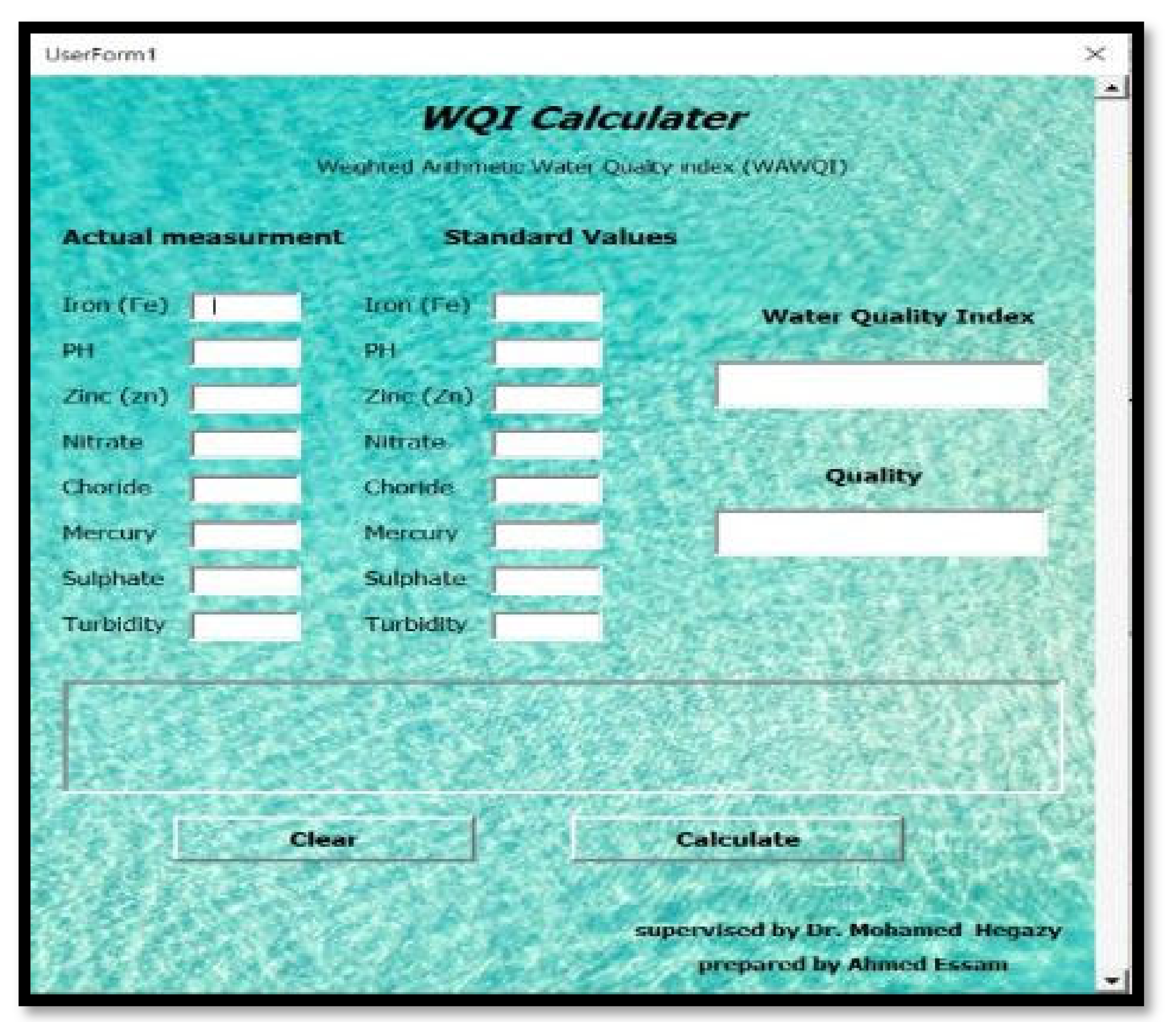
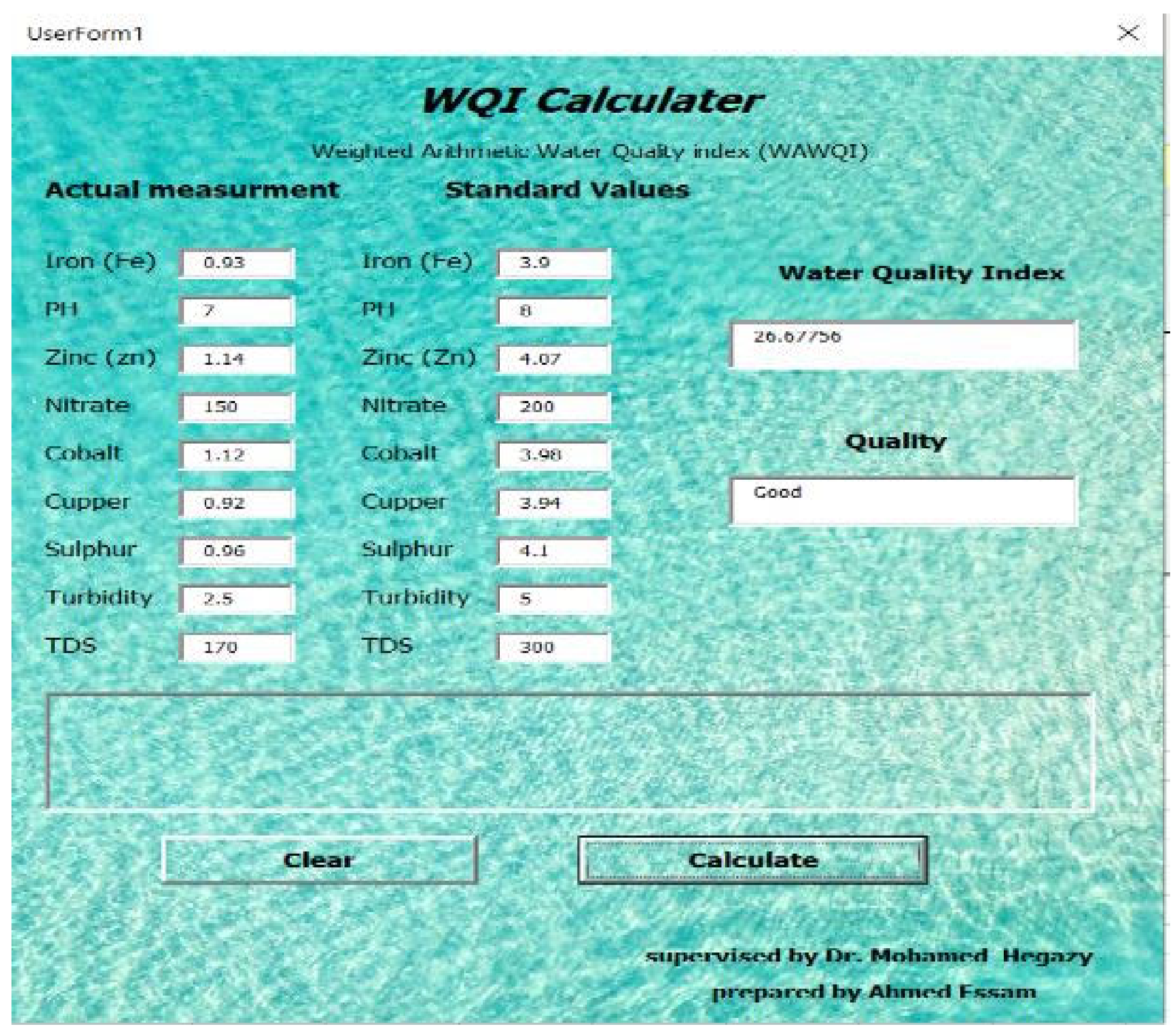
This software shown in
Figure 3 is designed to identify the quality of effluent water obtained from the riverbank filtration unit. The main aim of this software is to determine the quality of water and water quality index quickly without doing manual calculations. Also, to identify the parameter issues or to identify which parameter does not follow the standards, this calculator is designed by using visual basics technology and based on the weighted arithmetic method for water quality (WAWQI) calculation which is one of the widely used water quality indicators (1).
The inputs of this software contain standard values for the parameters suggested by the “World Health Organization” and actual measurement of factors (parameters) that were carried out during the experiment. Software inputs parameters can include the concentration of different parameters such as in our study heavy metals (Fe, Zn,….), and also some water characteristics that include pH value and water turbidity.
On the other hand, there are three outputs obtained from the software; the first one is the water quality index, where there are five categories for water quality index (A, B, C, D, and E). These categories can be determined from software by using a group of if-else statements. The second output is the quality of water (first-rate, good, barren, very poor, and not potable). The last output is the parameter issue to determine which parameter does not follow the standards by calculating the difference between the standards values and actual measurement.
5. Conclusions
In Egypt, there is an increase in population and hence types of contaminations, for instance, fund pollution and accumulating pollution. These types of contaminations cause a negative effect on the quality of water due to the many parameters of water quality [
31]. That is why the spreading need for drinkable water is increased compared to the past years. There are many characteristics or parameters of water quality, such as physical, chemical, and biological characteristics, and these water quality characteristics deicide if the water can be used or not [
32]. There is a gap around the water demand and water supply not only in Egypt but in the world [
33]; therefore, responsible authorities must improve and identify every detail in the water treatment process. Also, we must start to use effective and economical methods to purify the water, such as riverbank filtration. Many acceptable previous examples have proven that the RBF method is considered as a very effective method against heavy metals [
20]. The efficiency of the RBF process in the removal of organic micropollutants is considerably affected by the redox conditions in the aquifer such as carbamazepine, and sulfamethoxazole.
The utilization of riverbank filtration has expanded widely in Egypt, especially in Upper Egypt. The use of riverbank filtration as an easy and natural purification technique has demonstrated that it is beneficial for removing many types of contaminations and heavy metals. RBF is considered as a regulatory system used to eliminate heavy metals and microorganisms and is environmentally friendly because of the absence of chemicals in this technique [
34]. Governments and responsible parties must seek encouragement to use this process by doing the following: (1) Post guidelines or codes by specialist parties containing all requirements to reach the best outcomes. (2) Develop software, especially for RB systems, that contain good interface and various tools to make it easy to import all factors that affect the quality of RBF such as porosity of medium, temperature, quality of water. (3) Establish an institution concerned with RBF.
This research provides a detailed analysis of the riverbank filtration technique; it represents the outcome quality of the outlet water. To determine the outcomes of water quality, there are various procedures that can be done. There are two main lab experiments used to identify the power of RBF against heavy metals. The first experiment is applied to the soil located in the column of the RBF unit. Before placing the soil in the column, we must determine the properties and classification of the soil by using the soil classification test and permeability test. The second lab experiment is applied to determine the concentration of heavy metals of the outcome water samples by using a spectrophotometer.
In order to start the filtration process, the backwashing process, which will help in the maturation process to improve biological mechanisms during the filtration process, must be completed first. The filtration process is divided into five runs, and each run takes three days. The result-taking technique is performed as follows. First, we put a hundred liters on the CE 579-unit raw tank (this value is constant in all five runs). Then prepare the heavy metals in the lab. The concentration of heavy metals increases in each run or every three days. The results of the research’s experiment show the average percentage of heavy metal removal for iron, cobalt, lead, zinc, and copper are 74.04%, 74.44%, 70.72%, 75.1%, and 70.8%, respectively.
After the outcome results of the experiments, the water quality was calculated by using the water quality index calculator that was mentioned previously. This program is designed to determine the quality of the resulting water that riverbank filtration units receive. The main objective of this software is to quickly calculate the water quality and water quality index without conducting many manual calculations, also to identify the parameter problems or to identify which parameter does not meet the requirements.